Abstract
Apoptotic cells are opsonized by complement components such as C1q and C3b, which increases their susceptibility to phagocytosis. Soluble complement inhibitors such as factor H (fH) also recognize apoptotic cells to minimize the pro-inflammatory effects of downstream complement activation. We used four radiolabeled protein constructs that span different regions of the 20 complement control protein (CCP) modules that make up fH and found that fragments comprising CCPs 6–8, CCPs 8–15, and CCPs 19–20 but not CCPs 1–4, bound to apoptotic Jurkat T cells. There are four possible ligand types on apoptotic cells that could recruit fH: proteins, carbohydrates, lipids, and DNA. We found that CCPs 6–8 of fH bind to annexin-II, a trypsin-insensitive protein that becomes exposed on surfaces of apoptotic cells. The second ligand of fH, which interacts with CCPs 6–8 and 19–20, is DNA. Confocal microscopy showed co-localization of fH with antibodies specific for DNA. fH also binds to histones devoid of DNA, and CCPs 1–4, 6–8, and 8–15 mediate this interaction. Treatment of apoptotic cells with neuraminidase, chondroitinase, heparitinase, and heparinase did not change fH binding. Treatment of apoptotic cells with phospholipase A2 dramatically increased both binding of fH and cell-surface DNA. We also excluded the possibility that fH interacts with lysophospholipids using surface plasmon resonance and flow cytometry with lipid-coated beads. Identification of annexin-II as one of the fH ligands on apoptotic cells together with the fact that autoantibodies against annexin-II are found in systemic lupus erythematosus provides further insight into understanding the pathogenesis of this disease.
Keywords: Apoptosis, DNA, Histones, Immunology/Innate Immunity, Annexin-II, Complement, Factor H
Introduction
Programmed cell death, or apoptosis, is an essential constituent of immune system homeostasis assuring “silent” and non-inflammatory clearance of cells. It is characterized by cell shrinkage, cytoplasmic and nuclear chromatin condensation, intranucleosomal “ladder-type” DNA fragmentation, and membrane blebbing (1). Furthermore the asymmetric distribution of membrane lipids changes: phosphatidylserine (PS)2 that is normally exclusively present in the inner leaflet of the plasma membrane translocates to the outside (2) and cardiolipin, which is mostly confined to the mitochondrial membrane, appears on the surface (3). The hydrolysis of sphingomyelin to ceramide in the plasma membrane also accompanies apoptosis (4). During apoptosis the profile of cell-surface molecules changes in that molecules normally absent on the cell surface become exposed, whereas others disappear. Some of these changes induce phagocytosis of apoptotic cells either directly or via extracellular bridging molecules.
Complement is a central part of the innate immune system. Besides its classic functions such as opsonization of pathogens, generation of inflammatory mediators, and cell lysis (5), its role in the recognition and removal of apoptotic and necrotic cells is of significant importance (6). C1q, the initiator molecule of the classic complement pathway, and mannose-binding lectin (MBL) that activates the lectin pathway, both bind to dying (apoptotic and necrotic) cells (7–9). Binding of C1q and MBL leads to opsonization and facilitation of phagocytosis (10). The alternative complement pathway is activated directly by properdin bound to dying cells (11, 12), and it also acts as the amplification loop for the classic and lectin pathways. A balance between activation and inhibition of complement is essential, because both erroneous/excessive as well as missing/decreased complement activation contribute significantly to the pathology of many diseases (13). Therefore, it is no surprise that a whole set of fluid-phase and membrane-bound inhibitors exist that tightly regulate the system. We showed previously that both major fluid-phase complement inhibitors, factor H (fH) of the alternative pathway and C4b-binding protein (C4BP) of the classic and lectin pathways, bind to apoptotic and necrotic cells, and limit complement activation by these cells (14, 15). This allows the binding of C1q/MBL and a certain degree of complement activation but prevents overt inflammation. C4BP binds to dying cells mainly via protein S with which it forms a tight complex and thus binds to the PS that appears on the surface of dying cells (15). The one or more ligands for fH on apoptotic cells on the other hand are, as yet, unknown.
fH, a 155-kDa glycoprotein, is one of the most abundant human plasma proteins (∼550 μg/ml). It is composed of 20 complement control protein (CCP) domains, of which the four N-terminal CCPs contain the complement regulatory activity (9) and the C-terminal two CCPs mediate surface binding and target recognition (16). The facts that fH does not bind to live Jurkat T cells and its binding increases with the degree of apoptosis (14) indicate that the fH ligand becomes exposed on the cell surface during the apoptotic process. Apoptotic cells that are not removed efficiently undergo secondary necrosis, a pro-inflammatory and pro-immunogenic event that can lead to the development of autoimmune diseases. It is therefore of great importance to understand which surface changes result in fH binding with the concomitant prevention of the generation of anaphylatoxins and membrane attack complexes but retention of opsonization and phagocytosis.
In this study we searched for fH ligands on apoptotic cells. We found at least three ligands for fH on apoptotic cells: DNA, histones, and the phospholipid-binding protein annexin-II, which has recently been suggested as a fH-binding partner in a mouse ischemia/reperfusion model (17).
EXPERIMENTAL PROCEDURES
Cells and Induction of Cell Death
Jurkat T cells (ATCC) were grown in RPMI media containing glutamine and 10% heat-inactivated fetal calf serum (all from Invitrogen). Apoptosis was induced using 1 μm staurosporine (Sigma) for 4 h (if not stated otherwise) in RPMI media without fetal calf serum at 37 °C and 5% CO2. Necrosis was induced by 30-min incubation at 56 °C (15).
Proteins, Antibodies, and Sera
fH (18), C4BP (19), and prothrombin (obtained as a side fraction during purification of C4BP) were purified from human plasma as described; the purity was at least 95%, as judged by Coomassie Brilliant Blue staining of proteins separated by SDS-PAGE. fH fragments were produced in Pichia pastoris as previously described (20, 21). Histones were isolated from Jurkat T-cells using a histone isolation kit (Active Motif) following the manufacturer's instructions. Eluted fractions were separated by 15% SDS-PAGE and stained with Coomassie Brilliant Blue. Fraction IV from the H2A/H2B plus H1 elution and fraction II from H3/H4 elution were considered most suitable for binding assays. For flow cytometric analysis and confocal microscopy, the proteins were labeled either with Alexa Fluor 488 (AF488, Molecular Probes) or with DyLight 488 and 633 (DL488/DL633, Pierce), respectively, according to the manufacturer's instructions. fH and its fragments were also labeled with 125I using the chloramine T method. The specific activity was 0.4–0.5 MBq/μg of protein.
The following antibodies were used: mouse anti-human dsDNA (Immunotools), goat anti-human fH (Quidel), goat anti-human fH (Calbiochem), rabbit anti-human annexin-II (Abcam), mouse anti-human CD45, mouse anti-human CD4 (Immunotools), fluorescein isothiocyanate (FITC)-labeled swine anti-rabbit, FITC-labeled rabbit anti-human C3c (Dako) Alexa Fluor 647 (AF647)-labeled goat anti-mouse, AF647-labeled rabbit anti-goat (Invitrogen), horseradish peroxidase (HRP)-labeled rabbit anti-goat and swine anti-rabbit secondary antibodies (Dako), and IgG1 and IgG2a isotype controls (Immunotools). Normal human serum (NHS) was prepared from blood of six healthy volunteers as described previously (22).
Binding of fH Fragments to Dying Cells
Jurkat T cells were rendered apoptotic and washed twice with 10 mm HEPES, 150 mm NaCl, 5 mm KCl, 1 mm MgCl2, 2 mm CaCl2 (binding buffer (BB)), and 1.5 × 106 cells were incubated with 106 cpm of different fH fragments labeled with 125I for 1 h at 37 °C. The cells were spun down through 20% sucrose in PBS and immediately frozen at −80 °C for 30 min. The pellet (cells) was cut off, and the radioactivity of pellet and supernatant were measured in a 1277 GammaMaster (LKB Wallac).
