Abstract
The thyroid-stimulating hormone receptor (TSHR), activated by either TSH or the newly discovered glycoprotein hormone thyrostimulin, plays a central role in the control of body metabolism. Interestingly, in addition to its thyroid expression, we discovered that the mRNA level of TSHR is periodically regulated in rat ovary by gonadotropins. Ovarian microdissection followed by real-time PCR analysis indicated that granulosa cells show the highest level of TSHR expression. Cultures of follicles and primary granulosa cells demonstrated that the level of TSHR is up-regulated and decreased by the gonadotropin-driven cAMP cascade and estradiol production, respectively. Furthermore, in contrast to the negligible expression of TSH in the ovary, we also found by real-time PCR and immunohistochemical analysis that thyrostimulin is expressed mainly in oocytes. Evolving before the appearance of gonadotropins, thyrostimulin is considered the most ancestral glycoprotein hormone. Therefore, the presence of thyrostimulin in the ovary suggests that it may have a primitive function in reproduction when it activates ovarian TSHR. Next, we generated recombinant thyrostimulin protein and characterized its non-covalent heterodimeric nature. Using purified recombinant thyrostimulin, we show that the human ovarian cell line NIH:OVCAR-3 also expresses endogenous and functional TSHR. Using cultured rat granulosa cells isolated from different ovarian stages, we found that treatments with thyrostimulin significantly increase cAMP production and the c-fos gene response in the presence of gonadotropins. Thus, this study demonstrates that oocyte-derived thyrostimulin and granulosa cell-expressed TSHR compose a novel paracrine system in the ovary, where the activity is tightly controlled by gonadotropins.
Keywords: G Protein-coupled Receptors (GPCR), Hormones/Peptide, Reproduction, Signal Transduction/Cyclic Nucleotides/Cyclic AMP, Signal Transduction/G proteins, Ovary, Thyroid-stimulating Hormone Receptor
Introduction
Together with the follicle-stimulating hormone receptor (FSHR)2 and luteinizing hormone receptor (LHR), the thyroid-stimulating hormone receptor (TSHR) belongs to a subfamily of G protein-coupled receptors that can be activated by heterodimeric glycoprotein hormones (1, 2). However, in contrast to FSHR and LHR, which are expressed in gonads and involved in the animal reproductive cycle (3), TSHR is expressed mainly in thyroid follicular cells and is involved in regulating the body metabolic rate (4, 5). Upon TSH (also named thyrotropin) binding, activated TSHR facilitates the synthesis as well as the release of thyroid hormones through the cAMP cascade; this increases the expression of thyroglobulin, thyroid peroxidase, and the sodium/iodine symporter (5, 6). Interestingly, in addition to TSH, the newly discovered glycoprotein hormone thyrostimulin, which is composed of glycoprotein α2 (GPA2) and glycoprotein β5 (GPB5) subunits, has been demonstrated to be a more potent ligand of TSHR than TSH itself (7). In contrast to TSH, thyrostimulin exhibits a different and wider distribution across many tissues, where it is suggested to act as a local but yet uncharacterized regulator (8, 9).
In addition to its expression in thyroid tissues, the presence of TSHR in many other mammalian tissues has also been documented. For example, high affinity TSH-binding sites and the existence of the TSHR transcript have been demonstrated in lymphocytes, brown adipose tissues, and erythrocytes, implying that TSHR has non-metabolic roles in immunoregulation (10), thermogenesis (11, 12), and local circulatory control (13), respectively. TSHR expression has also been found in folliculostellate cells in human anterior pituitary, where TSHR signaling seems to be regulated in a paracrine manner by thyrotroph-derived TSH (14). In addition, TSHR expression can also be detected in a number of non-thyroidal cells such as retro-ocular fibroblasts (15), osteocytes (16), hepatocytes (17), and neuronal cells and astrocytes (18), where the potential physiopathological roles of TSHR still await elucidation.
TSHR is phylogenetically close to FSHR and LHR, which are crucial to the control of animal reproduction. Therefore, one may speculate that TSHR may also play a role in regulating reproductive processes. It is interesting in this context that transcripts encoding TSHR have been found to be abundant in gonads of various teleosts (19–22). Recently, the ovarian expression of TSHR has also been demonstrated in mammals. Immunohistochemical staining and a cAMP assay indicated the existence of functional TSHR in mature human granulosa cells, suggesting that TSHR may participate in the regulation of ovarian function (23). The ovarian localization of TSHR protein has also been confirmed in the mature bovine corpus luteum, where it has been postulated to be involved in the synthesis of thyroid hormones or the modulation of progesterone synthesis locally (24). Nevertheless, how TSHR expression is controlled and the exact roles of TSHR in mammalian ovary remain unclear. Therefore, in this study, we characterized the regulatory pattern of TSHR during the ovarian cycle and demonstrated the existence of a potential ligand pair, thyrostimulin, but not TSH, in the ovary. We further generated recombinant thyrostimulin and used the protein to evaluate the paracrine action of oocyte-derived thyrostimulin on TSHR-containing granulosa cells.
EXPERIMENTAL PROCEDURES
Animals
Sprague-Dawley rats were obtained from the Laboratory Animal Center of the National Yang-Ming University. For time course analyses of the TSHR mRNA levels using a superovulation model, immature female rats (26 days old) were primed with 15 IU of pregnant mare serum gonadotropin (PMSG) at 0900–1000 h and received an intraperitoneal injection of 10 IU of human chorionic gonadotropin (hCG) 48 h later. Rats were then killed at different time points, and the ovaries were collected for total RNA extraction. All animals were housed under a controlled humidity, temperature, and light regimen and fed standard rat chow ad libitum. The rats were anesthetized and killed using CO2. Animal care was consistent with institutional guidelines for the care and use of experimental animals.
Reagents and Hormones
McCoy's 5A medium, Leibovitz L-15 medium, RPMI 1640 medium, l-glutamine, penicillin, and streptomycin were obtained from Invitrogen. PMSG, hCG, and human FSH were purchased from Calbiochem. The anti-progesterone antibody, anti-FLAG monoclonal antibody M1, anti-FLAG M1 affinity gel, progesterone, estradiol, androstenedione, 3-isobutyl-1-methylxanthine, 8-bromo-cAMP, forskolin, bovine serum albumin/fluorescein isothiocyanate-conjugated progesterone 3-(O-carboxymethyl)oxime, and other chemicals (unless noted) were purchased from Sigma. The anti-GPB5 antibody was obtained from immunized rabbits using the purified recombinant GPB5 protein from Escherichia coli (7).
Preparation of Ovarian Cells and Culture of Granulosa Cells
To assess the gene distribution in each ovarian compartment, the ovaries from immature rats (26 days old) were microdissected in Leibovitz L-15 medium by puncturing under a stereomicroscope (Nikon Instruments). Oocytes, granulosa cells, and theca-containing shells were individually collected. The purity of granulosa and theca cells was confirmed based on the differential expression of FSHR and LHR transcripts determined by real-time PCR (25). Oocytes were further treated with 350 units/ml hyaluronidase (Sigma) to remove additional cumulus cells. Corpora lutea were isolated after hCG treatment for 72 h.
