Abstract
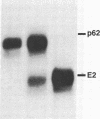
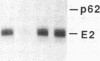
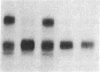
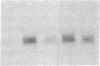
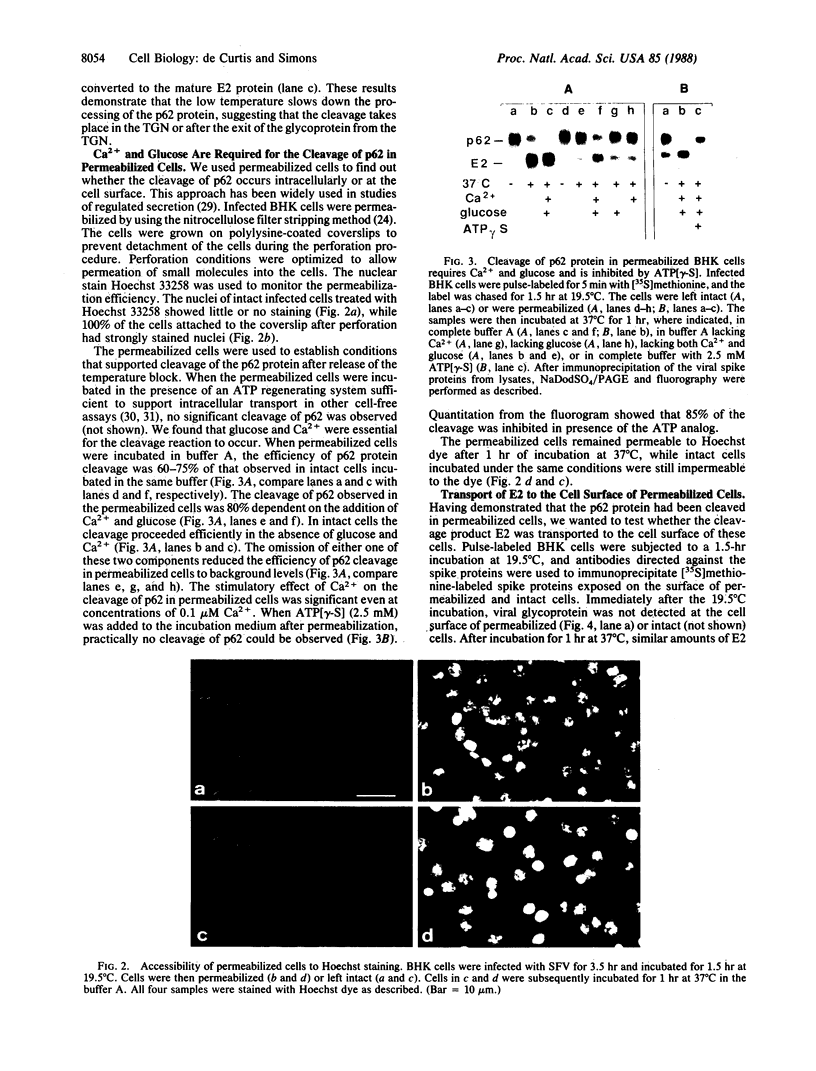
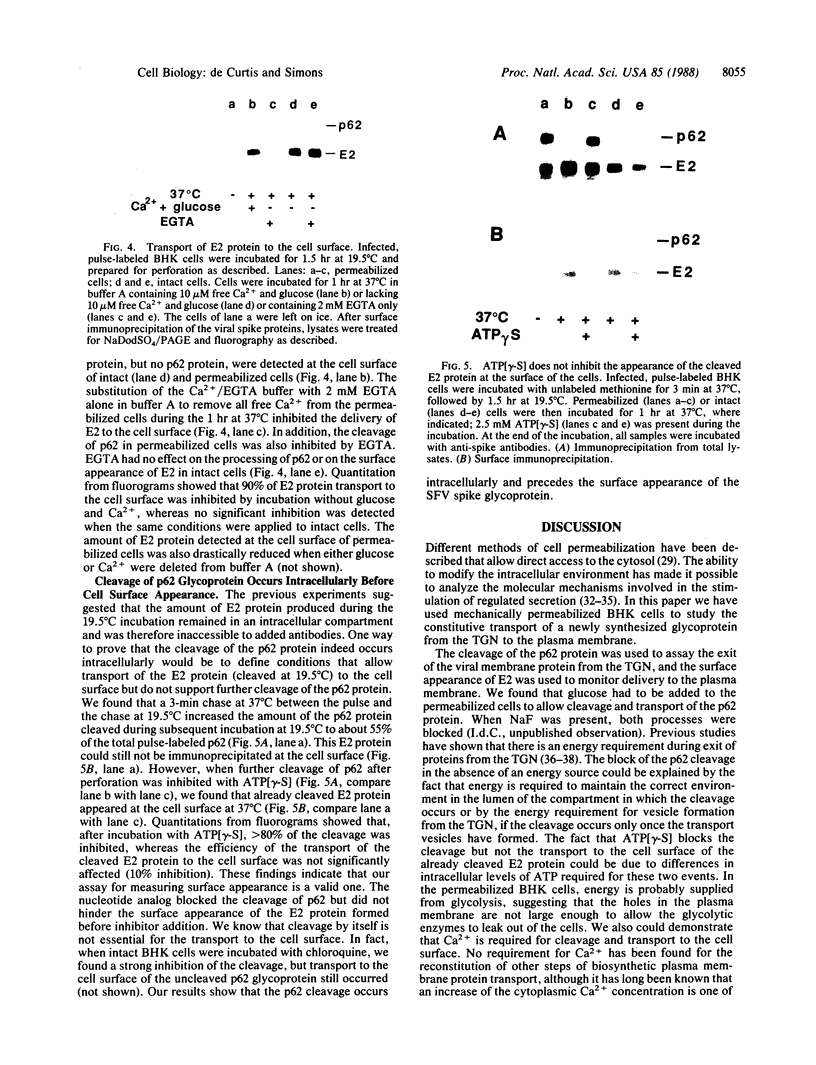
In this paper mechanically permeabilized cells have been used to dissect the transport of Semliki Forest virus glycoproteins from the trans-Golgi network to the plasma membrane. Transport from the Golgi complex was monitored by measuring the proteolytic cleavage of the Semliki Forest virus p62 glycoprotein into the E2 and E3 polypeptide chains. Cell surface appearance was measured by the exposure of the exoplasmic domain to antibodies directed against the viral glycoprotein. Both the cleavage of the p62 protein and the transport of the glycoprotein to the cell surface were reconstituted in permeabilized BHK cells when calcium and glucose were present in the medium. Detailed analysis showed that the cleavage of the p62 protein occurred before arrival to the plasma membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Knight D. E. Calcium-dependent exocytosis in bovine adrenal medullary cells with leaky plasma membranes. Nature. 1978 Dec 7;276(5688):620–622. doi: 10.1038/276620a0. [DOI] [PubMed] [Google Scholar]
- Balcarova J., Helenius A., Simons K. Antibody response to spike protein vaccines prepared from Semliki Forest virus. J Gen Virol. 1981 Mar;53(Pt 1):85–92. doi: 10.1099/0022-1317-53-1-85. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Dunphy W. G., Braell W. A., Rothman J. E. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984 Dec;39(2 Pt 1):405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Glick B. S., Rothman J. E. Sequential intermediates in the pathway of intercompartmental transport in a cell-free system. Cell. 1984 Dec;39(3 Pt 2):525–536. doi: 10.1016/0092-8674(84)90459-8. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Keller D. S. ATP-coupled transport of vesicular stomatitis virus G protein. Functional boundaries of secretory compartments. J Biol Chem. 1986 Nov 5;261(31):14690–14696. [PubMed] [Google Scholar]
- Balch W. E., Wagner K. R., Keller D. S. Reconstitution of transport of vesicular stomatitis virus G protein from the endoplasmic reticulum to the Golgi complex using a cell-free system. J Cell Biol. 1987 Mar;104(3):749–760. doi: 10.1083/jcb.104.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke B., Walter C., Griffiths G., Warren G. Viral glycoproteins at different stages of intracellular transport can be distinguished using monoclonal antibodies. Eur J Cell Biol. 1983 Sep;31(2):315–324. [PubMed] [Google Scholar]
- Docherty K., Steiner D. F. Post-translational proteolysis in polypeptide hormone biosynthesis. Annu Rev Physiol. 1982;44:625–638. doi: 10.1146/annurev.ph.44.030182.003205. [DOI] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. D., Bravo R., Simons K. An enzymatic assay reveals that proteins destined for the apical or basolateral domains of an epithelial cell line share the same late Golgi compartments. EMBO J. 1985 Feb;4(2):297–307. doi: 10.1002/j.1460-2075.1985.tb03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. Nucleotide sequence of cdna coding for Semliki Forest virus membrane glycoproteins. Nature. 1980 Nov 20;288(5788):236–241. doi: 10.1038/288236a0. [DOI] [PubMed] [Google Scholar]