Protein-Protein Binding Assay
Proteins (annexin-II, histones H2A/H2B plus H1 and H3/H4, osteoadherin (OSAD) as positive control and bovine serum albumin (BSA) as negative control) were coated overnight at 4 °C onto Maxisorp microtiter plates (Nunc) at a concentration of 5 μg/ml for annexin-II and OSAD and 4 μg/ml for histones in 75 mm sodium carbonate buffer, pH 9.6 (coating buffer, 50 μl/well). Between each step, the wells were washed extensively with 50 mm Tris-HCl, 150 mm NaCl, 0.1% (v/v) Tween 20, pH 7.5 (immunowash). All wells were blocked with 100 μl/well immunowash plus 3% fish gelatin (quenching solution, Nordic) for 2 h at room temperature or for 1 h at 37 °C for histone assay. fH, fH fragments, biotinylated fH, or radiolabeled full-length fH as well as fH fragments were added at increasing concentrations in 50 mm HEPES, pH 7.4, 100 mm NaCl for annexin-II or 150 mm NaCl for histone assay, 2 mm CaCl2, and incubated overnight or for 2–3 h at room temperature, respectively. For binding studies from NHS, 0.5% heat-inactivated NHS in BB was used. The amount of bound protein was assessed using 100 μl/well StreptABComplex/HRP for biotinylated fH or 50 μl/well goat anti-fH followed by rabbit anti-goat-HRP for unlabeled fH, fH fragments, and serum. Both were developed with OPD development kit (Dako), according to the manufacturer's instructions. Absorbance at 490 nm was measured to quantify protein binding in a Cary50 Bio UV spectrometer connected to a 50MPR microplate reader (Varian). Radiolabeled bound proteins were measured using a 1277 GammaMaster.
Flow Cytometry
For the determination of fH binding, the cells were washed twice with BB and incubated with Alexa Fluor- or DyLight-labeled proteins for 30 min at 37 °C with gentle shaking. After washing twice with BB supplemented with 1% BSA and 30 mm NaN3, the cells were re-suspended in the same buffer and analyzed in a CyFlow space (Partec). To discriminate between live, apoptotic, and necrotic populations, cells were stained with annexin V-PE (AV) and Via-probe, according to the manufacturer's instructions (BD Biosciences) after the protein incubation. For the determination of extracellular annexin-II expression, cells incubated with AF488-labeled fH were washed twice with ice-cold PBS, fixed for 10 min at room temperature with 1:100-diluted CellFix (BD Biosciences), washed twice with PBS supplemented with 1% BSA and 30 mm NaN3 (PBS++), and incubated for 30 min on ice with rabbit anti-human annexin-II polyclonal antibody diluted 1:500. After incubation with secondary goat anti-rabbit AF647, the cells were washed in PBS++ and analyzed using flow cytometry. For DNA analysis, apoptotic cells were treated with 0–50 units of DNase-1 (Roche Applied Science) in 10 mm Tris-HCl, pH 7.5, 50 mm NaCl, 10 mm MgCl2, and 2 mm CaCl2 for 30 min at 37 °C, washed twice in BB, and incubated with 20 μg/ml AF488-labeled fH. For DNA staining cells were washed once in BB and once in PBS++ and incubated with 1:500 mouse anti-dsDNA in PBS++ for 30 min at 4 °C, washed twice and incubated with AF647-labeled goat anti-mouse for 30 min at 4 °C in PBS++, washed in PBS++, and analyzed by flow cytometry. For fH-DNA interaction in the presence of serum, linearized pcDNA3 vector (Invitrogen) was biotinylated with Biotin-LC-Hydrazide (Pierce) dissolved in imidazole. The vector was added to 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (Pierce) followed by addition of the biotin solution and incubated for 1.5 h at 50 °C. Unbound biotin was removed using Zeba desalting spin columns (Pierce) according to the manufacturer's instruction. AF488-labeled fH was incubated with 2% heat-inactivated NHS with or without biotinylated DNA in BB for 45 min at 37 °C. M-280 streptavidin-coated Dynabeads (Invitrogen), blocked for 30 min at 37 °C in BB with 1% BSA were added to the samples, incubated for 30 min, washed, and analyzed by flow cytometry.
Immunoprecipitation
Apoptosis was induced and 4 × 106 cells were incubated with or without 150 μg/ml fH at 37 °C for 1 h in BB. The cells were washed once and suspended in 200 μl of 1% Triton X-100, 20 mm Tris-HCl, pH 8.0, 150 mm NaCl, 5 mm EDTA, 1% Trasylol (solubilization buffer) containing 2 mm phenylmethylsulfonyl fluoride added immediately before use, incubated 30 min on ice and spun down at 15,000 rpm for 10 min at 4 °C. The supernatant was incubated with 20 μg of rabbit anti-factor I 9205 (homemade) at 4 °C overnight. Then, 4 μl of protein A-Sepharose (Sigma) was pre-mixed with 16 μl of Sephacryl S-100 (GE Healthcare) and incubated for 1 h at 4 °C. The formed antibody-protein A complex was spun down, and the supernatant was incubated with 10 μg of rabbit anti-annexin-II at 4 °C overnight. Protein A-Sepharose was pre-mixed with Sephacryl S-100 and incubated with the samples at 4 °C for 1 h. The samples were then spun down and washed three times with solubilization buffer, resuspended in 40 μl of SDS-PAGE loading buffer, and samples were heated at 95 °C for 5 min before loading on a 10% SDS-PAGE gel. The gel was subsequently blotted onto a polyvinylidene difluoride membrane, blocked in quenching solution, and incubated with goat anti-human fH (Calbiochem) diluted in quenching solution, washed four times with immunowash, incubated with HRP-conjugated rabbit anti-goat antibodies diluted in quenching solution, washed four times with immunowash, and developed with 5 mg of 3,3′-diaminobenzidine, 0.06 mm NiCl2, in 50 ml of Tris-buffered saline supplemented with 5 μl of 30% H2O2 immediately before use.
Trypsinization of the Plasma Membrane
Jurkat T cells were rendered apoptotic (3.5 h), washed twice with PBS and 1 × 106 cells were incubated for 30 min on ice with 15 μg of trypsin-l-1-tosylamido-2-phenylethyl chloromethyl ketone (Worthington) in a total volume of 150 μl. Control cells were treated with PBS. All cells were washed with ice-cold PBS and incubated for 30 min on ice with 4 mm Pefabloc (Roche Applied Science) to inhibit remaining trypsin. After washing twice with BB, 2.5 × 105 cells were incubated for 30 min either on ice or at 37 °C with AF488-labeled fH or AF488-labeled C4BP in addition to BB alone as a control. Protein binding was determined using flow cytometry.
To confirm successful trypsinization, cells were analyzed in parallel for CD45 (trypsin-insensitive) (23) and CD4 (trypsin-sensitive) (24) surface expression using staining with the specific monoclonal antibodies and isotype controls. Expression of annexin-II was analyzed in parallel with CD4 and CD45 to ensure expected trypsin activity. FITC-labeled goat anti-mouse or swine anti-rabbit secondary antibody were used for detection. Cells were washed twice with PBS++ after each antibody staining and finally suspended in PBS++ and analyzed by flow cytometry.
Deglycosylation of the Cell Surface
Apoptotic Jurkat T cells were washed twice with BB and incubated at 37 °C with a combination or with the single enzyme of 0.04–0.34 unit/ml neuraminidase-F, 0.003–0.025 unit/ml heparitinase, 0.003–0.02 unit/ml heparinase, and 0.08–0.6 unit/ml chondroitinase ABC (all from Seikagaku) diluted in BB or PBS. After 1 h, samples in PBS were washed with BB before fH-AF488 was added to a final concentration of 25 μg/ml, for samples in BB fH-AF488 was added straight to samples and incubated for another 30 min. As a control, sialic acid-binding, FITC-labeled Sambucus nigra lectin (Vector Laboratories) was added to neuraminidase-treated cells, and the mixture was incubated for 30 min at 4 °C in PBS++. For fH-binding and C3b-deposition experiments, 3-h apoptosis-induced cells were deglycosylated, washed twice in 0.1% gelatin, 144 mm NaCl, and 5 mm veronal buffer, and incubated at 37 °C for 45 min with 10% NHS. After incubation, samples were washed twice in PBS++, incubated with FITC-labeled rabbit anti-C3c, goat anti-fH followed by AF647-labeled rabbit anti-goat, washed, and analyzed by flow cytometry.