For granulosa cell cultures, rat ovaries after the indicated treatments were punctured in Leibovitz L-15 medium. Ovarian debris and oocytes were removed, and the remaining medium containing granulosa cells was collected after low speed centrifugation at 500 × g for 10 min. Granulosa cells were dispersed by repeated washing and resuspended into McCoy's 5A medium supplemented with 10−7 m androstenedione, 2 mm l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 0.25 mm 3-isobutyl-1-methylxanthine. Cell numbers were adjusted to 2 × 105 viable cells/ml.
cDNA Isolation, Gene Amplification, and Quantification
To determine the c-fos transcript levels in granulosa cells, cells harvested from rats with different treatments were preincubated under serum-free conditions for 20 h before stimulation with 10 nm thyrostimulin in the presence or absence of gonadotropins for an additional 30 min. To analyze the changes in TSHR transcript levels in granulosa cells, cells isolated from immature 26-day-old rats were incubated with forskolin, 8-bromo-cAMP, or estradiol for 12 h before RNA extraction. The target tissues and treated cells were harvested, and their total RNAs were extracted using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. For first-strand cDNA synthesis, 2 μg of total RNA were reverse-transcribed using a RevertAidTM first-strand cDNA synthesis kit (Fermentas, Hanover, MD) with an oligo(dT) primer.
For semiquantitative PCR, the primer pairs for each gene were as follows: rat TSHR, ACCAGAAGCTTGACTTACATAGACC (forward) and CAGGTTCCGGATACTACTCTCATTA (reverse); Gpa2, AGGCAGCCGTCCCAATC (forward) and TCACTTCGCACTGTCACGTTAA (reverse); Gpb5, TGACGGTGAAGCTGCCTAACT (forward) and GGACAGCCATAGGGTAGGTGTAGA (reverse); Gpa1, CACGTGCTGTGTGGCCAA (forward) and CAGTGGCAGTCCGTGTGGT (reverse); TSHβ, CATCTGCCTGACCATCAACA (forward) and CCTGAGAGAGTGCGTACTTG (reverse); and β-actin, TGACAGACTACCTCATGAAGATCC (forward) and CTGCTTGCTGATCCACATCTG (reverse).
For quantitative TaqMan real-time PCR, a QuantiTect probe PCR kit (Qiagen, Valencia, CA) was used. The primer pairs and fluorescent probes for each gene were as follows: TSHR, ACCAGAAGCTTGACTTACATAGACC (forward) and CATGTAAGGGTTGTCTGTGATTTCT (reverse); TSHR probe, CAGAGCTCCCCTTGCTCAAGTTTCTTGG; β-actin, CTCTGTGTGGATTGGTGGCTC (forward) and CTGCTTGCTGATCCACATCTG (reverse); and β-actin probe, CCTGGCCTCACTGTCCACCTTCC.
For quantitative SYBR Green real-time PCR, a Maxima SYBR Green qPCR Master Mix kit (Fermentas) was used, and the primer pairs for each gene were as follows: Gpb5, TGACGGTGAAGCTGCCTAACT (forward) and GGACAGCCATAGGGTAGGTGTAGA (reverse), c-fos, GGGACAGCCTTTCCTACTACCAT (forward) and TGCGCAAAAGTCCTGTGTGT (reverse); and β-actin, CTCTGTGTGGATTGGTGGCTC (forward) and CTGCTTGCTGATCCACATCTG (reverse).
Expression and Purification of Recombinant Human Thyrostimulin Proteins
The full-length human GPA2 and GPB5 cDNAs were amplified by PCR from human ovarian cDNA (Clontech, Palo Alto, CA). To facilitate protein purification, GPA2 was further tagged with a FLAG epitope at the N terminus by replacing the endogenous signal peptide with a prolactin signal peptide and the epitope tag. The GPA2 and GPB5 cDNAs were then subcloned into the bipromoter expression vector pBudCE4.1 (Invitrogen). Fidelity of the PCR products was confirmed by sequencing of the final construct.
To generate recombinant thyrostimulin from the mammalian expression system, transfected 293T cells were clonally selected and confirmed to express both the GPA2 and GPB5 subunit proteins using antibodies against the FLAG epitope and GPB5. Conditioned medium was then purified using an anti-FLAG M1 affinity gel against FLAG-GPA2. Protein purity and biochemical characteristics were analyzed by electrophoresis using a 15% SDS-polyacrylamide gel.
Immunohistochemical Analyses
The ovaries from immature rats were fixed using Bouin's fixative for 8 h before paraffin embedding. Blocks were sectioned at 8 μm thickness. Immunohistochemical analyses were performed using the rabbit polyclonal antibody against GPB5 diluted at 1:100. Substitution for the primary antibody with rabbit preimmune serum served as a negative control. Staining was visualized using the VECTASTAIN ABC-AP kit (Vector Laboratories, Burlingame, CA) following the manufacturer's instructions. To neutralize specific epitope binding, the anti-GPB5 antibody was presaturated with purified thyrostimulin protein (1 μm) for 2 h at room temperature. The mixtures were then used on sections instead of the anti-GPB5 antibody alone. To eliminate any endogenous biotin background, the sections were pretreated using an avidin/biotin blocking kit (Vector Laboratories) following the manufacturer's instructions before adding the primary antibody.
Assessment of the Changes in cAMP and Progesterone Levels
To assess the bioactivity of purified thyrostimulin on human NIH:OVCAR-3 cells, cells were treated with increasing doses of thyrostimulin in RPMI 1640 medium supplemented with 2 mm l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 0.25 mm 3-isobutyl-1-methylxanthine for 16 h. To analyze the changes in cAMP and progesterone in granulosa cells, cells from rats after different treatments were resuspended in McCoy's 5A medium and dispensed into 12-well plates (Corning, Corning, NY) in triplicate. Following treatment with purified thyrostimulin in the presence or absence of gonadotropins for 48 h, the conditioned media were collected and stored at −80 °C until their cAMP and progesterone levels were measured. The amount of cAMP in the media was determined using the cAMP-Glo assay kit (Promega, Madison, WI) following the manufacturer's instructions. The amount of progesterone was measured by enzyme-linked immunosorbent assay using the specific anti-progesterone antibody.
Data Analysis
All experimental results are presented as the means ± S.D. of triplicate cultures or samples; at least two extra experiments showed similar results. Statistical significance was determined using Student's t test for multiple group comparisons. Significance was accepted at p < 0.05 and is indicated by asterisks in the figures unless noted otherwise.
