Abstract
We combined fluorescence recovery after photobleaching (FRAP) beam-size analysis with biochemical assays to investigate the mechanisms of membrane recruitment and activation of phospholipase C-β2 (PLCβ2) by G protein αq and βγ dimers. We show that activation by αq and βγ differ from activation by Rac2 and from each other. Stimulation by αq enhanced the plasma membrane association of PLCβ2, but not of PLCβ2Δ, which lacks the αq-interacting region. Although αq resembled Rac2 in increasing the contribution of exchange to the FRAP of PLCβ2 and in enhancing its membrane association, the latter effect was weaker than with Rac2. Moreover, the membrane recruitment of PLCβ2 by αq occurred by enhancing PLCβ2 association with fast-diffusing (lipid-like) membrane components, whereas stimulation by Rac2 led to interactions with slow diffusing membrane sites. On the other hand, activation by βγ shifted the FRAP of PLCβ2 and PLCβ2Δ to pure lateral diffusion 3- to 5-fold faster than lipids, suggesting surfing-like diffusion along the membrane. We propose that these different modes of PLCβ2 membrane recruitment may accommodate contrasting functional needs to hydrolyze phosphatidylinositol 4,5-bisphosphate (PtdInsP2) in localized versus dispersed populations. PLCβ2 activation by Rac2, which leads to slow lateral diffusion and much faster exchange, recruits PLCβ2 to act locally on PtdInsP2 at specific domains. Activation by αq leads to lipid-like diffusion of PLCβ2 accompanied by exchange, enabling the sampling of larger, yet limited, areas prior to dissociation. Finally, activation by βγ recruits PLCβ2 to the membrane by transient interactions, leading to fast “surfing” diffusion along the membrane, sampling large regions for dispersed PtdInsP2 populations.
Keywords: G Proteins, Heterotrimeric G Proteins, Membrane Biophysics, Membrane Lipids, Phospholipase C, FRAP, Lateral Diffusion, Rac
Introduction
Phospholipase C-β (PLCβ)4 isozymes hydrolyze phosphatidylinositol 4,5-bisphosphate (PtdInsP2) to produce inositol 1,4,5-trisphosphate and diacylglycerol (1–3). They are activated to different extents by heterotrimeric G protein αq subunits (αq) and βγ dimers (βγ) (1–3). PLCβ2 is also activated by the Rho GTPases Rac and Cdc42 (4–7). Activation by Rac has also been demonstrated for PLCγ2 (8). PLCβ2 has long been known to be expressed in hematopoietic cells (2, 3) but is also encountered in a variety of other cell types and tissues, including smooth muscle cells (9) and several brain regions (10, 11). Moreover, PLCβ2 was shown to be essential for taste perception via certain G protein-coupled oral taste receptors (12).
Activation of PLCβ2 by αq and related α subunits requires the C-terminal region of the enzyme; mutants with deletions in this region (e.g. PLCβ2Δ, that lacks the Phe819–Glu1166 segment) are resistant to stimulation by αq, but undergo activation by βγ and Rac/Cdc42 (7, 13–15). Recent results show that βγ and Rac/Cdc42 activate PLCβ2 by interacting, at least in part, with different regions of the effector enzyme. Thus, although the pleckstrin homology (PH) domain of PLCβ2 is dispensable for activation of the enzyme by βγ dimers (6), this domain also interacts with βγ, suggesting that the latter binds to at least two sites on the enzyme (16). In contrast, direct interaction of the PH domain with activated Rho GTPases is both necessary and sufficient for their stimulatory function (6, 17, 18). Functional evidence for a connection between PLCβ2 and Rho GTPases in cells is provided by the chemoattractant receptor system, which activates Rac/Cdc42 and PLCβ2 (19–22).
The mechanisms by which heterotrimeric G proteins and Rho GTPases regulate PLCβ isozymes are only partially understood (16, 23, 24), especially in live cells. Because both PLCβ substrate(s) and stimulators are membrane-bound (16, 23, 24), recruitment of PLCβ from the cytoplasm to the membrane is clearly required for effective enzyme activation. This view is consistent with the loss of PLCβ activation following mutations that interfere with the membrane association of αq or βγ (25, 26). PtdInsP2, the substrate of PLCβ enzymes, is located at the internal plasma membrane leaflet in both dispersed and localized populations (27–33) and diffuses in the cytoplasmic leaflet of cell membranes with a lateral diffusion coefficient (D) of 0.5–1 μm2/s (32, 34). The PH domains of several proteins (e.g. PLCδ1 and the Dictyostelium discoideum CRAC protein) were found to undergo dynamic membrane-cytoplasm exchange along with lateral diffusion over short distances (35, 36). Interestingly, we found a similar behavior for PLCβ2 (7), although this protein is likely to interact with the membrane through several distinct sites; these include the catalytic triosephosphate isomerase barrel, the C-terminal region, and possibly the EF hands motif and the C2 domain, as well as its PH domain, which, unlike that of PLCδ, appears to be unable to bind phosphoinositides (16, 18).
We have formerly studied the interactions of green fluorescent protein (GFP)-tagged PLCβ2 (PLCβ2-GFP) and PLCβ2Δ-GFP with the plasma membrane in live cells (7). Using FRAP beam-size analysis (37), which discriminates between recovery by lateral diffusion and exchange, we demonstrated that activation by Rac2 enhances the association of PLCβ2 and PLCβ2Δ with the plasma membrane via binding to slow diffusing membrane proteins. However, the effects of the G protein subunits αq and βγ on the membrane interactions of PLCβ2 and their potential relevance to PLCβ2 stimulation were not explored. Here, we show that αq and βγ recruit PLCβ2 to the plasma membrane by distinct mechanisms, which differ from the mechanism employed by Rac2 and from each other. Each stimulator leads to a specific ratio between the rates of exchange and lateral diffusion characterizing the interaction of PLCβ2 with the membrane, and this in turn allows the enzyme to act preferentially on localized or dispersed PtdInsP2 populations.
EXPERIMENTAL PROCEDURES
Materials and Plasmids
Murine anti-GFP antibodies were from Roche Applied Science, and peroxidase-conjugated goat anti-mouse IgG was from Sigma. The cDNAs of wild-type (wt) and G12V mutant human Rac2 (Rac2(wt) and Rac2(G12V), respectively), mouse αq(wt) and αq(R183C), human β1, bovine γ2(wt) and γ2(C68S), and human PLCβ2 were ligated into pcDNA3.1(+) or pcDNA3.1(−) (Invitrogen). Bovine and human γ2 have identical amino acid sequences, and mouse αq differs from human αq in but one residue (S141A). There are several indications that this residue has no role in αq function: (i) In mouse αq, Ser141 is in a long loop connecting helices αE and αF of the helical domain (PDB accession codes 2BCJ and 2RGN); it does not reside in any of the three switch domains or in regions known to interact with PLCβ isozymes and is not among the residues that contact the bound guanine nucleotide and Gβγ (38, 39). (ii) Secondary structure prediction algorithms do not predict a structural difference between mouse and human αq αE-loop-αF region (40, results not shown). (iii) Mouse αq has been successfully reconstituted with many signaling proteins from several other species, including human, for functional and structural analyses (e.g. 41–43). The αq(wt) and αq(R183C) cDNAs were a gift from B. R. Conklin and H. R. Bourne (University of California, San Francisco, CA). The cDNAs of PLCβ2 and the deletion mutant PLCβ2Δ, which lacks a C-terminal region necessary for stimulation by αq (Phe819–Glu1166), were inserted in-frame with the cDNA of GFP into the EcoRI/SalI site of pEGFP-N1 (Clontech) to generate the plasmids encoding PLCβ2-GFP and PLCβ2Δ-GFP (7). Plasmids (pcDNA3.1) encoding the N-terminal half (residues 1–155) of the venus fluorescent protein fused to the N terminus of human γ2 (venus 1–155-γ2) or venus residues 156–239 fused to the N terminus of human β1 (venus 156–239-β1) were described earlier (44) and donated by N. A. Lambert (Medical College of Georgia, Augusta, GA). A vector encoding the Salmonella typhimurium SigD protein cloned in pEGFP-N1 lacking the EGFP-encoding sequences (45), with SigD starting at residue 28 to increase stability, was a gift from B. Brett Finlay (University of British Columbia, Vancouver, Canada).