Gel Shift Analysis
fH, fH fragments, or prothrombin (5 μg of each) were incubated with 0.2 μg of linearized pSEC TagA vector (Invitrogen) in BB (20 μl of total volume) at 37 °C for 30 min. The samples were mixed with loading buffer (0.6 mm bromphenol blue, 0.8 mm xylene cyanol, and 5% glycerol) and loaded onto a 0.5% agarose gel with 30 mm Tris-HCl, 0.1% acetic acid, and 1 mm EDTA pH 8.0 (TAE buffer) supplemented with 0.3 μg/ml ethidium bromide and subjected to electrophoresis at 80 V for 30 min in TAE buffer. DNA was visualized using UV, and the intensities of the bands were analyzed using the ImageGauge software (Fujifilm).
Confocal Microscopy
Apoptosis was directly induced on SuperFrostPlus object slides (Menzel). 8 × 104 cells diluted in RPMI media without fetal calf serum containing 1 μm staurosporine were pipetted in a DakoPen circle and incubated for 4 h at 37 °C in 5% CO2. After washing twice with BB, 25–30 μg/ml AF488 or DL488-labeled fH was added in a final volume of 30 μl, and the mixture was incubated for 5 min at 37 °C. After washing three times for 5 min in PBS, the cells were fixed with 3.7% paraformaldehyde, washed again, and centrifuged for 1 min at 500 rpm in a Cellspin (Tharmac). After blocking with 1% BSA for 30 min, the cells were incubated with mouse anti-dsDNA overnight at 4 °C. After washing, the cells were incubated with the goat anti-mouse-AF647 for 1 h at room temperature. Then, 100 ng/ml propidium iodide (PI) was added for 15 min at room temperature. After the final wash, the slides were very briefly immersed in 99% ethanol and, after drying, mounted with DakoCytomation Fluorescent Mounting Medium. After sealing the glass plates with nail polish, the slides were analyzed in a Zeiss LSM 510 microscope. Co-localization was calculated using CoLocalizer Express (CoLocalization Research Software).
Enzymatic Hydrolysis of the Plasma Membrane Lipids
Apoptotic Jurkat T cells were incubated with 0.1–1 unit of secreted Phospholipase A2 (PLA2) from bee venom and 0.1–5 units of phospholipase D (PLD) from cabbage, both from Sigma, for 30 min at 37 °C. PLA2-treated cells were also stained for dsDNA as described above.
10–20 μm of the iPLA2-specific inhibitor, bromoenol lactone (BEL), 10–20 μm of the general PLA2 inhibitor methyl arachidonyl fluorophosphonate (MAFP, both from Sigma) and the apoptosis-inducing agent were applied simultaneously. To investigate potential solvent effects, DMSO, the solvent of the inhibitors, was applied alone at the same concentrations.
Oxidation of the Plasma Membrane
Apoptotic Jurkat T cells (2 × 105 cells/sample) were treated for 30 min at 37 °C with 2 μg/ml myeloperoxidase (Sigma) in 100 μl of PBS supplemented with 0.5 mm H2O2. Cells were washed with BB, incubated with 25 μg/ml fH-AF488 in 50 μl of BB for 30 min at 37 °C, washed with BB, and analyzed by flow cytometry.
Binding of fH to Liposome-coated Dynabeads
All lipids were purchased from Avanti Polar Lipids and suspended in chloroform with 10% methanol. Synthentic lipids were: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine, 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-l-serine, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine, 1-oleoyl-2-hydroxy-sn-glycero-3-phosphocholine, 1-oleoyl-2-hydroxy-sn-glycero-3-phospho-l-serine, and 1-oleoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine. Natural lipids were: l-α-phosphatidylcholine (egg, chicken), l-α-phosphatidylserine (brain, porcine), l-α-phosphatidylethanolamine (egg, chicken), l-α-lysophosphatidylcholine (egg, chicken), l-α-lysophosphatidylserine (brain, porcine), l-α-lysophosphatidylethanolamine (egg, chicken), ceramide (brain, porcine), sphingomyelin (brain, porcine), and cardiolipin (heart, bovine). For liposome preparation, 1 μmol of lipids was mixed with 10 nmol of N- (biotinoyl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium (Invitrogen), and 6.4 nmol of 2-(3-(diphenylhexatrienyl)propanoyl)-1-hexadecanoyl-sn-glycero- 3-phosphocholine (Invitrogen). Lipids were desiccated under N2 followed by vacuum until they were completely dry. Lipids were re-suspended in 25 mm HEPES, 150 mm NaCl, pH 7.7 (HN buffer) and sonicated for 10 min at grade 3 in an Ultrasonic processor XL (Misonix). The lipids were mixed with 100 μl of 10 mg/ml M-280 streptavidin-coated Dynabeads. The beads were preincubated in 1 ml of HN buffer plus 1% BSA at 37 °C for 2 h. Beads and lipids were incubated for another 2 h at 37 °C in HN buffer plus 1% BSA followed by two washing steps in HN buffer plus 1% BSA and re-suspension in 1 ml of HN buffer plus 1% BSA. Liposome-coated beads (50 μl/sample) were washed twice and re-suspended in BB. Liposomes were, for some experiments, preincubated with 10 μg/ml annexin-II or 10 μg/ml purified histones before another 30-min incubation at 37 °C with fH-AF488 at a final concentration of 25–50 μg/ml; the beads were washed once and re-suspended in HN buffer plus 1% BSA or BB and analyzed by flow cytometry. To explicitly analyze the liposomes, only the 2-(3-(diphenylhexatrienyl)propanoyl)-1-hexadecanoyl-sn-glycero-3-phosphocholine-positive population was analyzed.
RESULTS
Localization of the fH Region Responsible for Binding Apoptotic and Necrotic Cells
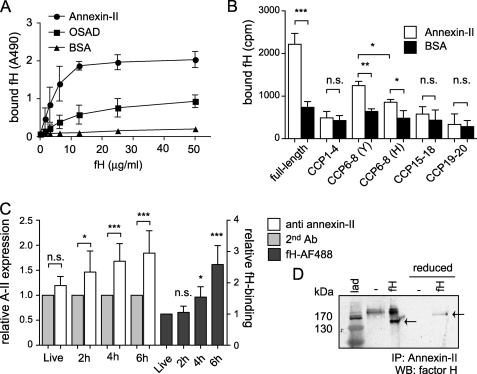
To determine which domains of fH are involved in binding to dying cells we used recombinantly expressed fragments of fH representing the CCPs 1–4, 6–8, 8–15, and 19–20. The 125I-labeled fH fragments were incubated with dying cells, and we found that the full-length fH bound to both apoptotic and necrotic cells and the binding to necrotic cells was 4-fold higher in comparison to apoptotic cells (Fig. 1, A and B). For CCPs 1–4, binding to neither apoptotic nor necrotic cells could be detected. CCPs 6–8 and CCPs 19–20 bound well to both types of cells. CCPs 8–15 showed no significant binding to necrotic cells but bound to apoptotic cells. Taken together, these results show that several regions of fH bind to dying cells, which may be related to the presence of several fH ligands on the surfaces of these cells.
FIGURE 1.