- Green J., Griffiths G., Louvard D., Quinn P., Warren G. Passage of viral membrane proteins through the Golgi complex. J Mol Biol. 1981 Nov 15;152(4):663–698. doi: 10.1016/0022-2836(81)90122-4. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Pfeiffer S., Simons K., Matlin K. Exit of newly synthesized membrane proteins from the trans cisterna of the Golgi complex to the plasma membrane. J Cell Biol. 1985 Sep;101(3):949–964. doi: 10.1083/jcb.101.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986 Oct 24;234(4775):438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Gumbiner B., Kelly R. B. Two distinct intracellular pathways transport secretory and membrane glycoproteins to the surface of pituitary tumor cells. Cell. 1982 Jan;28(1):51–59. doi: 10.1016/0092-8674(82)90374-9. [DOI] [PubMed] [Google Scholar]
- Howell T. W., Cockcroft S., Gomperts B. D. Essential synergy between Ca2+ and guanine nucleotides in exocytotic secretion from permeabilized rat mast cells. J Cell Biol. 1987 Jul;105(1):191–197. doi: 10.1083/jcb.105.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. IV. Metabolic requirements. J Cell Biol. 1968 Dec;39(3):589–603. doi: 10.1083/jcb.39.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou W. M., Verhoeyen M., Devos R., Saman E., Fang R., Huylebroeck D., Fiers W., Threlfall G., Barber C., Carey N. Complete structure of the hemagglutinin gene from the human influenza A/Victoria/3/75 (H3N2) strain as determined from cloned DNA. Cell. 1980 Mar;19(3):683–696. doi: 10.1016/s0092-8674(80)80045-6. [DOI] [PubMed] [Google Scholar]
- Kelly R. B. Pathways of protein secretion in eukaryotes. Science. 1985 Oct 4;230(4721):25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Tonge D. A., Baker P. F. Inhibition of exocytosis in bovine adrenal medullary cells by botulinum toxin type D. Nature. 1985 Oct 24;317(6039):719–721. doi: 10.1038/317719a0. [DOI] [PubMed] [Google Scholar]
- Käriäinen L., Simons K., von Bonsdorff C. H. Studies in subviral components of Semliki Forest virus. Ann Med Exp Biol Fenn. 1969;47(4):235–248. [PubMed] [Google Scholar]
- Matlin K. S., Simons K. Reduced temperature prevents transfer of a membrane glycoprotein to the cell surface but does not prevent terminal glycosylation. Cell. 1983 Aug;34(1):233–243. doi: 10.1016/0092-8674(83)90154-x. [DOI] [PubMed] [Google Scholar]