RESULTS
Ovarian TSHR Expression and Its Regulation by Gonadotropins
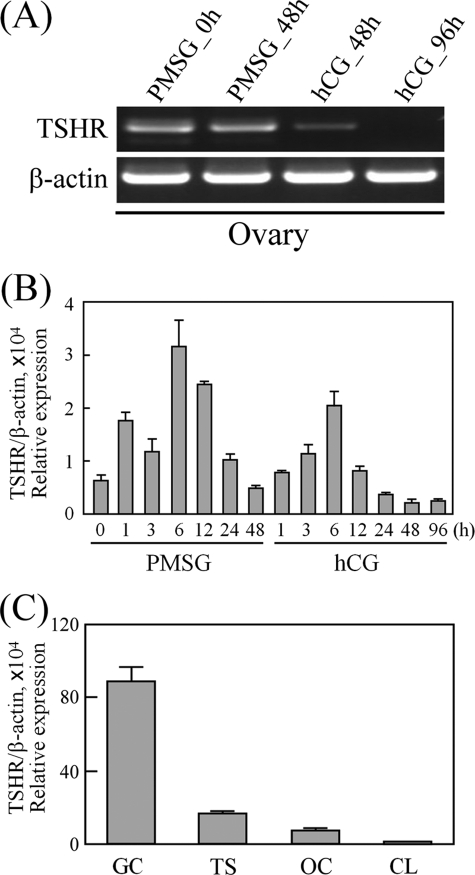
Using PCR amplification, the TSHR transcript could be detected in both the ovaries and adjacent oviducts of immature and mature rats (Fig. 1A). Interestingly, quantitative real-time PCR analysis showed that the TSHR mRNA level in immature ovaries was 6.5-fold higher than that in mature ovaries (Fig. 1B), which suggests that TSHR expression is regulated during ovarian maturation. To demonstrate this, the ovaries from superovulated immature rats treated with PMSG followed by hCG for different intervals were collected to assess changes in TSHR expression. Using PCR amplification, the ovarian TSHR transcripts after hCG treatment were found to be decreased (Fig. 2A). Therefore, the gonadotropin effects on TSHR expression were further characterized by quantitative real-time PCR. As shown in Fig. 2B, the TSHR mRNA level was up-regulated during the early stages immediately after injection of either PMSG or hCG but then gradually decreased after injection for 6 h. TSHR expression showed the lowest level at 48 and 96 h after hCG treatment. We subsequently analyzed TSHR expression in the various ovarian compartments by isolating cDNA from granulosa cells, theca-containing shells, luteal cells, and oocytes. As shown in Fig. 2C, the TSHR transcript was present mainly in granulosa cells and was lowest in the corpus luteum.
FIGURE 1.
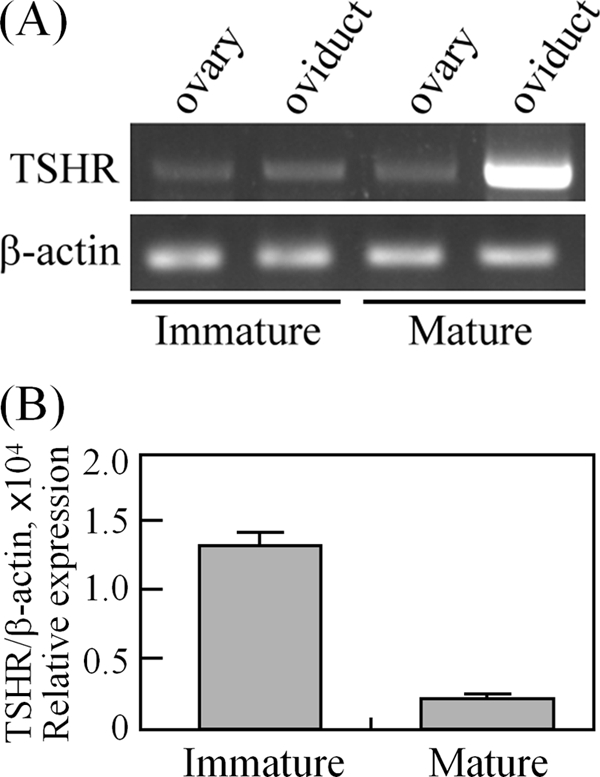
TSHR is expressed in the ovary and oviduct. cDNAs from immature (26 days old) and mature (8 weeks old) rat tissues were used for PCR amplification (A) and real-time quantification of TSHR expression levels (B). Data are expressed as the means ± S.D. The levels of β-actin served as loading and normalized controls.
FIGURE 2.
Expression and regulation of TSHR in the ovary. Rats at 26 days of age were treated with PMSG, followed 48 h later with hCG. The ovaries collected at the indicated intervals were used for cDNA preparation. The TSHR transcripts were compared by PCR amplification (A) and real-time PCR quantification (B). C, comparison of TSHR expression in different ovarian cell types by real-time PCR quantification. Granulosa cells (GC), theca shells (TS), and oocytes (OC) were isolated from rat ovaries (26 days old), whereas corpora lutea (CL) were obtained from PMSG-primed rats at 72 h after hCG treatment. Data are expressed as the means ± S.D. For all data, at least three individual repeats were done, and the β-actin levels served as internal and normalized controls.
Mechanism Underlying the Modulation of TSHR Levels by Gonadotropins
The changes in TSHR transcript levels in the superovulated rat model (Fig. 2B) suggest that ovarian TSHR expression is tightly controlled by gonadotropins. It is known that the gonadotropin actions lead to a prompt increase in cAMP levels in the ovary. In turn, these modulate steroidogenic genes that facilitate the synthesis and release of steroid hormones, mainly estradiol, as a late response to gonadotropins (26, 27). To further clarify the possible mechanisms underlying the modulation of ovarian TSHR levels by gonadotropins, the effects of cAMP and estradiol on TSHR expression were evaluated. As shown in Fig. 3A, treatments with graded doses of forskolin or 2 mm 8-bromo-cAMP increased the TSHR transcript level in cultured immature granulosa cells. Furthermore, we isolated early antral follicles and cultured them with estradiol (10−8 m) for different time intervals. The TSHR transcript was suppressed by 67% at 3 h after estradiol treatment and maintained this low level when incubated for longer periods (Fig. 3B). The effect of estradiol on isolated immature granulosa cells was also analyzed. Treatment with estradiol led to a dose-dependent suppression of the TSHR transcript in cultured granulosa cells (Fig. 3C).
FIGURE 3.