Localization of binding sites for dying cells in fH. Apoptotic (A) and necrotic (B) cells were incubated with radiolabeled full-length fH as well as its recombinantly expressed fragments. Cell-bound fH fraction was separated from free fH in solution by centrifugation over 20% sucrose, and the binding is expressed as % of total radioactivity added. Negative control represents sample with full-length fH but no cells. Data are presented as mean values of three separate experiments performed in doublets ± S.D. Significance of the observed binding in comparison to control was assessed using ANOVA: ns, not significant; *, p < 0.05; **, p < 0.01; and ***, p < 0.001.
fH Binds to Annexin-II
A direct binding assay in which annexin-II was immobilized in microtiter plates was used to assess interactions of fH with annexin-II. fH bound specifically and in a dose-dependent and saturable manner to annexin-II (Fig. 2A). The small-leucine rich repeat proteoglycan OSAD was used as a positive control for fH binding (25). To investigate which region of fH mediates the binding to annexin-II, annexin-II was immobilized on microtiter plates and tested for its ability to interact with 125I-labeled full-length, and fragments of, fH. Binding to full-length fH and to CCPs 6–8 was detected, whereas the other tested fragments showed no significant binding (Fig. 2B). Substitution of tyrosine to histidine, as in the common polymorphism Y402H, resulted in an increase of binding for CCPs 6–8. These results indicate that annexin-II is one of possibly multiple fH ligands on apoptotic cells and that it binds to the CCPs 6–8 region of fH.
FIGURE 2.
fH binds to annexin-II. A, annexin-II, OSAD (positive control), and BSA (negative control) were immobilized in microtiter plates, and increasing concentrations of biotinylated fH were added. Bound protein was detected with a streptavidin peroxidase kit. Background signal obtained with streptavidin-HRP alone was subtracted from the original values. Data are presented as mean values (n = 3) ± S.D. B, radiolabeled full-length fH and its fragments were incubated with immobilized annexin-II and BSA (negative control). After washing, the amount of bound protein was determined in a γ-counter. Data are presented as mean values (n = 3) ± S.D. The significance of observed differences between annexin-II or BSA-coated wells for each construct was assessed using unpaired t test: ns, not significant; *, p < 0.05; **, p < 0.01; and ***, p < 0.001. C, annexin-II was detected with specific antibodies on surface of live cells, and cells were rendered apoptotic for increasing periods of time. Significance of observed differences in binding of annexin-II to cells in comparison with signal obtained with secondary antibody only (2nd Ab) or compared with signal from fH incubated with live cells was assessed using ANOVA. D, immunoprecipitation using lysates of apoptotic cells preincubated with and without fH. Annexin-II was pulled down from apoptotic cell lysate using specific antibodies, and fH was detected on Western blot using goat anti-fH antibody; arrows mark the position of fH; lad, molecular weight marker.
fH Interacts with Annexin-II Exposed on the Surface of Apoptotic Cells
Because fH does not bind to live Jurkat T cells, but its binding increases with the degree of apoptosis (14), we next investigated if annexin-II is expressed on the surface of these cells. No surface expression of annexin-II could be detected on live cells (Fig. 2C), but significant quantities of the protein appeared on the cell surface 2 h after apoptosis induction. The expression increased during the first 6 h of apoptosis induction; during this time fH binding also increased (Fig. 2C). To confirm that fH in fact interacts with annexin-II on apoptotic cells, we performed an immunoprecipitation using lysates of apoptotic cells incubated with fH. We used a rabbit anti-annexin-II antibody to pull down the potential fH-annexin-II complex, and we detected fH on a Western blot (Fig. 2D), confirming that fH interacts with annexin-II on apoptotic cells.
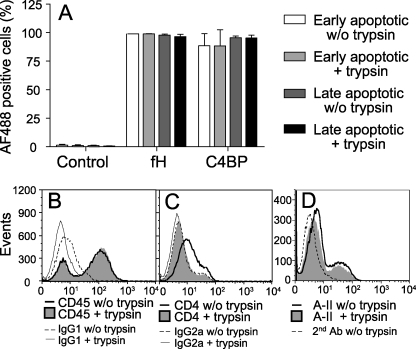
fH Does Not Bind to Trypsin-sensitive Proteins Present on the Surface of Apoptotic Cells
Because fH binding to apoptotic cells is not only mediated via annexin-II, we wanted to systematically investigate whether proteins, carbohydrates, DNA, or lipids comprise other potential ligands. To analyze whether the additional ligands are proteins, we treated cells with trypsin. Trypsin cleaves peptide chains at the carboxyl side of the amino acid residues lysine and arginine, but these are not accessible in all surface proteins. Hence some proteins are not removed from the plasma membrane after trypsinization. CD45 is one example of a trypsin-insensitive protein (23) (Fig. 3B), whereas CD4 is a trypsin-sensitive surface protein (24) (Fig. 3C). Jurkat T cells were rendered apoptotic, trypsinized on ice, and binding of fH and C4BP was determined by flow cytometry. C4BP interacts with PS on apoptotic cells (26) and to a minor degree also with DNA exposed on the surface of necrotic cells (15). The live, early, and late apoptotic/necrotic cell populations were differentiated using staining with AV and Via-probe. No binding of fH or C4BP occurred to live cells, whereas significant binding of fH and C4BP was detected to both the early and the late apoptotic cell populations (data not shown) in agreement with published data (14). Trypsinization had, as expected, no effect on binding of C4BP and, interestingly, no effect on binding of fH to both cell populations (Fig. 3A). These observations lead to the conclusion that fH does not bind to any trypsin-sensitive protein that is expressed on the surface of apoptotic cells. We also verified that annexin-II is a trypsin-insensitive protein (Fig. 3D).
FIGURE 3.
fH does not interact with trypsin-sensitive proteins but with the trypsin-insensitive annexin-II expressed on the surface of apoptotic cells. A, apoptotic Jurkat T cells were treated with trypsin, which was then stopped with Pefabloc. Binding of AF488-labeled fH and C4BP was determined using flow cytometry. Control cells were incubated in binding buffer alone. The results are presented as mean AF488-positive cells (n = 3) ± S.D. Histograms showing surface expression of trypsin-insensitive CD45 (B) and trypsin-sensitive CD4 (C) are shown to confirm that trypsin had expected effect. D, amount of annexin-II found on the cell surface did not change upon incubation of cells with trypsin. In B and C background signals obtained with isotype-matched control antibodies with and without trypsin treatment are also shown. Experiments shown in B–D were repeated three times, and representative histograms are shown.
fH Does Not Bind to GAGs and Sialic Acid Present on the Surface of Apoptotic Cells
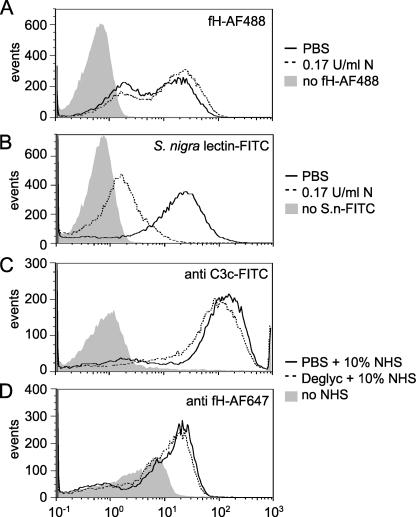
With the intention of clarifying whether fH binds to carbohydrate ligands that are covalently linked to proteins or lipids that are expressed on the surface of dying cells, cells were rendered apoptotic and treated with enzymes degrading specific carbohydrates. Because fH is known to bind polyanionic structures such as GAGs as well as glycoproteins containing sialic acid (27–29), a treatment with a combination of neuraminidase-F, chrondroitinase ABC, heparitinase, and heparinase was chosen. The deglycosylated cells were incubated with AF488-labeled fH, and we found that neither separate treatments with the individual deglycosylation enzymes nor combined treatment with all four enzymes affected the binding of fH to apoptotic cells (data not shown). Binding of fH to apoptotic cells after neuraminidase treatment, which removes sialic acid, was not affected (Fig. 4A), whereas the same treatment caused a dramatic decrease in binding of the sialic acid-binding lectin S. nigra (Fig. 4B). Deglycosylation neither affected C3b deposition nor fH binding to apoptotic cells from NHS (Fig. 4, C and D).