Cell Culture and Transfection
Cells were grown in Dulbecco's modified Eagle's medium with 10% fetal calf serum (7). For FRAP experiments, COS-7 cells grown on glass coverslips in 35-mm dishes for 24 h were transfected using DEAE-dextran (46) with 150 ng of plasmid DNA encoding one of the PLCβ2-GFP derivatives together with 850 ng of empty vector or expression vectors encoding the various activating proteins: human β1 along with bovine γ2(wt) (β1γ2), β1 plus the isoprenylation-defective γ2(C68S) (β1γ2(C68S)), murine αq(wt), constitutively active αq(R183C), and human Rac2(wt) or Rac2(G12V). After 24 h, the cells were taken for the FRAP studies.
For studies of inositol phosphate formation, COS-7 cells (1.5 × 105/well) were seeded into 12-well plates. After 24 h, the cells were incubated with fresh medium (1 ml, 1 h), and co-transfected with vector encoding a GFP-tagged PLCβ2 derivative (250 ng) together with 750 ng of vectors encoding the various activating proteins. Transfection was done with LipofectamineTM 2000 (Invitrogen) according to the manufacturer's instructions. At 48 h post-transfection, the cells were taken for experiments on inositol phosphate formation. For experiments on subcellular fractionation of PLCβ2, COS-7 cells (2.5 × 106/10-cm dish) were co-transfected as above with PLCβ2-GFP or PLCβ2Δ-GFP (7 μg of DNA) together with 7 μg of vectors encoding one of the PLCβ2 stimulators or empty vector.
FRAP
FRAP studies (47, 48) were conducted as described (7). The experiments were performed 24–26 h post-transfection on COS-7 cells transfected with PLCβ2-GFP derivatives as described above. All experiments were conducted at 22 °C, in Hanks' balanced salt solution supplemented with 20 mm HEPES, pH 7.2. The monitoring argon ion laser beam (488 nm and 1.2 microwatts) was focused through the microscope (Zeiss Universal, Carl Zeiss MicroImaging) to a Gaussian spot with a radius ω = 0.85 ± 0.02 μm (63×/1.4 numerical aperture (NA) oil-immersion objective) or 1.36 ± 0.04 μm (40×/0.75 NA objective). Experiments were conducted with each beam size (beam-size analysis; described previously (7, 37)). The ratio between the illuminated areas (ω2(40×)/ω2(63×)) was 2.56 (n = 39). After a brief measurement at the monitoring intensity, a 5-milliwatt pulse (4–6 ms or 10–20 ms for the 63× and 40× objectives, respectively) bleached 50–70% of the fluorescence in the spot. Fluorescence recovery was followed by the monitoring beam. The apparent characteristic fluorescence recovery time (τ) and the mobile fraction (Rf) were derived from the FRAP curves by nonlinear regression analysis, fitting to a lateral diffusion process with a single τ value (49).
Statistical Analysis of FRAP Data
The significance of differences between τ values measured with the same laser beam size was evaluated by Student's t test. To compare ratio measurements (τ(40×)/τ(63×) and ω2(40×)/ω2(63×)), we employed bootstrap analysis, which is preferable for comparison between ratio values (50). The τ(40×) and τ(63×) values were resampled with replacement using Excel, and average values from each group of resampled data (τ(40×)Boot and τ(63×)Boot) were derived. For each beam size, 1000 averaged samples were generated, followed by calculation of the bootstrap ratio dividing τ(40×)Boot by τ(63×)Boot. To evaluate whether the τ ratios thus obtained differ significantly from the beam-size ratio calculated by the same method (ω2(40×)Boot/ω2(63×)Boot), the set of the τ bootstrap ratios was divided by the set of beam area bootstrap ratios, and the p value was derived from the spread of the resulting histogram at ∼1.
Radiolabeling of Inositol Phospholipids and Analysis of Inositol Phosphate Formation
Twenty-four hours after transfection, COS-7 cells were washed once with 0.5 ml/well of buffer A (10 mm Na2HPO4, 1.8 mm KH2PO4, 140 mm NaCl, 2.7 mm KCl, pH 7.4), followed by addition of 0.4 ml/well of Dulbecco's modified Eagle's medium containing 10% fetal calf serum, 10 μCi/ml myo-[2-3H]inositol (Amersham Biosciences) and 10 mm LiCl. After incubation for 20 h, the cells were washed once with 0.4 ml/well of buffer A and lysed in 0.2 ml/well 10 mm ice-cold formic acid (51). Samples were incubated on ice for 30 min, neutralized with 0.3 ml/well of 10 mm NH4OH, and centrifuged for 5 min at 15,000 × g. The total inositol phosphates in the supernatants were separated on columns of Dowex® 1 × 8–200 ion exchange resin (Sigma), as described (51, 52), and the radioactivity was quantified by liquid scintillation counting.
Subcellular Fractionation
COS-7 cells grown in 10-cm dishes were transiently transfected as described above by vectors encoding wt or mutant GFP-PLCβ2 and various activators. After 20 h, the cells were serum-starved (24 h) to avoid serum-induced activation, scraped into 8 ml of buffer A, and centrifuged at 300 × g for 5 min. The cells were lysed in 70 μl of ice-cold buffer B (20 mm Tris/HCl, pH 7.5, 2 mm EDTA, 2 μg/ml soybean trypsin inhibitor, 3 mm benzamidine, 0.1 mm phenylmethylsulfonyl fluoride, 1 μm pepstatin, 1 μm leupeptin, and 1 μg/ml aprotinin) by freezing in liquid nitrogen and thawing followed by homogenization, forcing the suspension ten times through a 0.40- × 20-mm syringe needle. After removal of unbroken cells and nuclei (300 × g, 10 min, 4 °C), particulate (P) and soluble (S) fractions were separated by centrifugation at 100,000 × g for 60 min at 4 °C. The P fraction was washed once with 100 μl of buffer B and resuspended in 25 μl of buffer B. To enable direct comparison of the relative distribution of PLCβ2-GFP (wt or mutant) between the two fractions, equal proportions (identical percentages by volume, v/v) of pairs of the P and S fractions were analyzed by SDS-PAGE and immunoblotting using anti-GFP antibodies followed by peroxidase goat anti-mouse and ECL. The bands were quantified by densitometry using EZQuant-Gel 2.2 (EZQuant Ltd.).
Miscellaneous
Protein concentrations were determined according to Bradford (53) using bovine IgG as standard. SDS-PAGE and immunoblotting were as described before (7); immunoreactive proteins were visualized using the ECLTM Western blotting Detection System (Amersham Biosciences).