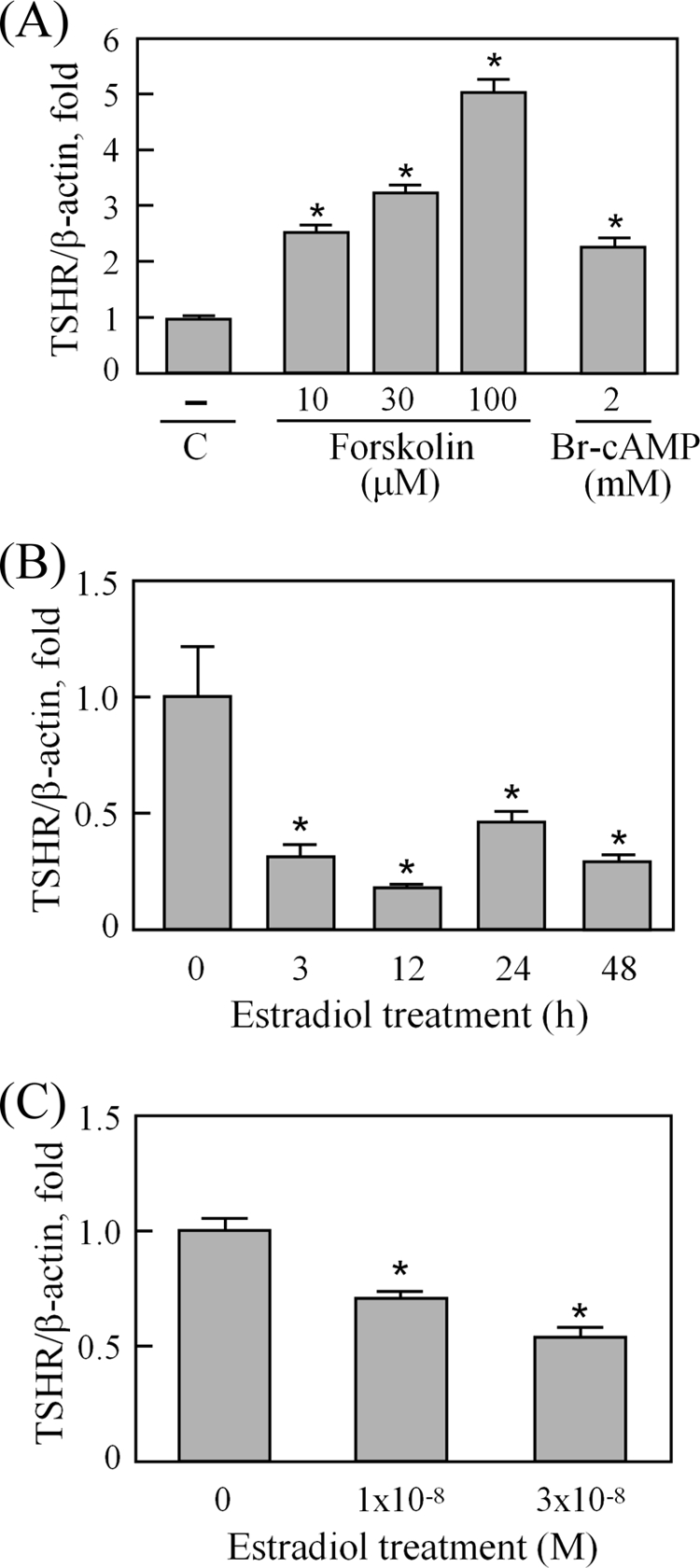
Forskolin, 8-bromo-cAMP, and estradiol effects on the TSHR transcript in the ovary. To evaluate the cAMP effects on TSHR expression, granulosa cells from 26-day-old rats were treated with graded doses of forskolin or 2 mm 8-bromo-cAMP (Br-cAMP) for 12 h (A). C, control. To test the estradiol effect, early antral follicles isolated from immature rats were incubated in the presence of 10−8 m estradiol for different intervals (B), and granulosa cells from 26-day-old rats were cultured in the presence of graded doses of estradiol for 12 h (C). After reverse transcription, the TSHR transcript levels were determined by real-time PCR and normalized using β-actin levels. Data were obtained from triplicate experiments and are shown as the means ± S.D. *, p < 0.05.
Thyrostimulin (GPA2/GPB5) Acts as a Paracrine Ligand for TSHR in the Ovary
TSHR can be activated by two heterodimeric glycoprotein hormones, TSH and thyrostimulin. To assess whether the ovary has the endogenous ligand for ovarian TSHR, the transcript of each glycoprotein subunit, Gpa1 and TSHβ for TSH and Gpa2 and Gpb5 for thyrostimulin, was evaluated using rat ovaries. The primer set for each gene has been previously tested using rat pituitary cDNA as a positive control. Primer pairs that were capable of amplifying a single-band correct gene product (data not shown) were then used to assess the gene transcript profile in the ovary. In contrast to the negligible expression of the TSHβ subunit for TSH, PCR amplification indicated that both Gpa2 and Gpb5 were expressed constitutively in ovaries treated with PMSG or with PMSG followed by hCG for different intervals (Fig. 4A). Using PCR amplification, we demonstrated that both the Gpa2 and Gpb5 transcripts can be detected in every isolated ovarian compartment (Fig. 4B). It is known the α-subunit genes exhibit a wider and more abundant distribution than the β-subunit genes; thus, the location of functional heterodimeric glycoprotein hormone depends on the expression of restricted β-subunits (9, 28). We therefore quantified the expression levels of Gpb5 in different ovarian compartments. As shown in Fig. 4C, oocytes contained the highest levels, whereas granulosa cells showed a moderate level of the Gpb5 transcript.
FIGURE 4.
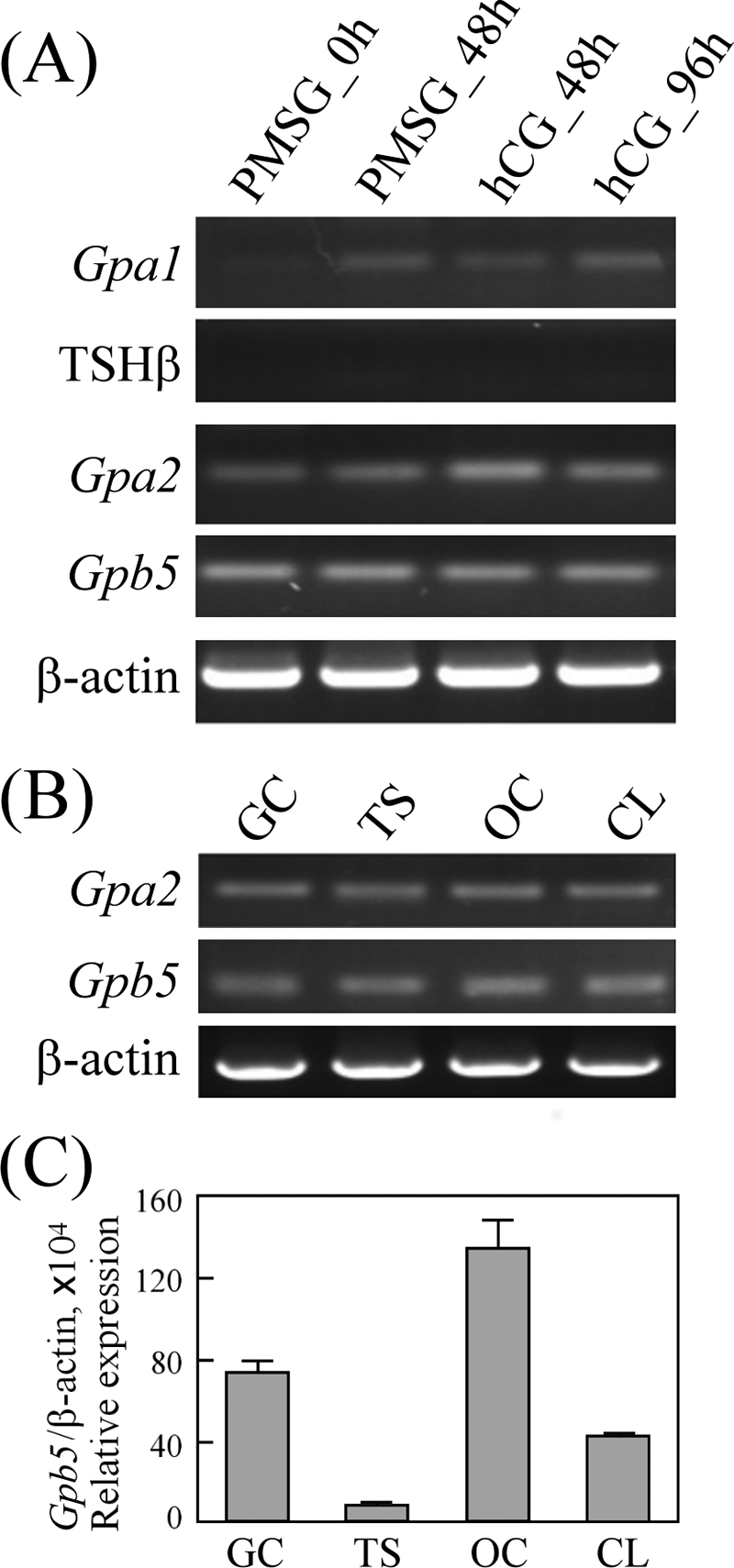
Thyrostimulin, but not TSH, is expressed in the ovary. A, ovaries collected at the indicated times from superovulated rats were used for cDNA preparation. The subunit genes, Gpa1 and TSHβ for TSH and Gpa2 and Gpb5 for thyrostimulin, were PCR-amplified. To assess the transcript levels of Gpa2 and Gpb5 in various ovarian compartments, cDNAs from different ovarian cell types were isolated for PCR amplification of the Gpa2 and Gpb5 genes (B) and real-time quantification of the Gpb5 gene (C). Data are expressed as the means ± S.D. For all data, at least three individual repeats were done, and β-actin levels served as internal and normalized controls. GC, granulosa cells; TS, theca shells; OC, oocytes; CL, corpora lutea.
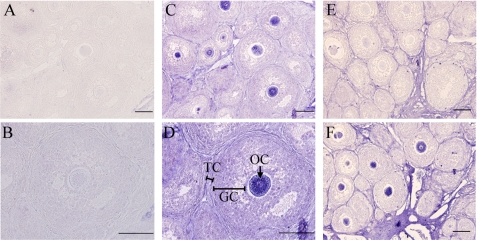
Interestingly, immunohistochemical staining indicated a strong GPB5 immunoreactivity in oocytes but not in granulosa cells (Fig. 5, A–D). Similar results were also obtained using the horseradish peroxidase system counterstained with hematoxylin (supplemental Fig. 1). The synthesis of GPB5 protein in granulosa cells is thus likely to be low or blocked at the translational level by a yet uncharacterized mechanism. To confirm the positive GPB5 staining found in oocytes, an antigen blocking assay was performed. As shown in Fig. 5E, GPB5 staining was completely abolished when the antibody was preabsorbed with the purified GPA2/GPB5 heterodimer. In addition, the intensity of GPB5 staining was increased after the sample was pretreated with avidin/biotin-blocking reagents that reduce the nonspecific signal from endogenous biotin or biotin-binding molecules (Fig. 5F). It is also known that thyrostimulin is ∼30-fold more potent than TSH in terms of activating TSHR (8). Therefore, these results suggest the thyrostimulin, but not TSH protein, is expressed in oocytes and acts as a paracrine factor for TSHR signaling in the ovary.