FIGURE 4.
fH does not bind to glycosaminoglycans and sialic acid on the surface of apoptotic cells. Binding of fH-AF488 (A) or S. nigra lectin-FITC (B) to apoptotic cells after treatment with 0.17 unit/ml neuraminidase-F (N, dashed line) or PBS (solid line) as a control. No difference in binding was detected for fH, whereas a decrease in binding was detected for S. nigra lectin. C, deposition of C3b and fH (D) from NHS after deglycosylation with a combination of 0.17 unit/ml neuraminidase-F, 0.0125 unit/ml heparitinase, 0.01 unit/ml heparinase, and 0.3 unit/ml chondroitinase ABC or in PBS as a control. Deposited C3b- and bound-fH were detected with specific antibodies. No difference in deposition could be detected. All graphs show representative results of three independent experiments.
fH Binds DNA
Because binding of fH to DNA has been reported previously (30) and because DNA becomes exposed on the cell surface during apoptosis (31) we investigated whether fH also interacts with DNA on apoptotic cells. To decrease the amount of DNA on apoptotic cells we treated cells with DNase. This intriguingly resulted in an increase of the fH-binding cells (Fig. 5A). When measuring the amount of DNA on the surface of DNase-treated cells using an antibody against dsDNA, we found, unexpectedly, an increase of cells with detectable DNA (Fig. 5B). It appears that DNase treatment renders exposed DNA more accessible rather then removing it by degradation and that fH interacts with this DNA. To confirm that fH co-localizes with DNA on apoptotic cells, we used confocal microscopy (Fig. 5C), and we calculated the co-localization for different areas on three microscope slides from separate experiments by using the overlap coefficient (R) according to Manders (32). fH and DNA displayed a mean coefficient of 0.7 ± 0.1, which indicates co-localization. To determine the binding site for DNA on fH, we performed a gel-shift assay using the different fH fragments and a linearized plasmid. Full-length fH showed binding, as did CCPs 6–8 and CCPs 19–20 (Fig. 5, D and E). The negative control prothrombin did not interact with DNA. Using flow cytometry, fH-AF488 was shown to bind biotinylated DNA attached to beads, both in the absence and in the presence of 2% heat-inactivated NHS (Fig. 5F). The signal obtained in the presence of serum was presumably lower because of the competition between added fH-AF488 and fH as well as other DNA-binding molecules present in NHS. fH-AF488 was added in 2-fold molar excess over NHS fH.
FIGURE 5.
fH binds DNA and co-localizes with DNA on surface of apoptotic cells. Apoptotic cells were incubated with DNase (10 or 50 units), and binding of fH (A) as well as DNA-specific antibodies (B) was assessed using flow cytometry. Binding of secondary antibody alone is also shown (2nd Ab). C, confocal microscopy images of apoptotic cells incubated with AF488-labeled fH (top left panel) and anti-DNA antibodies (top right panel) and propidium iodide (bottom left panel). The bottom right panel shows an overlay of the three signals indicating co-localization. Experiments shown in A–C were repeated three times, and representative results are shown. D and E, plasmid DNA was incubated with fH fragments and separated using agarose electrophoresis. Full-length fH, CCPs 6–8, and CCPs 19 and 20 bound DNA, which affected their migration. Prothrombin was used as negative control. Graphs (D) show % of DNA retained in the wells (n = 3) ± S.D. The significance of observed differences between sample containing only DNA and those also including fH fragments was assessed using ANOVA: ns, not significant; ***, p < 0.001. F, binding of fH-AF488 to biotinylated DNA attached to streptavidin-coated beads. Binding of purified fH was detected in the presence or absence of 2% heat-inactivated NHS. Graphs show means (n = 3) ± S.D. Significance was calculated using unpaired t test: *, p < 0.05; ***, p < 0.001.
fH Binds to Histones
Because DNA becomes exposed on apoptotic cells in nucleosomes containing histones, we tested whether fH can interact directly with histones. Histones from Jurkat cells were isolated and collected in fractions containing either H2A/H2B and H1 (the latter not visible upon Coomassie staining) or H3/H4 (Fig. 6A). Binding of fH to the different histones was investigated in a direct binding assay. fH and fH fragments were detected using a goat anti-fH antibody; the antibody showed similar binding to all fH fragments (data not shown). Binding was observed in a concentration-dependent manner mainly to H3/H4 complexes but also to H2A/H2B plus H1 (Fig. 6B). Binding of fH to the histones could also be observed from heat-inactivated NHS (Fig. 6C). All fH fragments except CCPs 19 and 20 could bind to the histone complexes (Fig. 6D). The H version of the common polymorphism Y402H displayed increased binding to both histone complexes (Fig. 6D, inset). To rule out the possibility that fH was interacting with traces of DNA still bound to histones, the histones were treated with DNase prior to fH incubation; no difference in fH binding was detected (data not shown).
FIGURE 6.
fH binds histones. A, histones were purified from Jurkat T cells in two fractions containing H2A/H2B plus H1 or H3/H4 and separated on 15% SDS-PAGE followed by staining with Coomassie Brilliant Blue. B, histones or BSA were immobilized in microtiter plates and incubated with increasing concentrations of fH, binding of which was detected with goat anti-fH followed by HRP-conjugated rabbit anti-goat. Background signal obtained with rabbit anti-goat-HRP alone was subtracted from the original values. Data are presented as mean values (n = 3) ± S.D. C, binding of fH from heat-inactivated NHS to immobilized histones was detected using polyclonal antibodies against fH, and the significance was calculated using ANOVA (n = 3) ± S.D. D, binding of fH fragments to immobilized histones was also detected (n = 3) ± S.D. using polyclonal antibodies against fH. Background binding to BSA was subtracted for each fH construct. D (inset): the two versions of CCPs 6–8 (Tyr-402 and His-402) were compared in binding to histones. The significance of observed differences between signals obtained for wells coated with histones or BSA as negative control was assessed using ANOVA: ns, not significant; **, p < 0.01; and *** p < 0.001.
fH Does Not Interact with Lipids on the Surface of Apoptotic Cells
Next we investigated whether fH interacts with specific lipids that appear on the surface of apoptotic cells. Specific phospholipases are activated during cell death and cause changes in the surface lipid pattern in addition to the exposure of PS (33). We treated apoptotic cells with secreted PLA2 (sPLA2) and PLD. sPLA2 catalyzes the hydrolysis of the sn-2 fatty acyl bond to form lysophospholipids. Exposure of apoptotic cells to sPLA2 increased fH binding dramatically (Fig. 7A). However, the treatment with PLD, which catalyzes the removal of phospholipid head groups, had no effect on fH binding (Fig. 7B). To exclude the possibility that the concentration of PLD was too low, 2.5 and 5 units were also tested, but this had no influence on the binding of fH (data not shown). These results indicated that fH might interact with lysophospholipids that appear on the cell surface during the course of apoptosis but that the binding is not confined to the lipid head group.
FIGURE 7.
fH does not interact with lipids. Apoptotic Jurkat T cells were treated with phospholipases PLA2 (A) or PLD (B) for 30 min at 37 °C and thereafter incubated with buffer AF488-labeled fH. fH binding was analyzed by flow cytometry. Results are presented as mean fluorescent intensity (n = 3) ± S.D. and show that treatment with PLA2 increased binding of fH while PLD had no effect. Statistical differences were calculated in comparison to cells incubated with buffer only and those fH-incubated (Tukey test); ns, not significant; **, p < 0.01; and ***, p < 0.001. C, Jurkat T cells were rendered apoptotic in the presence of phospholipase inhibitors BEL or MAFP. fH binding to the cells was analyzed in a flow cytometer using AF488-labeled protein. Annexin-V and Via-Probe were used to define apoptotic stages, because BEL itself, when applied in higher concentrations, exhibits pro-apoptotic features. None of the added PLA2 inhibitors affected fH binding in a statistically significant manner (n = 3). D, apoptotic cells were treated with PLA2 as above and stained with specific antibodies against dsDNA. Treatment of PLA2 resulted in an increase in dsDNA-positive cells compared with no treatment. The figure shows representative histogram for three experiments.