RESULTS
PLCβ2 Activation by Different Stimulators Can Occur at Varying Extents of Membrane Recruitment
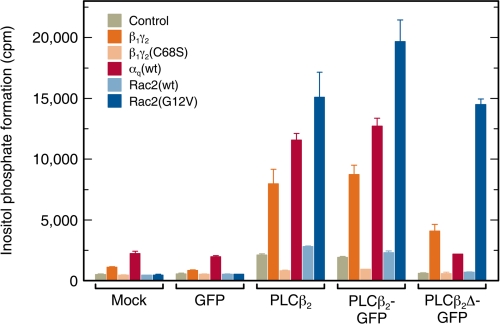
PLCβ2 can be activated by G protein subunits (αq, βγ) and by Rac with different orders of efficacy and potency (1, 2, 6). Because the different stimulators interact with distinct regions of the enzyme, they may differ in their ability to recruit PLCβ2 to the membrane, as well as in the resulting mode of membrane interactions. We therefore compared, in cells and under identical conditions, the activation of PLCβ2 and the PLCβ2Δ mutant by the different stimulators (Fig. 1). Because a major part of the studies involves PLCβ2-GFP chimeric proteins, we validated that the GFP tagging does not interfere with the activation of the enzyme by all the stimulators. To this end, we compared side-by-side (Fig. 1) the ability of β1γ2, αq, and Rac2 (wt or mutated) to stimulate inositol phosphate formation by PLCβ2, PLCβ2-GFP, and PLCβ2Δ-GFP. Inositol phosphate formation was measured in COS-7 cells co-transfected with vectors encoding one of the above PLCβ2 constructs (replaced by empty or GFP-encoding vectors as controls) together with: (i) β1γ2 (β1 along with γ2(wt)); (ii) β1γ2(C68S) (β1 along with the isoprenylation mutant γ2(C68S)); (iii) αq(wt); (iv) Rac2(wt); or (v) Rac2(G12V). Comparison between the inositol phosphate formation levels in cells transfected with PLCβ2 versus PLCβ2-GFP (third and fourth bar groups in Fig. 1) clearly demonstrates that PLCβ2-GFP was activated by all stimulators to an extent similar to that of untagged PLCβ2. These results confirm the earlier demonstration that PLCβ2-GFP is effectively stimulated by activated Rac2 (7), and extend them to show that attachment of GFP to the C terminus of PLCβ2 does not affect the regulation of the enzyme by the heterotrimeric G protein subunits. For both PLCβ2 and PLCβ2-GFP, the degree of stimulation was Rac2(G12V) > αq(wt) > β1γ2. Considering that only a portion of αq(wt) is activated under the conditions used here, this is consistent with the rank order of potencies (which cannot be directly determined in cells) of the three stimulators in in vitro assays: activated αq > activated Rac2 > β1γ2 (6, 14, 54). As shown in Fig. 1, stimulation by β1γ2 required membrane anchorage of γ2, as suggested by the lack of PLCβ2 activation upon replacement of γ2 by the isoprenylation-resistant mutant γ2(C68S). Rac2(wt) had only a weak effect, in accord with the notion that it should undergo activation to stimulate PLCβ2 (7). It should be noted that αq(wt) induced a marked stimulatory response in cells expressing PLCβ2 or PLCβ2-GFP, but not in cells expressing PLCβ2Δ-GFP, in line with the inability of the latter to bind αq. However, PLCβ2Δ-GFP remained responsive to Rac2(G12V) or β1γ2, in accord with earlier in vitro studies on the untagged form of this mutant (6). Constitutively active αq(R183C) gave a very high stimulation of inositol phosphate formation (up to ∼60-fold; not shown in Fig. 1 due to the out-of-range value); this robust activation was obtained already in mock- or GFP-transfected cells, suggesting that it reflects activation of endogenous PLCβ isozymes other than PLCβ2, which are present in COS-7 cells (13, 55). Constitutively active Rac2(G12V) did not stimulate inositol phosphate formation in the absence of a co-transfected PLCβ2 construct (Fig. 1), because it specifically activates PLCβ2, whose endogenous expression level in these cells is very low.
FIGURE 1.
Regulation of wild-type PLCβ2, PLCβ2-GFP, and PLCβ2Δ-GFP by heterotrimeric G protein subunits and Rac2 in intact cells. COS-7 cells were co-transfected as indicated at the abscissa with 250 ng per well of either empty vector (Mock) or vector encoding GFP, PLCβ2, PLCβ2-GFP, or PLCβ2Δ-GFP together with empty vector (Control) or vectors encoding the activating proteins as shown in the inset (750 ng in all cases, composed of 375 ng each for β1 and γ2; Rac2-expressing vectors were added at 50 ng, completed to 750 ng of DNA by empty vector). At 24-h post-transfection, the cells were incubated for 20 h in the presence of myo-[2-3H]inositol (10 μCi/ml) and 10 mm LiCl, and the levels of inositol phosphates were determined as described under “Experimental Procedures.” The values shown correspond to the means ± S.D. of triplicate determinations. Co-transfection with αq(R183C) (data not shown) yielded saturating levels of inositol phosphate formation in all cases (25,000–34,000 cpm), including the mock- and GFP-transfected controls, because high levels of activated αq stimulate the activity of PLCβ isozymes endogenously present in COS-7 cells.
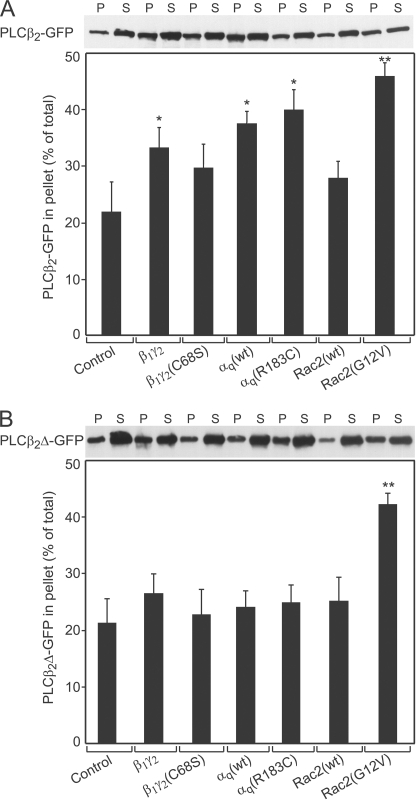
PLCβ2 must be recruited to the membrane to interact with its substrate, PtdInsP2, which is membrane-associated. We therefore investigated the ability of the different stimulators to translocate PLCβ2-GFP and PLCβ2Δ-GFP to the membrane fraction (Fig. 2). To this end, COS-7 cells were co-transfected with a vector encoding PLCβ2-GFP or PLCβ2Δ-GFP together with either empty vector or with vectors encoding various PLCβ2 activators, and the relative distribution of PLCβ2-GFP or PLCβ2Δ-GFP between the cytosolic (S) and membrane (P) fractions was determined by cell fractionation as described under “Experimental Procedures.” Fig. 2A shows that singly expressed PLCβ2-GFP was mostly in the cytosol (S fraction). Co-expression with the different PLCβ2 activators increased the proportion (%) of PLCβ2-GFP in the particulate fraction to varying degrees (Fig. 2A), to extents that correlated with their abilities to stimulate the enzymatic activity of PLCβ2 (cf. Fig. 1): Rac2(G12V) > αq(wt) > β1γ2. Because the fractionation experiment specifically detects the localization of PLCβ2-GFP, it is not masked by activation of endogenous PLCβ enzymes (unlike the stimulation of PLCβ activity), enabling us to measure the effects of constitutively active αq(R183C) on PLCβ2-GFP membrane association. This mutant had a slightly higher effect than αq(wt), which was hard to detect due to the limited sensitivity of the fractionation assay, but is supported by the more sensitive biophysical FRAP studies of PLCβ2 membrane interactions (see Fig. 4). Interestingly, even though both Rac2(G12V) and αq(R183C) are both constitutively active, Rac2(G12V) recruited PLCβ2-GFP to the particulate fraction to a higher extent than αq(R183C). Together with the lower membrane recruitment and lower activation of PLCβ2 by β1γ2 (Figs. 1 and 2A), this raises the possibility that the extent and/or mode of membrane recruitment mediated by the distinct stimulators are different. This view gains strong support from the FRAP beam-size analysis experiments (Fig. 4), which detect with high sensitivity the membrane association dynamics of PLCβ2-GFP in live cells; it is corroborated by fractionation studies on cells expressing PLCβ2Δ-GFP (Fig. 2B). Here, β1γ2 did not induce a measurable increase in the percentage of PLCβ2Δ-GFP in the membrane pellet, contrasting with its ability to induce mild, but clear stimulation of PLCβ2Δ-GFP enzymatic activity (Fig. 1). The ability of β1γ2 to activate PLCβ2 or PLCβ2Δ despite its weak effect on their recruitment to the particulate fraction is likely to reflect transient (as opposed to stable) membrane recruitment, enabling significant dissociation of the enzyme from the membrane during fractionation. The notion that β1γ2 elicits transient association of the enzyme with the membrane was validated by the biophysical studies described later (see Figs. 4 and 5). In line with the inability of PLCβ2Δ to bind αq, neither αq(wt) nor αq(R183C) affected its membrane association. On the other hand, Rac2(G12V) was highly effective in recruiting PLCβ2Δ to the particulate fraction, in accord with the concept that activated Rac/Cdc42 GTPases interact with the N-terminal PH domain also present in the mutant (6, 7, 17, 18).