FIGURE 5.
Distribution of the thyrostimulin protein in the ovary. Ovarian sections from immature rats (26 days old) were incubated under different conditions as indicated, A and B, normal rabbit serum at different magnifications; C and D, anti-GPB5 antibody at different magnifications; E, anti-GPB5 antibody preneutralized with the purified thyrostimulin protein; F, addition of an additional avidin/biotin-blocking step to quench the endogenous non-specificity of the biotin background before incubation with the anti-GPB5 antibody. GC, granulosa cells; TC, theca cells; OC, oocytes. Bars = 100 μm.
Purification of Recombinant Thyrostimulin and Its Effects on Cultured Ovarian Cells
To study the ovarian functions of thyrostimulin, conditioned media collected from transfected 293T cells stably expressing FLAG-tagged GPA2/GPB5 were used for recombinant protein purification. Following affinity purification against the FLAG tag appended to GPA2, the purity and heterodimeric property of thyrostimulin were confirmed by Coomassie Blue staining and Western blotting. Under reducing conditions, purified thyrostimulin could be separated into two subunits, namely GPA2 and GPB5, and migrated as bands at ∼22 and ∼18 kDa, respectively (supplemental Fig. 2A). These two bands were confirmed to be GPA2 and GPB5 monomers using the specific antibodies against the FLAG epitope on GPA2 and the GBP5 epitope (supplemental Fig. 2B, first and third lanes). To further demonstrate the non-covalent heterodimeric nature of purified thyrostimulin, we performed a cross-linking analysis using disuccinimidyl suberate. After cross-linking, both Coomassie Blue staining (supplemental Fig. 2A, fourth lane) and Western blotting (supplemental Fig. 2B, second and fourth lanes) revealed the presence of a heterodimer, migrating as a band at ∼40 kDa.
The bioactivity of purified thyrostimulin was confirmed by the protein's ability to stimulate cAMP production in TSHR-transfected 293T cells (data not shown). Furthermore, to test the potency of purified thyrostimulin and the existence of endogenous TSHR in ovarian-derived cells, human NIH:OVCAR-3, an ovarian cell line from a patient with progressive adenocarcinoma of the ovary (29), was selected. Treatments with purified thyrostimulin stimulated cAMP production in a dose-dependent manner. PCR amplification also showed the presence of the TSHR transcript in NIH:OVCAR-3 cells (supplemental Fig. 2C). These results confirm that recombinant thyrostimulin is bioactive and the existence of functional TSHR in some ovarian cells.
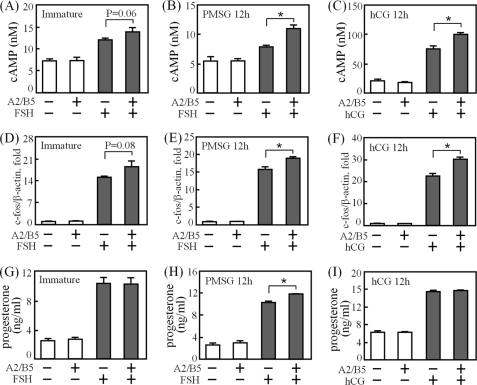
Based on the TSHR expression pattern in the ovary, TSHR is abundant in rat granulosa cells and shows higher transcript levels after injection of gonadotropins for 6 h (Fig. 2). In consideration of the gonadotropin effects and the potential delay in TSHR translation, granulosa cells from untreated rats, rats primed with PMSG for 12 h, and rats primed with PMSG for 48 h followed by hCG injection for 12 h were harvested and treated with either buffer or thyrostimulin in the presence or absence of gonadotropins (Fig. 6). In contrast to the negligible effect on cells treated with thyrostimulin alone, we demonstrated that thyrostimulin was able to augment cAMP production (Fig. 6, A–C) and c-fos gene transcription (Fig. 6, D–F) to a statistically significant level when the cells were co-treated with gonadotropins, suggesting that the expression of functional TSHR protein is gonadotropin-inducible. However, progesterone production was not affected by the thyrostimulin treatments except for a slight increase when PMSG-primed granulosa cells were co-treated with FSH (Fig. 6, G–I). This phenomenon, which will be discussed below, is likely to be a result of the tight control of ovarian TSHR expression by gonadotropins.