PLA2 has been implicated in membrane remodeling during apoptosis (34, 35). To further determine whether endogenous phospholipases were responsible for inducing the cell membrane changes that lead to the binding of fH, inhibitors of PLA2 were used to attenuate PLA2 hydrolysis. Neither BEL (an inhibitor of the Ca2+-independent type VI PLA2, iPLA2), nor MAFP (a general PLA2 inhibitor) significantly decreased the binding of fH to apoptotic cells (Fig. 7C). In Fig. 5 we show that fH interacts with DNA on the apoptotic cell surface. Therefore, we investigated whether PLA2 treatment renders more DNA-positive cells by staining for surface DNA with an antibody against dsDNA. A dramatic increase of DNA-positive cells was observed after the PLA2 treatment (Fig. 7D).
To also explore the putative interaction of fH and lysophospholipids, we used liposome-coated Dynabeads, flow cytometry, and surface plasmon resonance (SPR, Biacore). For both methods liposomes containing distinctive compositions of phospholipids and/or lysophospholipids were analyzed (100% PC, 10% PS, 10% LysoPS, and 3, 10, and 20% LysoPC), and no binding of fH to any of the tested liposomes was detected, using neither liposome-coated Dynabeads in flow cytometry (Fig. 8A) nor SPR (data not shown). For the SPR experiments an L1 chip was coated with PC-based liposomes of varying composition (100% PC, 10% PE, 10% PS, 30% PE and PS, 3–60% LysoPC, 60% LysoPE, and 10–60% LysoPS), and fH was injected over the lipid surface as previously described (36). Under the same conditions C4BP bound strongly to a chip surface containing 10% PS (data not shown). By flow cytometry we also investigated binding of fH to PC liposomes containing 10% (data not shown) and 30% ceramide, sphingomyelin, and cardiolipin without detecting any interaction (Fig. 8B). A liposome was constructed to resemble a plasma membrane of apoptotic neutrophils (24:27:13:20:8:8 molar ratio of PC/PE/PS/sphingomyelin/ceramide/cardiolipin), with small adjustments from previous studies (37, 38), but fH binding was not detected. The slight increase in fH binding to all tested compositions of liposome-coated Dynabeads was caused by the use of strongly labeled fH. When the liposome resembling apoptotic neutrophils was preincubated with annexin-II or histones, a clear binding of fH could be detected to the liposome (Fig. 8, C and D). Taken together, these data suggest that sPLA2 causes changes in membrane structure or morphology resulting in an increased number of DNA-expressing cells. This resulted in an increased fH binding; furthermore this binding did not seem to be mediated by lysophospholipids. Addition of annexin-II or histones was, however, enough to associate fH to an apoptotic plasma membrane-like liposome.
FIGURE 8.
fH only binds to liposomes in the presence of annexin-II or histones. A, AF488-labeled fH was incubated for 30 min at 37 °C with liposome-coated Dynabeads containing distinctive compositions of phospho- and/or lysophospholipids; the binding was analyzed using flow cytometry gating on the bead population (DPH+). B, binding of AF488-labeled fH to ceramide (Cer), sphingomyelin (Sph), cardiolipin (Card), and liposomes intended to resemble an apoptotic neutrophil plasma membrane (with some modifications) with the molar ratio of 24:27:13:20:8:8 for PC/PE/PS/sphingomyelin/ceramide/cardiolipin (Apo). C and D, the liposome resembling an apoptotic neutrophil (Apo) was incubated with annexin-II (C) or histones (D) followed by AF488-labeled fH. Binding of fH was detected using flow cytometry. Experiments shown were repeated three times, and representative results are shown.
DISCUSSION
Opsonization with complement components such as C1q and C3b is fundamental to ensure rapid and silent removal of apoptotic cells. We showed previously that fH, one of the main fluid-phase complement inhibitors, binds to apoptotic cells to protect them from excessive complement activation (14). Blocking of fH binding to apoptotic cells resulted in increased opsonization with C3b and C9 as well as an increase in lysis by human serum (14), suggesting that fH retains its complement inhibitory activity when bound to apoptotic cells. Interactions between fH and C3b, heparin, C-reactive protein, cell surface glycosaminoglycans, and microbial virulence factors have been extensively studied (39), but the ligand for fH on the surface of apoptotic cells has not yet been discovered. Here we further characterize the binding of fH to apoptotic cells and describe for the first time three of the ligands expressed on the surface of dying cells that interact with fH.
We first investigated which of the 20 CCP domains of fH are involved in the interaction with apoptotic cells. CCPs 1–4 are known to contain the site responsible for both decay acceleration and factor I cofactor activities. CCPs 1–4 also contain one of the major binding sites for C3b, the other being located in CCPs 19 and 20 (21). We revealed that CCPs 1–4 do not mediate binding to apoptotic cells, which is in agreement with our previous observation that fH binds equally well to apoptotic cells in the presence and absence of serum and hence is not C3b-mediated (14). We found that CCPs 6–8, 8–15, and 19–20 of fH all contain sites involved in interaction with apoptotic cells, suggesting that more than one cell-surface ligand is involved. Interestingly we found that sites within CCPs 6–8 and 19–20 are also involved in binding of fH to necrotic cells, whereas CCPs 8–15 do not appear to participate in this interaction. Therefore it seems that the ligand on apoptotic cells recognized by a site within CCPs 8–15 is either absent or obscured on the surface of necrotic cells. In general, we found binding of fH to necrotic cells is stronger than to apoptotic cells, as has been observed previously (14). We used double staining with AV and Via-probe to discriminate between apoptotic cells (AV+ Via-probe−) and those entering post-apoptotic necrosis (AV+ Via-probe+) to exclude the possibility that the binding observed in a heterogeneous apoptotic cell population is mediated exclusively through the small population of late apoptotic/necrotic cells (data no shown).
We found that the calcium-dependent phospholipid-binding protein annexin-II is one of the fH ligands on apoptotic cells. Annexin-II is expressed in many different cell types and exists both in the cytosol and in the plasma membrane (40). Annexin-II is responsible for communication between cellular membranes and the cytoplasmic environment. It also plays a role in membrane trafficking and remodeling of the membrane cytoskeleton (41). The serum protein, β2 globulin, has been proposed to bind endothelial cells through annexin-II (42). Like apoptotic cells, mature macrophages also express PS on their surface, which is a prerequisite for adequate phagocytosis. Interestingly, annexins I and II are thought to function as bridging molecules serving both as ligands and receptors in promoting phagocytosis through their interactions with PS (43). The relevance of the role of annexin-II in pathology is unclear, but autoantibodies against annexin-II have been found in patients with systemic lupus erythematosus and other autoimmune diseases (44). Annexin-II has recently been identified as an fH binding partner in a mouse ischemia/reperfusion model (17). The authors hypothesized that annexin-II directs fH to ischemic renal cells and mediates their protection. In ischemia/reperfusion many cells die, and therefore this study is in good accordance with our observation that annexin-II becomes expressed on the surface of human apoptotic cells and functions there as a ligand for fH. We established that annexin-II is translocated to the surface of Jurkat T cells upon induction of apoptosis. We also showed that annexin-II is insensitive to digestion of cells with trypsin, which is in accordance with our current observation that incubation of apoptotic cells with trypsin does not affect binding of fH. We also revealed that annexin-II alone is capable of recruiting fH to an apoptotic plasma membrane-like structure. Thus, annexin-II appears to be an important fH ligand on surfaces of apoptotic cells due in part to its interaction with a binding site within CCPs 6–8. CCP 7 harbors the common single-nucleotide polymorphism Y402H, which affected the affinity of CCPs 6–8 for annexin-II. Because the binding of CCPs 6–8 to annexin-II was lower than that of full length fH, it remains possible that there are additional binding sites for annexin-II in fH that are not covered by the recombinant fragments tested in the current study.