FIGURE 2.
Effect of G protein subunits and Rac2 on the subcellular distribution of PLCβ2-GFPand PLCβ2Δ-GFP. COS-7 cells grown in 10-cm dishes were co-transfected with 7 μg per dish of vector encoding PLCβ2-GFP (A) or PLCβ2Δ-GFP (B) together with either empty vector (Control) or vectors encoding the activating proteins shown in the abscissa (7 μg of DNA, composed of 3.5 μg each for β1 and γ2). After 20 h, the cells were serum-starved for another 24 h, homogenized, and fractionated into postnuclear particulate (P) and soluble (S) fractions as described under “Experimental Procedures.” The two fractions were well separated, as controlled by immunoblotting for RhoGDIα (cytosolic) and Gβ1–5 (membrane-bound, not shown). Equal proportions (v/v) of the P and S fractions of each sample were subjected to SDS-PAGE and quantified by immunoblotting and densitometry using anti-GFP antibodies. The regions shown are those around 160 kDa (A) and 125 kDa (B); no other immunoreactive bands were detected. The immunoblots shown (upper panels) are of a representative experiment, whereas the bar graphs (lower panels) depict the means ± S.E. (n = 3) of multiple experiments quantified by densitometry. Asterisks indicate a significant increase in the percentage of PLCβ2-GFP (A) or PLCβ2Δ-GFP (B) in the membrane fraction (P) relative to the control (**, p < 0.01; *, p < 0.05; Student's t test).
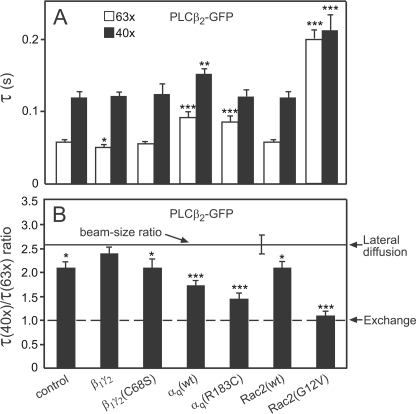
FIGURE 4.
FRAP beam-size analysis suggests activator-dependent distinct modes of PLCβ2-GFP interactions with the plasma membrane. FRAP experiments were conducted at 22 °C as in Fig. 3, on COS-7 cells transfected with PLCβ2-GFP, and an excess of empty vector (Control) or vectors encoding the indicated proteins as described under “Experimental Procedures.” Two beam sizes were generated using a 63× and 40× objectives (see “Experimental Procedures”), and the τ values were determined with each. The ratio between the areas illuminated by the two beams, ω2(40×)/ω2(63×), was 2.56 (n = 39). This ratio is expected for FRAP by lateral diffusion, whereas a ratio of 1 is expected for recovery by exchange (37). The Rf values were high in all cases (≥0.93). A, τ values. Bars are means ± S.E. of 40–60 measurements, each conducted on a different cell. Comparing τ values measured with the same beam size, Rac2(G12V) and αq induced significant increases in τ of PLCβ2-GFP relative to the control (***, p < 10−6; **, p < 0.005; Student's t test). β1γ2 had no significant effect on τ(40×), but reduced τ(63×) (*, p < 0.02). B, τ(40×)/τ(63×) ratios. The ratio values (τ ratios and the beam-size ratio) and their S.E. were calculated from the experimentally measured values (τ(40×) and τ(63×) for τ ratio, ω2(40×) and ω2(63×) for the beam-size ratio) using bootstrap analysis. The bootstrap analysis (see “Experimental Procedures”) showed that the τ ratios of PLCβ2 differ significantly from the 2.56 beam-size ratio predicted for FRAP by lateral diffusion in all cases (***, p < 10−6; *, p < 0.02), except for co-expression with β1γ2 (p > 0.3). Comparison of the τ ratios to 1 (the value expected for FRAP by exchange) using bootstrap analysis shows that in the presence of Rac2(G12V) the τ ratio of PLCβ2-GFP is not significantly different from 1 (p > 0.4).
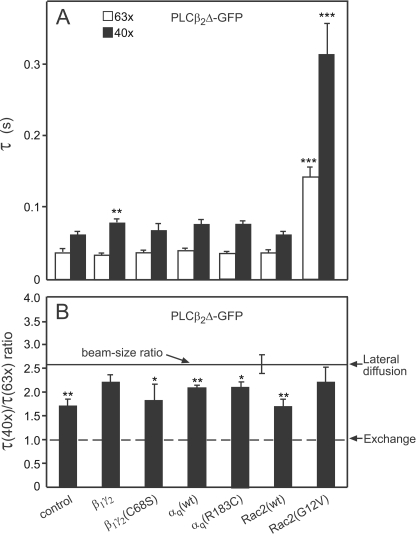
FIGURE 5.
FRAP beam-size analysis of the effects of various activators on the mode of the membrane interactions of PLCβ2Δ-GFP. FRAP experiments and beam-size analyses were conducted as in Fig. 4, except that PLCβ2Δ-GFP replaced PLCβ2-GFP. The Rf values were high in all cases (≥95%; not shown). A, τ values. Bars are means ± S.E. of 40–60 measurements. Comparison of the values measured for cells co-transfected with the various activators relative to control (cells singly transfected with PLCβ2Δ-GFP) showed highly significant increase in both τ(63×) and τ(40×) for PLCβ2Δ-GFP co-expressed with Rac2(G12V) (***, p < 10−6; Student's t test). β1γ2 had a weaker but significant effect on τ(40×) of PLCβ2Δ-GFP (**, p < 0.005). In all other cases, the differences from the τ values measured with the same beam size in the control cells were not significant (p > 0.05). B, τ(40×)/τ(63×) ratios. All the ratio values and their S.E. were calculated using bootstrap analysis. This analysis (see “Experimental Procedures”) showed that co-expression with β1γ2 or with Rac2(G12V) shifts the τ ratio of PLCβ2Δ-GFP to values essentially similar to the 2.56 ratio expected for FRAP by lateral diffusion (p > 0.15 for co-expression with β1γ2, and p > 0.3 for co-expression with constitutively active Rac2). In all other cases, τ(40×)/τ(63×) of PLCβ2Δ differed significantly from the 2.56 beam-size ratio (**, p < 0.005; *, p < 0.02), except for co-expression with β1γ2 (p > 0.3), indicative of a significant contribution of exchange to the FRAP mechanism.