FIGURE 6.
Effects of thyrostimulin on cAMP, c-fos gene, and progesterone biosyntheses in granulosa cells treated with or without gonadotropins. Granulosa cells from immature rats (left panels) or from rats injected with PMSG for 12 h (middle panels) or with PMSG for 48 h followed by hCG for 12 h (right panels) were treated with thyrostimulin (indicated as A2/B5) in the presence or absence of gonadotropins as indicated. For the cAMP (A–C) and progesterone (G–I) assays, cells were incubated for an additional 48 h before measurements. For c-fos gene induction (D–F), cells were serum-starved for 20 h before thyrostimulin and gonadotropin treatments for an additional 30 min. Cells were then collected for cDNA preparation. The c-fos gene levels were quantified by real-time PCR and normalized against the β-actin expression level. At least three individual repeats were done. Data were obtained from triplicate experiments and are shown as the means ± S.D. *, p < 0.05.
DISCUSSION
To our knowledge, this is the first report that demonstrates the coexistence of TSHR and its cognate ligand thyrostimulin in mammalian ovary, where they compose a novel paracrine system. Taken together with our results, a model regarding the regulatory control of TSHR and the action of thyrostimulin in the ovary is presented in Fig. 7. We have shown that thyrostimulin, but not TSH, is located in oocytes, where it can be secreted and act as a local paracrine factor to activate the cAMP cascade and nuclear c-fos response in granulosa cells through TSHR. Contrary to the constitutive expression of thyrostimulin in the ovary, the TSHR transcript is tightly regulated during ovarian development. At the early stages of gonadotropin actions, either the transcript or the functional protein of TSHR is up-regulated. Such an up-regulation is likely to be triggered by an increase in the secondary messenger cAMP derived from activated gonadotropin receptors. This hypothesis is further supported by our results showing that there is an increase in the TSHR transcriptional levels in granulosa cells when treated with forskolin or 8-bromo-cAMP (Fig. 3A). Interestingly, a functional cAMP-response element between −139 and −131 bp upstream of the translational initiation site of the human TSHR gene has also been characterized and is known to be conserved in mammals (30). It is known that an increase in gonadotropins will stimulate follicle growth and luteinization, which is accompanied by a significant increase in the estradiol level. Using cultured rat granulosa cells treated with estradiol, we have also shown that estradiol is likely to be the major regulator that decreases ovarian TSHR expression (Fig. 3C). Such a result is consistent with the marked decrease in the TSHR transcript observed at the late stages of gonadotropin treatments in the superovulation model (Fig. 2B). We conclude that the ovarian TSHR level and signaling are tightly controlled by gonadotropins through the cAMP and steroid feedback loop. This implies that the induction of functional TSHR expression is periodic and transient during ovarian development. Furthermore, the down-regulation of TSHR expression by gonadotropin-driven estradiol production also explains why there is only <50% cAMP and c-fos augmentation and no apparent difference in progesterone production during long-term treatments with thyrostimulin in gonadotropin-primed granulosa cells (Fig. 6). Alternatively, TSHR signaling may serve to modulate different ovarian functions other than steroidogenesis. To characterize these, a more complete inventory of TSHR function with the elimination of estradiol interference will be needed to understand the exact roles of TSHR in folliculogenesis and luteinization.
FIGURE 7.
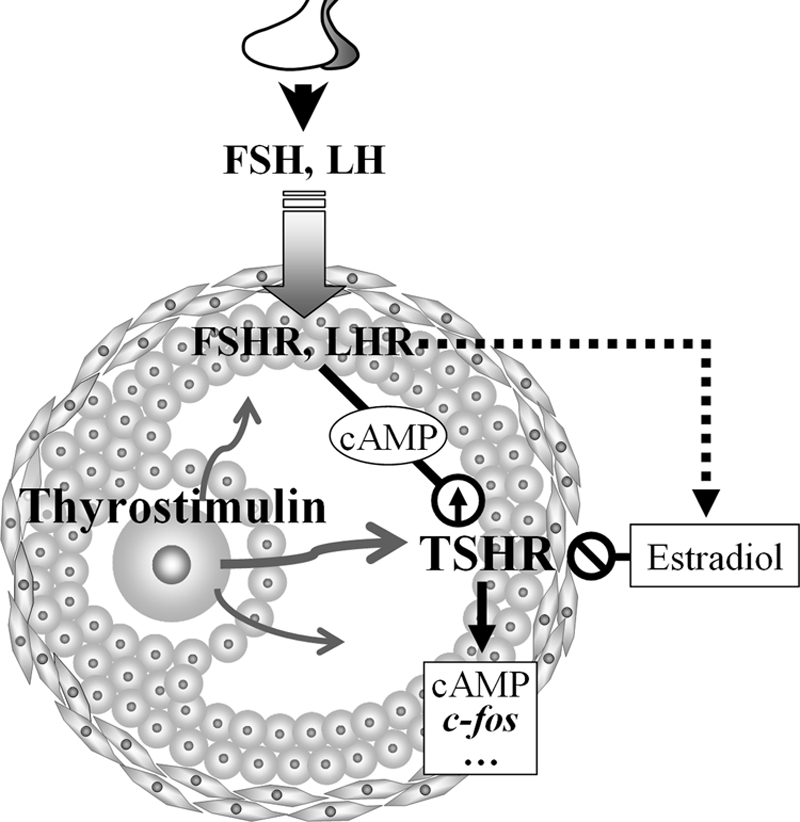
Paracrine action and regulatory mechanism of thyrostimulin-TSHR signaling in the ovary. Oocyte-derived thyrostimulin and granulosa cell-expressed TSHR compose a paracrine system in the ovary, where its action is tightly controlled by gonadotropin surges.
In addition to our results showing regulation of the TSHR transcript during the mammalian ovarian cycle, TSHR expression in fish ovaries has also been found and demonstrated to change significantly during the reproductive season. The ovarian expression of TSHR in channel catfish remains low throughout vitellogenesis but is markedly elevated at the spawning period and then gradually decreases afterward until the next run of ovarian recrudescence (22). A similar expression pattern of ovarian TSHR has also been reported in European seabass (21), suggesting that TSHR could participate in active vitellogenesis and in the regulation of gamete maturation and ovulation.