The site of interaction with annexin-II lies within CCPs 6–8 of fH, and therefore we searched for additional ligands that could bind to sites within CCPs 15–18 and 19 and 20. Glycosaminoglycans, represented by heparin, are known to bind several of the fH CCPs that we identified as interacting with dying cells in this study (45). On the other hand, we found that digestion of apoptotic cells with specific enzymes that degrade various classes of GAGs or remove sialic acid had no effect on binding of fH even though we could detect a 70% decrease of sialic acid recognized by S. nigra lectin. Moreover the removal of GAGs and sialic acid did not seem to affect complement activity on the apoptotic cells. Interestingly, heparan sulfate has been reported to be down-regulated during the course of apoptosis in Jurkat T cells (46), which supports the hypothesis that heparan sulfate is unlikely to be an fH ligand on apoptotic cells. This does not, however, contradict the important role played by fH interactions with negatively charged carbohydrates on living, normal cells that do not display the high affinity surface ligands found on apoptotic cells.
We have observed previously that CCPs 6–8 of fH interact with DNA (30). In the current study we confirmed this result and extended it with the observation that DNA also binds to CCPs 19 and 20. Surprisingly, CCPs 1–4, which bind C3b, did not interact with DNA despite the fact that they display a large patch of positively charged amino acid (47). The fH DNA interaction could also be detected in the presence of NHS. In a hemolytic assay adopted from Józsi et al. (48), we did not observe any effect of DNA at physiological concentrations (49) on fH regulatory functions or binding of fH to sheep erythrocytes while these cells were lysed in the presence of CCPs 19 and 20 (which competes with full-length fH) as expected (data not shown). Apoptotic cells paradoxically expressed stronger binding of fH after treatment with DNase. This could imply that DNase treatment does not in fact remove DNA molecules from the surface of apoptotic cells but rather makes the DNA more accessible, perhaps by exposing more DNA fragments through their partial release from histones. This is supported by the observation that DNase treatment also enhances binding of anti-DNA antibodies. Confocal microscopy provided evidence for co-localization of fH and DNA on apoptotic cells. It appears that DNA translocated to the surface of apoptotic cells is still wound around nucleosomes on the basis that DNA, individual core histones, histone-DNA complexes, and the native nucleosome core particles can all be detected on the surface of apoptotic cells (31). Therefore we also hypothesized that the increased fH binding that we observed upon DNase treatment could be due in part to interaction of fH with histones. In support of this, we showed that DNA-free histones isolated from Jurkat cells bound fH. Interestingly, all fH ligands identified on apoptotic cells so far, annexin-II, DNA, and histones bind with differential affinities to two common polymorphic variants of fH (Tyr-402 and His-402) located in CCPs 6–8 and associated with a propensity to develop age-related macular degeneration.
Phospholipids, particularly those found specifically on apoptotic cells, represented another potential ligand class for fH. Treatment of cells with PLA2 caused a significant increase of fH binding, implying that lysophospholipids may bind fH. A similar observation was previously reported for IgM (50), but, in that case, the binding was also sensitive to treatment with PLD, which was not observed in the case of fH. We used two different methods to investigate whether there is direct interaction between fH and lysophospholipids: liposome-coated beads from sonicated liposomes for flow cytometric analysis and sensor-chip L1 covered with extruded liposomes for SPR analysis. In both systems we found no significant interaction of fH with PS, PC, and PE or with their lysophospholipid form in various combinations and concentrations. We used both synthetic lipids as well as those isolated from natural sources with the same negative results. If lysophospholipids were involved in binding of fH to apoptotic cells, binding should be decreased by treatment with the intracellular phospholipase inhibitors MAFP and BEL. These inhibitors also have pro-apoptotic properties. We analyzed cells in which apoptosis had been induced in the presence of MAFP, and BEL, but detected no significantly reduced fH binding. Therefore, we suggest that lysophospholipids are not involved in binding of fH. The great increase in fH binding caused by PLA2 could be explained by the increase in DNA-expressing cells after such treatment. Creation of lysophospholipids also disrupts the membrane. This may give the cell a more necrotic profile, which might explain the increase of cell-surface DNA.
Oxidation of liposomes with myeloperoxidase in SPR did not result in binding to any of the tested phospholipids and lysophospholipids (data not shown). General oxidation of apoptotic cell surfaces has been reported as an important step for recognition by phagocytes. However, no increase in fH binding could be observed after oxidation of live and apoptotic cells with myeloperoxidase (data not shown). Finally, we could not detect any interaction of fH with cardiolipin, sphingomyelin, and ceramide that are known to be exposed on apoptotic cells (4, 51). Despite the extensive repertoire of lipids used in this study, we cannot completely rule out all phospholipids or lysophospholipids as potential ligands to fH. However, the evidence so far suggests that lipids are unlikely to be ligands for fH on apoptotic cells.
Taken together, we have identified annexin-II, DNA, and histones as three fH ligands found on cell surfaces during the course of apoptosis. Additional fH ligands may exist on apoptotic cells, but it is unlikely that these are lipids, trypsin-sensitive proteins, or GAGs. Furthermore, involvement of fH and annexin-II in the clearance of apoptotic cells and their role in autoimmune diseases such as systemic lupus erythematosus should be a subject of further studies.
This work was supported by the Swedish Research Council, the Swedish Foundation for Strategic Research, the Foundations of Österlund, Kock, King Gustav V's 80th Anniversary, Knut and Alice Wallenberg, and Inga-Britt and Arne Lundberg, and research grants from the University Hospital in Malmö. Work in Edinburgh was supported by the Wellcome Trust.
- PS
- phosphatidylserine
- AF488/647
- Alexa Fluor 488/647
- AV
- annexin V
- BEL
- bromoenol lactone
- BSA
- bovine serum albumin
- C4BP
- C4b-binding protein
- CCP
- complement control protein (domain)
- DL488
- DyLight 488
- fH
- factor H
- FITC
- fluorescein isothiocyanate
- GAG
- glycosaminoglycan
- HRP
- horseradish peroxidase
- MAFP
- methyl arachidonyl fluorophosphonate
- MBL
- mannose-binding lectin
- NHS
- normal human serum
- OSAD
- osteoadherin
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PLA2
- phospholipase A2
- sPLA2
- secreted PLA2
- PLD
- phospholipase D
- PBS
- phosphate-buffered saline
- dsDNA
- double strand DNA
- SPR
- surface plasmon resonance
- LysoPC
- lysophosphatidylcholine
- ANOVA
- analysis of variance.