FRAP Studies Demonstrate Distinct Modes of PLCβ2 Membrane Recruitment by the Different Stimulators
The subcellular fractionation experiments (Fig. 2) provide a measure for the population of PLCβ2 molecules that exhibit relatively stable association with the total membrane fraction. To investigate the effects of the various PLCβ2 activators on the mode and dynamics of PLCβ2 interactions with the plasma membrane of live cells, we expressed PLCβ2-GFP in COS-7 cells and employed FRAP to measure its lateral diffusion and membrane association dynamics in the presence or absence of the various stimulators. Typical FRAP experiments are depicted in Fig. 3; quantitative results on multiple cells using two different sizes of a Gaussian laser beam (FRAP beam-size analysis) are shown in Fig. 4. The beam-size analysis (7, 37) explores the membrane interaction mode of proteins capable of both lateral diffusion in the membrane and of exchange between membrane-associated and cytoplasmic pools. If FRAP occurs exclusively by diffusion, the characteristic fluorescence recovery time τ is identical to the characteristic diffusion time τD, which is proportional to the bleached area (τ = τD = ω2/4D, where ω is the Gaussian radius of the beam, and D is the lateral diffusion coefficient) (49). In the current studies, the ratio between the recovery times obtained with the two beam sizes generated using the 40× and 63× objectives, τ(40×)/τ(63×), should be 2.56 (the measured ratio between the illuminated areas). When FRAP occurs by exchange, τ reflects the chemical relaxation time, which is independent of the bleached area; i.e. τ(40×)/τ(63×) should equal 1. Intermediate τ ratios suggest mixed recovery, where the faster process has a higher contribution (7, 37).
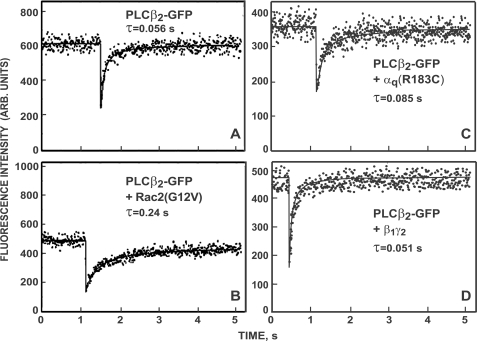
FIGURE 3.
Typical FRAP curves demonstrating that the FRAP rate of PLCβ2-GFP is modulated differently by the various activators. COS-7 cells were transfected with a plasmid encoding PLCβ2-GFP and an excess of empty vector (A) or vectors encoding Rac2(G12V) (B), αq(R183C) (C), or β1 and γ2 (D). FRAP experiments were conducted at 22 °C using a 63× objective (see “Experimental Procedures”). The solid lines show the best fit of a non-linear regression analysis (49). The τ values derived for the specific curves are depicted in each panel; the mobile fractions (Rf) were above 0.93 in all cases, and are therefore not shown.
Because PLCβ2-GFP (and PLCβ2 Δ-GFP) have a significant cytoplasmic fraction (cf. Fig. 2), we focused the laser beam on flat cell regions near the cell periphery, resulting in low contribution of cytoplasmic fluorescence due to the thin cell volume in such regions. Moreover, the FRAP rate of free PLCβ2-GFP in the cytoplasm is very fast, showing immediate recovery on the time scale of the current experiments, ensuring no contribution of cytoplasmic diffusion to the FRAP curves (7). The results shown in Figs. 3 and 4 demonstrate that the different stimulators each induce a distinct effect on the membrane interactions mode of PLCβ2. Prior to stimulation, FRAP beam-size analysis of PLCβ2-GFP yielded a τ(40×)/ τ(63×) ratio of 2.0, intermediate between the ratios characterizing FRAP by lateral diffusion (2.56 with the current beam sizes used) and by exchange (τ ratio = 1), suggesting a mixed contribution of the two mechanisms (7, 37). The contribution of exchange does not allow an accurate calculation of the lateral diffusion coefficient; yet, an estimate of D can be calculated from τ(63×) and the beam size with the 63× objective, because the recovery at this smaller beam size contains a higher contribution of lateral diffusion (56). This yields D = ω2/4τD = (3.2 ± 0.2) μm2/s, faster than D of the lipid probe DiIC16 (1 μm2/s) in the same cells (7) or the 0.5–1 μm2/s value reported for PtdInsP2 (32, 34), in accord with the lack of stable binding of the enzyme to the plasma membrane and the significant contribution of exchange to the FRAP measurements.
Stimulation of PLCβ2-GFP by co-expression with Rac2(G12V) led to a large and highly significant increase in the FRAP times (τ; slower recovery rates) of PLCβ2-GFP (Fig. 4A), in line with the marked increase in its membrane-associated fraction (Fig. 2A). Importantly, this was accompanied by a shift of the FRAP mechanism to recovery by nearly pure exchange (τ(40×)/τ(63×) ≈ 1; Fig. 4B), suggesting that the characteristic diffusion time (τD) must be at least an order of magnitude slower than τ for exchange (τex), resulting in a negligible contribution of the lateral diffusion to the fluorescence recovery. Thus, in the presence of Rac2(G12V), τD of PLCβ2-GFP is at least 2 s, 10-fold slower than the measured τ(63×) (0.2 s, reflecting exchange), providing an upper limit of D = 0.09 μm2/s. This D value is much lower than D of lipid probes in the plasma membrane, indicating that the enhanced association of PLCβ2 with the plasma membrane following activation by Rac2(G12V) is due to interactions with membrane proteins and/or localized protein/lipid clusters. In addition, because the dissociation rate governs fluorescence recovery due to exchange (57, 58), τ measured in the FRAP experiment under conditions where the recovery is due to exchange (τex) reflects the time constant for dissociation from the plasma membrane. This allows the calculation of the distance that a given fraction of the membrane-associated protein will diffuse laterally prior to dissociation into the cytoplasm (travel range); for a fraction comprising 63% of the protein population, this range (r) is given by r = (2 × D × τ)½ (59). For Rac2(G12V)-activated PLCβ2-GFP, this calculation yields r = (2 × 0.09 × 0.2)½ = 0.19 μm, suggesting that recruitment of PLCβ2 to the membrane by Rac2(G12V) targets the activated PLCβ2 mainly to laterally restricted small ranges, where it acts until it dissociates back to the cytoplasm.
To examine whether PtdInsP2 hydrolysis following PLCβ2 activation is involved in altering the membrane interaction dynamics of PLCβ2-GFP upon activation, we co-transfected COS-7 cells with PLCβ2-GFP (150 ng of DNA) together with a 7-fold excess of a plasmid encoding SigD, a bacterial inositol phosphatase shown to hydrolyze PtdInsP2 and reduce its cellular level (60, 61). FRAP studies conducted 24 h post-transfection showed no change in PLCβ2-GFP FRAP parameters, suggesting that PtdInsP2 hydrolysis per se does not significantly affect the dynamics of PLCβ2 membrane interactions. This notion is further supported by the finding that each PLCβ2 activator has a distinct effect on the FRAP kinetics of the stimulated PLCβ2 (Figs. 4 and 5; see below), although PtdInsP2 hydrolysis by PLCβ2 is elicited in all cases (Fig. 1).