- McCune J. M., Rabin L. B., Feinberg M. B., Lieberman M., Kosek J. C., Reyes G. R., Weissman I. L. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell. 1988 Apr 8;53(1):55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- Morrison T., Ward L. J., Semerjian A. Intracellular processing of the Newcastle disease virus fusion glycoprotein. J Virol. 1985 Mar;53(3):851–857. doi: 10.1128/jvi.53.3.851-857.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Storch M. J., Anderson R. G., Vassalli J. D., Perrelet A. Proteolytic maturation of insulin is a post-Golgi event which occurs in acidifying clathrin-coated secretory vesicles. Cell. 1987 Jun 19;49(6):865–868. doi: 10.1016/0092-8674(87)90624-6. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Paterson R. G., Harris T. J., Lamb R. A. Fusion protein of the paramyxovirus simian virus 5: nucleotide sequence of mRNA predicts a highly hydrophobic glycoprotein. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6706–6710. doi: 10.1073/pnas.81.21.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin D., Langley O. K., Aunis D. Anti-alpha-fodrin inhibits secretion from permeabilized chromaffin cells. Nature. 1987 Apr 2;326(6112):498–501. doi: 10.1038/326498a0. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Porter A. G., Barber C., Carey N. H., Hallewell R. A., Threlfall G., Emtage J. S. Complete nucleotide sequence of an influenza virus haemagglutinin gene from cloned DNA. Nature. 1979 Nov 29;282(5738):471–477. doi: 10.1038/282471a0. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Strauss J. H. Nucleotide sequence of the 26S mRNA of Sindbis virus and deduced sequence of the encoded virus structural proteins. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2062–2066. doi: 10.1073/pnas.78.4.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J., Kuismanen E. Pre- and post-Golgi vacuoles operate in the transport of Semliki Forest virus membrane glycoproteins to the cell surface. Cell. 1984 Sep;38(2):535–549. doi: 10.1016/0092-8674(84)90508-7. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Simons K., Keränen S., Käriänen L. Identification of a precursor for one of the Semliki forest virus membrane proteins. FEBS Lett. 1973 Jan 15;29(2):87–91. doi: 10.1016/0014-5793(73)80532-0. [DOI] [PubMed] [Google Scholar]
- Simons K., Virta H. Perforated MDCK cells support intracellular transport. EMBO J. 1987 Aug;6(8):2241–2247. doi: 10.1002/j.1460-2075.1987.tb02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Warren G. Semliki Forest virus: a probe for membrane traffic in the animal cell. Adv Protein Chem. 1984;36:79–132. doi: 10.1016/S0065-3233(08)60296-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J., Hollinshead M., Frank R., Burke B. An antibody specific for an endoproteolytic cleavage site provides evidence that pro-opiomelanocortin is packaged into secretory granules in AtT20 cells before its cleavage. J Cell Biol. 1987 Jul;105(1):155–162. doi: 10.1083/jcb.105.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- Ziemiecki A., Garoff H., Simons K. Formation of the Semliki Forest virus membrane glycoprotein complexes in the infected cell. J Gen Virol. 1980 Sep;50(1):111–123. doi: 10.1099/0022-1317-50-1-111. [DOI] [PubMed] [Google Scholar]
- de Curtis I., Howell K. E., Simons K. Isolation of a fraction enriched in the trans-Golgi network from baby hamster kidney cells. Exp Cell Res. 1988 Apr;175(2):248–265. doi: 10.1016/0014-4827(88)90190-5. [DOI] [PubMed] [Google Scholar]