REFERENCES
- 1.Fadeel B. (2003) Cell Mol. Life Sci. 60, 2575–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin S. J., Reutelingsperger C. P., McGahon A. J., Rader J. A., van Schie R. C., LaFace D. M., Green D. R. (1995) J. Exp. Med. 182, 1545–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorice M., Circella A., Misasi R., Pittoni V., Garofalo T., Cirelli A., Pavan A., Pontieri G. M., Valesini G. (2000) Clin. Exp. Immunol. 122, 277–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tepper A. D., Ruurs P., Wiedmer T., Sims P. J., Borst J., van Blitterswijk W. J. (2000) J. Cell Biol. 150, 155–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walport M. J. (2001) N. Engl. J. Med. 344, 1058–1066 [DOI] [PubMed] [Google Scholar]
- 6.Walport M. J. (2001) N. Engl. J. Med. 344, 1140–1144 [DOI] [PubMed] [Google Scholar]
- 7.Nauta A. J., Raaschou-Jensen N., Roos A., Daha M. R., Madsen H. O., Borrias-Essers M. C., Ryder L. P., Koch C., Garred P. (2003) Eur. J. Immunol. 33, 2853–2863 [DOI] [PubMed] [Google Scholar]
- 8.Nauta A. J., Trouw L. A., Daha M. R., Tijsma O., Nieuwland R., Schwaeble W. J., Gingras A. R., Mantovani A., Hack E. C., Roos A. (2002) Eur. J. Immunol. 32, 1726–1736 [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez de Córdoba S., Esparza-Gordillo J., Goicoechea de Jorge E., Lopez-Trascasa M., Sanchez-Corral P. (2004) Mol. Immunol. 41, 355–367 [DOI] [PubMed] [Google Scholar]
- 10.Ogden C. A., deCathelineau A., Hoffmann P. R., Bratton D., Ghebrehiwet B., Fadok V. A., Henson P. M. (2001) J. Exp. Med. 194, 781–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kemper C., Mitchell L. M., Zhang L., Hourcade D. E. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 9023–9028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu W., Berger S. P., Trouw L. A., de Boer H. C., Schlagwein N., Mutsaers C., Daha M. R., van Kooten C. (2008) J. Immunol. 180, 7613–7621 [DOI] [PubMed] [Google Scholar]
- 13.Sjöberg A. P., Trouw L. A., Blom A. M. (2009) Trends Immunol. 30, 83–90 [DOI] [PubMed] [Google Scholar]
- 14.Trouw L. A., Bengtsson A. A., Gelderman K. A., Dahlbäck B., Sturfelt G., Blom A. M. (2007) J. Biol. Chem. 282, 28540–28548 [DOI] [PubMed] [Google Scholar]
- 15.Trouw L. A., Nilsson S. C., Gonçalves I., Landberg G., Blom A. M. (2005) J. Exp. Med. 201, 1937–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oppermann M., Manuelian T., Józsi M., Brandt E., Jokiranta T. S., Heinen S., Meri S., Skerka C., Götze O., Zipfel P. F. (2006) Clin. Exp. Immunol. 144, 342–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coleman K., Renner B., Ferreira V., Cortes C., Pangburn M., Tomlinson S., Holers M., Thurman J. (2008) in XXII International Complement Workshop (Daha M. R., Villadangos J. A. eds) p. 411, Pergamon-Elsevier Science Ltd., Basel, Switzerland [Google Scholar]
- 18.Blom A. M., Kask L., Dahlbäck B. (2003) Mol. Immunol. 39, 547–556 [DOI] [PubMed] [Google Scholar]
- 19.Dahlbäck B. (1983) Biochem. J. 209, 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbert A. P., Uhrín D., Lyon M., Pangburn M. K., Barlow P. N. (2006) J. Biol. Chem. 281, 16512–16520 [DOI] [PubMed] [Google Scholar]
- 21.Schmidt C. Q., Herbert A. P., Kavanagh D., Gandy C., Fenton C. J., Blaum B. S., Lyon M., Uhrín D., Barlow P. N. (2008) J. Immunol. 181, 2610–2619 [DOI] [PubMed] [Google Scholar]
- 22.Happonen K. E., Sjöberg A. P., Mörgelin M., Heinegård D., Blom A. M. (2009) J. Immunol. 182, 1518–1525 [DOI] [PubMed] [Google Scholar]
- 23.Pulido R., Sánchez-Madrid F. (1989) J. Immunol. 143, 1930–1936 [PubMed] [Google Scholar]
- 24.Lehmann P. V. (1996) Nephrol. Dial Transplant 11, 952–955 [DOI] [PubMed] [Google Scholar]
- 25.Sjöberg A. P., Manderson G. A., Mörgelin M., Day A. J., Heinegård D., Blom A. M. (2009) Mol. Immunol. 46, 830–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webb J. H., Blom A. M., Dahlbäck B. (2002) J. Immunol. 169, 2580–2586 [DOI] [PubMed] [Google Scholar]
- 27.Clark S. J., Higman V. A., Mulloy B., Perkins S. J., Lea S. M., Sim R. B., Day A. J. (2006) J. Biol. Chem. 281, 24713–24720 [DOI] [PubMed] [Google Scholar]
- 28.Meri S., Pangburn M. K. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 3982–3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meri S., Pangburn M. K. (1994) Biochem. Biophys. Res. Commun. 198, 52–59 [DOI] [PubMed] [Google Scholar]
- 30.Sjöberg A. P., Trouw L. A., Clark S. J., Sjölander J., Heinegård D., Sim R. B., Day A. J., Blom A. M. (2007) J. Biol. Chem. 282, 10894–10900 [DOI] [PubMed] [Google Scholar]
- 31.Radic M., Marion T., Monestier M. (2004) J. Immunol. 172, 6692–6700 [DOI] [PubMed] [Google Scholar]
- 32.Manders E. M., Verbeek J. A., Aten J. A. (1993) J. Microsc. 169, 375–382 [DOI] [PubMed] [Google Scholar]
- 33.Cummings B. S., McHowat J., Schnellmann R. G. (2000) J. Pharmacol. Exp. Ther. 294, 793–799 [PubMed] [Google Scholar]
- 34.Atsumi G., Tajima M., Hadano A., Nakatani Y., Murakami M., Kudo I. (1998) J. Biol. Chem. 273, 13870–13877 [DOI] [PubMed] [Google Scholar]
- 35.Wissing D., Mouritzen H., Egeblad M., Poirier G. G., Jäättelä M. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 5073–5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oslakovic C., Krisinger M. J., Andersson A., Jauhiainen M., Ehnholm C., Dahlbäck B. (2009) J. Biol. Chem. 284, 5896–5904 [DOI] [PubMed] [Google Scholar]
- 37.Hinkovska-Galcheva V., Kjeldsen L., Mansfield P. J., Boxer L. A., Shayman J. A., Suchard S. J. (1998) Blood 91, 4761–4769 [PubMed] [Google Scholar]
- 38.Woodin A. M., Wieneke A. A. (1966) Biochem. J. 99, 493–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manuelian T., Hellwage J., Meri S., Caprioli J., Noris M., Heinen S., Jozsi M., Neumann H. P., Remuzzi G., Zipfel P. F. (2003) J. Clin. Invest. 111, 1181–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waisman D. M. (1995) Mol. Cell Biochem. 149–150, 301–322 [DOI] [PubMed] [Google Scholar]
- 41.Gerke V., Creutz C. E., Moss S. E. (2005) Nat. Rev. Mol. Cell Biol. 6, 449–461 [DOI] [PubMed] [Google Scholar]
- 42.Ma K., Simantov R., Zhang J. C., Silverstein R., Hajjar K. A., McCrae K. R. (2000) J. Biol. Chem. 275, 15541–15548 [DOI] [PubMed] [Google Scholar]
- 43.Fan X., Krahling S., Smith D., Williamson P., Schlegel R. A. (2004) Mol. Biol. Cell 15, 2863–2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salle V., Mazière J. C., Smail A., Cévallos R., Mazière C., Fuentes V., Tramier B., Makdassi R., Choukroun G., Vittecoq O., Goëb V., Ducroix J. P. (2008) J. Clin. Immunol. 28, 291–297 [DOI] [PubMed] [Google Scholar]
- 45.Józsi M., Zipfel P. F. (2008) Trends Immunol. 29, 380–387 [DOI] [PubMed] [Google Scholar]
- 46.Jones A. L., Poon I. K., Hulett M. D., Parish C. R. (2005) J. Biol. Chem. 280, 35733–35741 [DOI] [PubMed] [Google Scholar]
- 47.Hocking H. G., Herbert A. P., Kavanagh D., Soares D. C., Ferreira V. P., Pangburn M. K., Uhrín D., Barlow P. N. (2008) J. Biol. Chem. 283, 9475–9487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Józsi M., Strobel S., Dahse H. M., Liu W. S., Hoyer P. F., Oppermann M., Skerka C., Zipfel P. F. (2007) Blood 110, 1516–1518 [DOI] [PubMed] [Google Scholar]
- 49.Gormally E., Caboux E., Vineis P., Hainaut P. (2007) Mutat. Res. 635, 105–117 [DOI] [PubMed] [Google Scholar]
- 50.Kim S. J., Gershov D., Ma X., Brot N., Elkon K. B. (2002) J. Exp. Med. 196, 655–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sorice M., Circella A., Cristea I. M., Garofalo T., Di Renzo L., Alessandri C., Valesini G., Esposti M. D. (2004) Cell Death Differ. 11, 1133–1145 [DOI] [PubMed] [Google Scholar]