Unlike the robust effect of Rac2(G12V) on the FRAP dynamics of PLCβ2-GFP, co-expression with αq (wt or constitutively active) induced modest, albeit significant, effects on the FRAP parameters of the enzyme. This correlated with the biochemical fractionation experiments (Fig. 2A), where Rac2(G12V) induced a higher increase in the percentage of PLCβ2-GFP associated with the membrane fraction. Accordingly, αq(R183C) and αq(wt) modulated the FRAP parameters of PLCβ2 toward the same direction as Rac2(G12V), but to a lower extent (Fig. 4). They mildly increased the τ values of PLCβ2-GFP and shifted its τ(40×)/τ(63×) ratio to lower values, albeit still higher than 1. Note that the sensitivity of the FRAP beam-size analysis demonstrates that αq(R183C) has a stronger effect than αq(wt) on the τ ratio (Fig. 4B), a difference that was too mild to detect by the fractionation studies. Because the exchange rates of PLCβ2-GFP stimulated by αq (wt or mutant) were distinctively faster than after stimulation by active Rac2, the increase in the τ values was very mild. This is especially valid for τ(40×), which contains a higher contribution of exchange (due to the larger beam size) and was only weakly affected by αq(wt) and even less by αq(R183C). Therefore, the τ ratio can detect the stronger effect of αq(R183C) with higher sensitivity. The results depicted in Fig. 4 suggest that, although stimulation by αq(R183C) or αq(wt) enhances the mobility-retarding interactions of PLCβ2-GFP with the plasma membrane, the interactions are weaker than those induced by Rac2(G12V) (7; see Fig. 4), and may involve association with different targets in the membrane. Estimation of D for PLCβ2-GFP co-expressed with αq(R183C) or αq(wt) from the τ(63×) values (which are very similar for the two αq proteins) yields D = (2.1 ± 0.2) μm2/s, somewhat higher than lipid probe diffusion, most likely due to the residual contribution of exchange. Because the τ(40×)/τ(63×) ratio of PLCβ2-GFP upon co-expression with an αq protein is intermediate between the values expected for recovery by diffusion and exchange, the characteristic exchange time τex should be in the same range as τD and can be estimated from τ(40×) (τ measured with the larger beam size, where the relative contribution of exchange is higher). The τ(40×) values for αq(R183C) and αq(wt) are 0.12 and 0.15 s, respectively, suggesting that the travel range of PLCβ2 following activation by αq is roughly r = (2 × D × τex)½ = (2 × 2.1 × 0.135)½ = 0.75 μm, 4-fold larger than after stimulation with Rac2(G12V). Thus, activation by αq leads to a less confined recruitment of PLCβ2 to the plasma membrane, because the active enzyme can diffuse laterally a longer distance prior to dissociation to the cytoplasm.
Unexpectedly, the effects of β1γ2 on the FRAP parameters of PLCβ2-GFP were highly different from those of Rac2(G12V) and αq. Unlike the marked increase in τ of PLCβ2-GFP induced by the latter two stimulators, activation by β1γ2 had only very subtle effects on the τ values (Fig. 4A). Concomitantly, β1γ2 shifted the τ(40×)/τ(63×) ratio of PLCβ2 in a direction opposite to that mediated by Rac2(G12V) and αq, resulting in a τ ratio indistinguishable from lateral diffusion (Fig. 4B). This suggests that, following stimulation by β1γ2, the exchange rate of PLCβ2-GFP between the plasma membrane and the cytoplasm becomes much slower than its lateral diffusion rate, indicating enhanced membrane interactions. Yet, these interactions are highly transient. This notion is supported by the biochemical fractionation studies, which showed a markedly weaker recruitment of PLCβ2 to the membrane fraction by β1γ2 relative to Rac2(G12V) or αq (Fig. 2A). The D value of PLCβ2-GFP co-expressed with β1γ2 can be accurately calculated from the FRAP experiments, because in the presence of β1γ2 the τ ratio between the two beam sizes is as expected for lateral diffusion. Interestingly, the D value thus obtained, (3.6 ± 0.2) μm2/s, is markedly higher than D of lipid probes such as DiIC16 (1 μm2/s) or PtdInsP2 (0.5–1 μm2/s) (7, 32, 34). It is much higher than the lateral diffusion of β1γ2 at the plasma membrane of COS-7 cells, which we measured by FRAP on cells co-transfected with 850 ng DNA (1:1 ratio) of venus 1–155-γ2 and venus 155–239-β1. The two halves of the venus protein are not fluorescent separately and form a stable fluorescent complex due to bifunctional fluorescence complementation upon association (44, 62). These measurements yielded D = (0.21 ± 0.2) μm2/s (n = 31). This value, which is similar to the 0.23 μm2/s value reported for β1γ2 in HEK293 cells (62), was not altered by co-expressing the venus-tagged β1γ2 constructs with PLCβ2 (150 ng of plasmid DNA). This suggests that β1γ2-stimulated PLCβ2 exhibits a surfing-like diffusion along the plasma membrane, spending some of the time transiently bound to membrane lipids and/or fatty acid-anchored proteins, including the β1γ2 complex itself. The latter notion is supported by the failure of β1γ2(C68S), where γ2 cannot undergo isoprenylation, to modulate the FRAP parameters of PLCβ2 (Fig. 4). These findings have important implications for the travel range of PLCβ2 following stimulation by β1γ2. A lower limit for this travel range can be derived based on the assumption that the exchange time (τex) of PLCβ2-GFP co-expressed with β1γ2 is at least 10-fold slower than the diffusion time τD, which is essentially equal to the measured τ value (e.g. τ(63×) = τD(63×) = 0.045 s, and thus τex is at least 0.45 s). Therefore, the lower limit of the travel range is r = (2 × D × τex)½ = (2 × 3.6 × 0.45)½ = 1.8 μm, ∼10-fold larger than following stimulation with Rac2(G12V). We conclude that activation of PLCβ2 by βγ involves a mechanism that recruits the enzyme to the plasma membrane by inducing interactions that enable it to roam relatively large membrane regions prior to detachment to the cytoplasm, as required for hydrolysis of dispersed PtdInsP2 populations.
To validate the specificity of the effects of the various stimulators on the FRAP parameters of PLCβ2, we conducted analogous FRAP studies to measure their effects on PLCβ2Δ-GFP (Fig. 5), which does not respond to αq due to the F819-E1166 C-terminal deletion. In line with the loss of the response of this mutant to αq as measured by both activation and recruitment to the membrane (Figs. 1 and 2), neither αq(R183C) nor αq(wt) modulated the τ values or the τ(40×)/τ(63×) ratios of PLCβ2Δ-GFP (Fig. 5). On the other hand, the effects of β1γ2 on the FRAP dynamics of PLCβ2Δ closely resembled their effects on PLCβ2, inducing only a very minor effect on the τ values, while increasing the τ(40×)/τ(63×) ratio very close to the ratio expected for FRAP by pure lateral diffusion. These findings support the notion that β1γ2 expression induces highly transient interactions of PLCβ2 and PLCβ2Δ with the plasma membrane, in line with its inability to measurably enhance the fraction of PLCβ2Δ-GFP in the membrane pellet (Fig. 2B). Accordingly, the very fast lateral diffusion of β1γ2-stimulated PLCβ2Δ (D = (5.2 ± 0.3) μm2/s, calculated from the τ(63×) in Fig. 5A)) suggests surfing-like diffusion (even faster than that of β1γ2-stimulated PLCβ2), resulting in a high travel range prior to dissociation to the cytoplasm; assuming that τex for PLCβ2Δ-GFP is at least 10-fold slower than τ(63×), the lower limit of β1γ2-stimulated PLCβ2Δ travel range is r = (2 × D × τex)½ = (2 × 5.2 × 0.33)½ = 1.9 μm, similar to β1γ2-stimulated PLCβ2. Finally, activation of PLCβ2Δ-GFP by Rac2(G12V) markedly increased the τ values (Fig. 5A), as in the case of PLCβ2-GFP, supporting the notion that activation by Rac2 enhances the membrane association of PLCβ2Δ, in accord with the fractionation studies (Fig. 2). However, the effect of Rac2(G12V) on the τ(40×)/τ(63×) ratio differed markedly between PLCβ2 and PLCβ2Δ (cf. Figs. 5B and 4B), shifting the τ ratio of PLCβ2Δ toward recovery by pure lateral diffusion. As shown and discussed by us earlier (37), when recovery occurs by lateral diffusion and exchange, their contribution to the measured FRAP is determined by the relative rates of the two processes, with the faster process prevailing. Thus, the shift of Rac2-stimulated PLCβ2Δ to FRAP by lateral diffusion directly demonstrates that its exchange rate is at least 10-fold slower than its lateral diffusion rate. This situation differs from that observed for Rac2-stimulated PLCβ2, where exchange becomes the dominant mechanism, reflecting a much slower lateral diffusion rate for the full-length, Rac2-stimulated PLCβ2 (compare Figs. 5B and 4B). This difference, discussed by us extensively earlier (7), is in line with the suggestion (7) that the C-terminal region missing in PLCβ2Δ has a role in the membrane interactions of full-length PLCβ2, mainly with membrane proteins that diffuse slower than lipid probes. In its absence (PLCβ2Δ), the interactions with slow diffusing membrane proteins become weaker, resulting in a loss of the diffusion-restricting interactions with the above proteins and in faster, lipid-like diffusion of Rac2-stimulated PLCβ2Δ (D = (1.3 ± 0.2) μm2/s, calculated from τ(63×) of Rac2(G12V)-stimulated PLCβ2Δ in Fig. 5A). Under these conditions, the diffusion of PLCβ2Δ becomes fast relative to its exchange rate, as indicated by the diffusion-dominated FRAP mechanism (Fig. 5B). Thus, τex of Rac2-stimulated PLCβ2Δ should be higher than the measured τ(63×) by at least 10-fold (i.e. τex ≥ 1.4 s). This would increase the travel range of Rac2-stimulated PLCβ2Δ, with a lower limit estimate of r = (2 × D × τex)½ = (2 × 1.3 × 1.4)½ = 1.9 μm. This in turn indicates that, unlike the limited travel range of Rac2(G12V)-activated PLCβ2, the travel range on the plasma membrane of the Rac2-activated PLCβ2Δ mutant is 10-fold higher, strongly compromising the localized nature of the membrane recruitment.