In addition, TSHR expression has also been found in fish testes. In European seabass, the TSHR transcript in the testis increases and remains high during recrudescence but declines and reaches its lowest level at the post-spawning stage, suggesting that TSHR in the testis is involved in the processes of gonadal development and/or spermiation (21). It is known that TSHR has the same evolutionary origin as FSHR and LHR and that the ligands of these proteins are all glycoprotein hormones. Therefore, the findings of TSHR expression in both the testis and ovary suggest that, like FSHR and LHR, TSHR may play a direct role in the regulation of gonadal physiology.
Recent genomic analysis has indicated that glycoprotein hormones coevolved with their receptors and that their presence can be traced back to invertebrates (2, 28). Glycoprotein hormones are composed of a common α-subunit (GPA1) and a dissimilar β-subunit such as FSHβ, LHβ, CGβ, and TSHβ. In addition, two newly discovered glycoprotein subunits, GPA2 and GPB5, were found to form thyrostimulin (7). Amphioxus, a member of the phylum Chordata, is considered to be in an evolutionary lineage between invertebrates and vertebrates, and this organism has been reported to have no other glycoprotein hormone except for thyrostimulin (31). This evolutionary analysis indicates that the two heterodimeric glycoprotein subunits GPA2 and GPB5, which form thyrostimulin, are likely to be the most ancient origins of the gonadal and thyroid glycoprotein hormones. They may have appeared in the early Cambrian period. Furthermore, in Drosophila melanogaster, a pair of glycoprotein genes, Gpa2 and Gpb5, has been reported and shown to have high homology to mammalian Gpa2 and Gpb5 (32). Fly GPA2 is capable of forming a heterodimer with fly GPB5 to activate a fly receptor, the Drosophila leucine-rich repeat-containing G protein-coupled receptor-1, which shows high homology to mammalian TSHR. No other pair of gonadotropin-like molecules and receptors is found in the fly. These results, together with the study with amphioxus, strongly support the idea that the Gpa2 and Gpb5 genes, evolving before the appearance of the gonadotropin genes, are indeed the ancient origins of glycoprotein hormones. Therefore, one may also speculate that the primitive roles of the thyrostimulin-like molecule may also involve reproductive control and gonadal development in addition to regulation of the metabolic rate.
From the viewpoint of an in vitro bioactivity assay, thyrostimulin is likely to serve as an alternative ligand of TSH for activating TSHR. However, its exact roles are still unknown. Patients with lost-of-function mutations of TSHβ exhibit congenital central hypothyroidism (33, 34), whereas Gpb5-null mice show no overt thyroid-related phenotype (8, 35). Distinct from TSH expressed in thyrotrophs, thyrostimulin expression in the anterior pituitary is located at the corticotrophs, where its release has been demonstrated not to be in response to thyrotropin-releasing hormone (8, 9). These results indicate that thyrostimulin is unlikely to be involved in the thyrotropin-releasing hormone-TSH-thyroid hormone feedback loop and substitute for the TSH activity in animals. It is known that corticotrophs produce adrenocorticotropic hormone, which is secreted in response to the release of hypothalamus-derived corticotropin-releasing hormone and acts on the adrenal gland under stress conditions. The expression of thyrostimulin in corticotrophs suggests that thyrostimulin may also be controlled through the hypothalamus-pituitary-adrenal axis. Interestingly, the expression of TSHR has also been found in the adrenal gland (36). Future experiments will be critical to clarify whether thyrostimulin responds to corticotropin-releasing hormone stimulation and thus plays a non-thyroidal role in the control of homeostasis or immune suppressive effects.
Although activating the same receptor as TSH, the receptor-binding characteristics of thyrostimulin are quite different from those of TSH. Binding and competition assays indicate that there are two ligand-binding sites on TSHR. One site can interact with either thyrostimulin or TSH, whereas the other site can bind only thyrostimulin alone (8). The Scatchard plot for iodinated thyrostimulin binding fits a two-site model. The calculated kd values are 1.19 ± 0.68 nm for the high affinity site and 2.7 ± 2.2 mm for the low affinity site. In contrast, the kd for iodinated TSH binding is estimated at 41 nm. This indicates that thyrostimulin is a more potent ligand than TSH. Such a result is also supported by Nakabayashi et al. (7), who showed that thyrostimulin can stimulate cAMP production more efficiently than TSH in cells expressing recombinant TSHR.
Unlike TSH, which is expressed mainly in mammalian anterior pituitary, the existence of thyrostimulin has been found in many different tissues such as the anterior pituitary, skin, retina, adrenal gland, pancreas, central nervous system, and testis (8, 37), suggesting that thyrostimulin may act as a local regulator rather than as an endocrine hormone. Our results also indicate that thyrostimulin, but not TSH, can be expressed in rat oocytes as a paracrine factor for TSHR-expressing granulosa cells. Although we cannot rule out an endocrine effect of TSH on ovarian TSHR, on the basis of the ligand binding data described above, which show that the bioactivity of thyrostimulin is ∼30-fold more potent than that of TSH (8), one may discard the possibility of TSH interference with the paracrine action between thyrostimulin and TSHR in the ovarian compartments. Surprisingly, in situ hybridization results have indicated that the amphioxus Gpb5 transcript is abundant in previtellogenic oocytes (31), which also supports the hypothesis that thyrostimulin may have a primitive function in invertebrate ovary. In addition to the ovary, several studies also suggest that thyrostimulin and TSHR are possibly coexpressed in vertebrate testis (8, 19, 21, 37). Future experiments are needed to clarify their compartmental distribution and their signal transduction effect. These will provide a more comprehensive understanding of these proteins' paracrine and/or autocrine activity in testicular development and spermatogenesis.
Supplementary Material
This work was supported by Yen Tjing Ling Medical Foundation Grant CI-97-1, National Science Council Grant NSC97-2311-B-010-001-MY3, and the Ministry of Education Aim for the Top University Plan in Taiwan.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
- FSHR
- follicle-stimulating hormone receptor
- LHR
- luteinizing hormone receptor
- TSHR
- thyroid-stimulating hormone receptor
- PMSG
- pregnant mare serum gonadotropin
- hCG
- human chorionic gonadotropin.