DISCUSSION
The mechanisms regulating the membrane recruitment and activation of PLCβ isozymes by their activators are not fully understood. Here, we investigated these issues in live cells for PLCβ2 activated by several stimulators (Rac2, αq, and β1γ2). Our findings demonstrate that each activator causes a distinct mode of PLCβ2 membrane association, ranging between recruitment to confined regions (activation by Rac2, and to a lesser degree by αq) and fast, surfing-like diffusion of the enzyme along the cytoplasmic leaflet of the plasma membrane (activation by β1γ2). The diversity of these mechanisms has important implications for the PtdInsP2 populations targeted by PLCβ2, because the first mechanism targets the activated enzyme to act on discrete PtdInsP2 populations localized at or near the recruitment sites, while the second directs the enzyme to act on dispersed PtdInsP2 populations.
In the current study, we combined FRAP beam-size analysis with biochemical and signaling assays to investigate the mechanisms by which Rac2, αq, and β1γ2 mediate membrane recruitment and activation of PLCβ2. Because the FRAP studies and some of the biochemical studies employed GFP-tagged PLCβ2 or PLCβ2Δ, we first validated that the GFP-tagged enzymes are as responsive as their untagged counterparts to the various PLCβ2 activators (Fig. 1). Moreover, the specificity of the activation was kept in the GFP-tagged constructs, as shown by the loss of their response to β1γ2 upon mutational removal of the γ2 membrane anchor site (C68S) and the loss of αq activation in the PLCβ2Δ mutant (Fig. 1).
The fractionation studies demonstrate that PLCβ2-GFP and PLCβ2Δ-GFP are mainly cytosolic prior to activation (Fig. 2). However, transient association can be overlooked in such studies, which require relatively stable association with the membrane. Indeed, the FRAP beam-size analysis shows that unstimulated PLCβ2 and PLCβ2Δ do interact transiently with the plasma membrane, as indicated by the mixed contribution of lateral diffusion and exchange to their FRAP kinetics, which are much slower than that of free cytoplasmic GFP (Figs. 3–5; see also Ref. 7). Thus, although mainly cytoplasmic, unstimulated PLCβ2 (and PLCβ2Δ) experiences some mobility-retarding interactions with plasma membrane constituents, which are insufficient for stable association. The D values estimated from τ(63×) for PLCβ2 and PLCβ2Δ prior to activation (3.2 and 4.8 μm2/s, respectively) are ∼4-fold higher than those of lipids and of PtdInsP2 (7, 32, 34), most likely due to contributions of exchange and possibly of surfing-like diffusion along the cytoplasmic face of the membrane, a diffusion mode discussed later in the context of β1γ2-activated PLCβ2. It should be noted that the lack of significant enzymatic activity in the unstimulated enzymes (Fig. 1), despite their transient interactions with the plasma membrane, suggests that such interactions per se are not sufficient to activate PLCβ2, and that transient recruitment to the membrane (as observed following β1γ2 stimulation) should be accompanied by an additional event (e.g. a conformational change) to induce activation.
A striking finding of the current studies is that, although all the stimulators enhance the membrane interactions of PLCβ2, they induce these effects by different mechanisms. Expression of constitutively active Rac2(G12V) induced a robust recruitment of PLCβ2-GFP to the membrane fraction (Fig. 2A), accompanied by a major modulation of its membrane interaction dynamics (significantly longer τ values and a drastic shift of the τ(40×)/τ(63×) ratio to ∼1, indicative of exchange-dominated FRAP (Fig. 4)). The significantly slower τ values at both laser beam sizes indicate that both the lateral diffusion and exchange of PLCβ2-GFP are retarded following activation by Rac2(G12V), while the simultaneous shift to recovery dominated by exchange suggests that the lateral diffusion of PLCβ2 is inhibited at least 10-fold more than its exchange, resulting in a negligible contribution of lateral diffusion to the FRAP. Such slow diffusion (the upper limit estimate of D is 0.09 μm2/s; see “Results”) is well below the typical D values of lipid probes, which are in the 1 μm2/s range (7, 32, 34), indicating that Rac2(G12V) recruits PLCβ2 to the plasma membrane by enhancing its interactions with slow diffusing entities such as transmembrane proteins or protein-lipid clusters. This has important implications for the PtdInsP2 populations targeted by Rac2-stimulated PLCβ2; calculation of the resulting travel range prior to dissociation of the majority (63%) of the Rac-stimulated PLCβ2 molecules from the membrane (59) yields 0.19 μm (see “Results”), indicating that the enzyme is recruited to act on substrate populations localized in distinct limited regions or clusters. Interestingly, the localized nature of PLCβ2 recruitment and activation by Rac2(G12V) is disrupted in the PLCβ2Δ mutant, which is effectively recruited by activated Rac2 to the membrane fraction (Fig. 2B), but shifts to FRAP by lateral diffusion (τ ratio very close to the ratio expected for pure lateral diffusion (Fig. 5)). The D value obtained for Rac2-stimulated PLCβ2Δ is 1.3 μm2/s, similar to the typical values for lipid probe diffusion. These results indicate that the modulation of PLCβ2 membrane interactions by activated Rac2 involve not only the enzyme's PH domain, which was shown to interact with activated Rho GTPases (6, 17, 18), but also the C-terminal region, a major portion of which is missing in PLCβ2Δ. The C-terminal region appears to contribute to the interactions of full-length PLCβ2 with the slow diffusing membrane constituents, and its deletion in PLCβ2Δ interferes with these interactions, leading to faster lateral diffusion relative to exchange. Due to the higher D (1.3 μm2/s) and slower exchange time (τex ≥ 1.4 s), the travel range of Rac2-stimulated PLCβ2Δ increases by at least an order of magnitude (lower estimate, 1.9 μm).