REFERENCES
- 1.Kleinau G., Krause G. (2009) Endocr. Rev. 30, 133–151 [DOI] [PubMed] [Google Scholar]
- 2.Luo C. W., Hsueh A. J. (2006) Chang Gung Med. J. 29, 2–8 [PubMed] [Google Scholar]
- 3.Themmen A. P. N., Huhtaniemi I. T. (2000) Endocr. Rev. 21, 551–583 [DOI] [PubMed] [Google Scholar]
- 4.Davies T. F., Ando T., Lin R. Y., Tomer Y., Latif R. (2005) J. Clin. Invest. 115, 1972–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vassart G., Dumont J. E. (1992) Endocr. Rev. 13, 596–611 [DOI] [PubMed] [Google Scholar]
- 6.Ludgate M., Gire V., Crisp M., Ajjan R., Weetman A., Ivan M., Wynford-Thomas D. (1999) Oncogene 18, 4798–4807 [DOI] [PubMed] [Google Scholar]
- 7.Nakabayashi K., Matsumi H., Bhalla A., Bae J., Mosselman S., Hsu S. Y., Hsueh A. J. (2002) J. Clin. Invest. 109, 1445–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okada S. L., Ellsworth J. L., Durnam D. M., Haugen H. S., Holloway J. L., Kelley M. L., Lewis K. E., Ren H., Sheppard P. O., Storey H. M., Waggie K. S., Wolf A. C., Yao L. Y., Webster P. J. (2006) Mol. Endocrinol. 20, 414–425 [DOI] [PubMed] [Google Scholar]
- 9.Nagasaki H., Wang Z., Jackson V. R., Lin S., Nothacker H. P., Civelli O. (2006) J. Mol. Endocrinol. 37, 39–50 [DOI] [PubMed] [Google Scholar]
- 10.Francis T., Burch H. B., Cai W. Y., Lukes Y., Peele M., Carr F. E., Wartofsky L., Burman K. D. (1991) Thyroid 1, 223–228 [DOI] [PubMed] [Google Scholar]
- 11.Roselli-Rehfuss L., Robbins L. S., Cone R. D. (1992) Endocrinology 130, 1857–1861 [DOI] [PubMed] [Google Scholar]
- 12.Endo T., Kobayashi T. (2008) Am. J. Physiol. Endocrinol. Metab. 295, E514–E518 [DOI] [PubMed] [Google Scholar]
- 13.Balzan S., Del Carratore R., Nicolini G., Forini F., Lubrano V., Simili M., Benedetti P. A., Iervasi G. (2009) Cell Biochem. Funct. 27, 259–263 [DOI] [PubMed] [Google Scholar]
- 14.Brokken L. J., Bakker O., Wiersinga W. M., Prummel M. F. (2005) Exp. Clin. Endocrinol. Diabetes 113, 13–20 [DOI] [PubMed] [Google Scholar]
- 15.Mengistu M., Lukes Y. G., Nagy E. V., Burch H. B., Carr F. E., Lahiri S., Burman K. D. (1994) J. Endocrinol. Invest. 17, 437–441 [DOI] [PubMed] [Google Scholar]
- 16.Abe E., Marians R. C., Yu W., Wu X. B., Ando T., Li Y., Iqbal J., Eldeiry L., Rajendren G., Blair H. C., Davies T. F., Zaidi M. (2003) Cell 115, 151–162 [DOI] [PubMed] [Google Scholar]
- 17.Zhang W., Tian L. M., Han Y., Ma H. Y., Wang L. C., Guo J., Gao L., Zhao J. J. (2009) J. Cell. Mol. Med. 10.1111/j.1582-4934.2008.00670.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crisanti P., Omri B., Hughes E., Meduri G., Hery C., Clauser E., Jacquemin C., Saunier B. (2001) Endocrinology 142, 812–822 [DOI] [PubMed] [Google Scholar]
- 19.Kumar R. S., Ijiri S., Kight K., Swanson P., Dittman A., Alok D., Zohar Y., Trant J. M. (2000) Mol. Cell. Endocrinol. 167, 1–9 [DOI] [PubMed] [Google Scholar]
- 20.Vischer H. F., Bogerd J. (2003) J. Mol. Endocrinol. 30, 227–238 [DOI] [PubMed] [Google Scholar]
- 21.Rocha A., Gómez A., Galay-Burgos M., Zanuy S., Sweeney G. E., Carrillo M. (2007) Gen. Comp. Endocrinol. 152, 89–101 [DOI] [PubMed] [Google Scholar]
- 22.Goto-Kazeto R., Kazeto Y., Trant J. M. (2009) Gen. Comp. Endocrinol. 161, 313–319 [DOI] [PubMed] [Google Scholar]
- 23.Aghajanova L., Lindeberg M., Carlsson I. B., Stavreus-Evers A., Zhang P., Scott J. E., Hovatta O., Skjöldebrand-Sparre L. (2009) Reprod. Biomed. Online 18, 337–347 [DOI] [PubMed] [Google Scholar]
- 24.Mutinati M., Desantis S., Rizzo A., Zizza S., Ventriglia G., Pantaleo M., Sciorsci R. L. (2009) Anim. Reprod. Sci. doi:10.1016/j.anireprosci.2009.05.019 [DOI] [PubMed] [Google Scholar]
- 25.Luo C. W., Kawamura K., Klein C., Hsueh A. J. (2004) Mol. Endocrinol. 18, 2085–2096 [DOI] [PubMed] [Google Scholar]
- 26.Strauss J. F., 3rd, Golos T. G., Silavin S. L., Soto E. A., Takagi K. (1988) Prog. Clin. Biol. Res. 267, 177–200 [PubMed] [Google Scholar]
- 27.Hsueh A. J., Lapolt P. S. (1992) Trends Endocrinol. Metab. 3, 164–170 [DOI] [PubMed] [Google Scholar]
- 28.Trudeau V. L. (2009) Endocrinology 150, 3446–3447 [DOI] [PubMed] [Google Scholar]
- 29.Hamilton T. C., Young R. C., McKoy W. M., Grotzinger K. R., Green J. A., Chu E. W., Whang-Peng J., Rogan A. M., Green W. R., Ozols R. F. (1983) Cancer Res. 43, 5379–5389 [PubMed] [Google Scholar]
- 30.Saiardi A., Falasca P., Civitareale D. (1995) Biochem. J. 310, 491–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tando Y., Kubokawa K. (2009) Gen. Comp. Endocrinol. 162, 329–339 [DOI] [PubMed] [Google Scholar]
- 32.Sudo S., Kuwabara Y., Park J. I., Hsu S. Y., Hsueh A. J. (2005) Endocrinology 146, 3596–3604 [DOI] [PubMed] [Google Scholar]
- 33.Heinrichs C., Parma J., Scherberg N. H., Delange F., Van Vliet G., Duprez L., Bourdoux P., Bergmann P., Vassart G., Refetoff S. (2000) Thyroid 10, 387–391 [DOI] [PubMed] [Google Scholar]
- 34.Miyai K., Azukizawa M., Kumahara Y. (1971) N. Engl. J. Med. 285, 1043–1048 [DOI] [PubMed] [Google Scholar]
- 35.Macdonald L. E., Wortley K. E., Gowen L. C., Anderson K. D., Murray J. D., Poueymirou W. T., Simmons M. V., Barber D., Valenzuela D. M., Economides A. N., Wiegand S. J., Yancopoulos G. D., Sleeman M. W., Murphy A. J. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 2496–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dutton C. M., Joba W., Spitzweg C., Heufelder A. E., Bahn R. S. (1997) Thyroid 7, 879–884 [DOI] [PubMed] [Google Scholar]
- 37.Li C., Hirooka Y., Habu S., Takagi J., Gotoh M., Nogimori T. (2004) Endocr. Regul. 38, 131–142 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.