The C-terminal region of PLCβ2 is essential for activation by αq (7, 13–15), in line with the failure of αq or αq(R183C) to activate PLCβ2Δ and to modulate its membrane interactions (Figs. 1, 2B, and 5). This contrasts with the ability of activated Rac2 and β1γ2 to activate PLCβ2Δ, suggesting that the recruitment and activation mechanisms of PLCβ2 by αq may be different. Indeed, although αq(R183C) and αq enhanced the recruitment of PLCβ2-GFP to the membrane fraction (Fig. 2A), this effect was weaker than that mediated by activated Rac2, and the ability of the αq proteins to modulate the membrane interaction dynamics of PLCβ2 was much milder (Fig. 4). Thus, the effect of αq on prolonging the τ values was evident but much weaker, and the shift toward a τ ratio of 1 (recovery by exchange) was partial, indicating that, although stimulation by αq increases the contribution of exchange relative to diffusion, the latter still has a contribution to the fluorescence recovery. This suggests that the retardation of the lateral diffusion of PLCβ2 following stimulation by αq is less than that induced by Rac2(G12V); indeed, the D value estimated for αq-stimulated PLCβ2 (which may contain some contribution of exchange) is 2.1 μm2/s, close to but somewhat higher than D of lipid probes. This indicates that αq-stimulated PLCβ2 molecules interact with membrane constituents different from those targeted by Rac2 activation, possibly including lipids (e.g. via the PH and/or C2 domains) and/or lipid-anchored proteins. Direct association with αq, which can interact with the membrane via its single fatty-acyl residue, may also contribute to these interactions. Nevertheless, the association of αq-stimulated PLCβ2 with membrane constituents (including αq itself) must be dynamic, as indicated by the contribution of exchange to the FRAP kinetics (Fig. 4) and by the lower D value (0.47 μm2/s) measured for a Gα subunit (αoA) (63). Based on the D and τex values, the travel range of αq-stimulated PLCβ2 is estimated to be 0.75 μm, suggesting that PLCβ2 recruited by αq stimulation is less constrained than the Rac2-stimulated enzyme, roaming larger (albeit still limited) membrane regions.
β1γ2 dimers can interact with the PH domain of PLCβ2 (16) but also appear to interact with other portions of the enzyme (6). In line with the latter, distinct interactions, the modulation of the membrane interactions of PLCβ2 by β1γ2 are very different from those mediated by Rac2 or αq. Unlike activation by the latter two, activation by β1γ2 induced highly transient association with the membrane fraction, as evidenced by the fractionation experiments (Fig. 2). Yet, despite this highly transient nature, the FRAP of β1γ2-activated PLCβ2 (or PLCβ2Δ) is strongly dominated by fast lateral diffusion (Figs. 4 and 5), characterized by D values 3- to 5-fold faster than lipids or lipid-anchored proteins such as G protein α or βγ subunits (7, 32, 34, 62, 63). Combining the highly transient interactions and the very fast diffusion along the plasma membrane, we propose that stimulation by β1γ2 induces surfing-like diffusion of PLCβ2 enzymes along the cytoplasmic face of the plasma membrane. Thus, when a β1γ2-stimulated PLCβ2 molecule dissociates from the membrane, it diffuses a short distance and quickly re-associates with another membrane component, remaining in the juxtamembrane vicinity and diffusing along the membrane. Juxtamembrane diffusion was recently found for paxillin and vinculin above focal adhesions (58). This unique mechanism enables diffusion of β1γ2-stimulated PLCβ2 along the membrane at rates much faster than those of lipids or lipidated proteins, including the β1γ2 dimers, which diffuse with D = 0.2 μm2/s (62). The diffusion of β1γ2-stimulated PLCβ2 (or PLCβ2Δ) is also much faster than that of PtdInsP2, which has D values of 0.5–1 μm2/s (32, 34), enabling very fast spatial dispersal of the activated enzyme, not limited by the lateral diffusion of either lipidated protein targets or the substrate. This mechanism is likely relevant under physiological conditions, because PLCβs are typically much less abundant (at least 100-fold) in cells than βγ dimers (64, 65), enabling sequential interaction of a single PLCβ molecule with multiple βγ dimers by dissociation and fast re-association. An important consequence of this fast diffusion is a dramatic increase in the travel range of β1γ2-activated PLCβ2 (or PLCβ2Δ) to at least 1.9 μm. This implies that stimulation by β1γ2 recruits PLCβ2 to act on dispersed PtdInsP2 populations.
The divergent mechanisms described above may have evolved for stimulation of PLCβ2 (and possibly other PLCβ isozymes) to accomplish different tasks of cellular regulation. PtdInsP2 has long been known to occur in cells not only in dispersed populations, but also to form gradients and locally enriched regions (27–33). We propose that activation by Rac/Cdc42 recruits PLCβ2 to hydrolyze PtdInsP2 in discrete, spatially restricted zones. On the other hand, activation by β1γ2 dimers results in signals that rapidly propagate along the plasma membrane, because the activated PLCβ2 can roam large areas along the membrane, hydrolyzing PtdInsP2 with low spatial resolution. Activation by αq yields an intermediate situation, which may fit conditions where relatively shallow gradients of PtdInsP2 hydrolysis are beneficial.
Acknowledgements
We are grateful to Norbert Zanker and Susanne Gierschik for excellent technical assistance. We thank Drs. Bruce R. Conklin, Henry R. Bourne, Nevin A. Lambert, and B. Brett Finlay for their generous gifts of expression plasmids.
This work was supported in part by Grant I-730-46.13/2002 from the German-Israeli Foundation for Scientific Research and Development (to Y. I. H. and P. G.) and by the Deutsche Forschungsgemeinschaft (Grant SFB 497, TP C10, to P. G.).
- PLCβ
- phospholipase C-β
- D
- lateral diffusion coefficient
- GFP
- green fluorescent protein
- PtdInsP2
- phosphatidylinositol 4,5-bisphosphate
- r
- travel range
- Rf
- mobile fraction
- τ
- apparent characteristic fluorescence recovery time
- wt
- wild type
- FRAP
- fluorescence recovery after photobleaching
- PH
- pleckstrin homology.
REFERENCES
- 1.Singer W. D., Brown H. A., Sternweis P. C. (1997) Annu. Rev. Biochem. 66, 475–509 [DOI] [PubMed] [Google Scholar]
- 2.Rhee S. G. (2001) Annu. Rev. Biochem. 70, 281–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suh P. G., Park J. I., Manzoli L., Cocco L., Peak J. C., Katan M., Fukami K., Kataoka T., Yun S., Ryu S. H. (2008) BMB Rep. 41, 415–434 [DOI] [PubMed] [Google Scholar]
- 4.Illenberger D., Schwald F., Pimmer D., Binder W., Maier G., Dietrich A., Gierschik P. (1998) EMBO J. 17, 6241–6249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Illenberger D., Stephan I., Gierschik P., Schwald F. (2000) Methods Enzymol. 325, 167–177 [DOI] [PubMed] [Google Scholar]
- 6.Illenberger D., Walliser C., Nurnberg B., Diaz Lorente M., Gierschik P. (2003) J. Biol. Chem. 278, 3006–3014 [DOI] [PubMed] [Google Scholar]
- 7.Illenberger D., Walliser C., Strobel J., Gutman O., Niv H., Gaidzik V., Kloog Y., Gierschik P., Henis Y. I. (2003) J. Biol. Chem. 278, 8645–8652 [DOI] [PubMed] [Google Scholar]
- 8.Piechulek T., Rehlen T., Walliser C., Vatter P., Moepps B., Gierschik P. (2005) J. Biol. Chem. 280, 38923–38931 [DOI] [PubMed] [Google Scholar]
- 9.Coburn R. F., Labelle E. F., Griffiths T., 2nd, Baron C. B. (1997) J. Cell Physiol. 171, 271–283 [DOI] [PubMed] [Google Scholar]
- 10.López de Jesús M., Zalduegui A., Ruiz de Azúa I., Callado L. F., Meana J. J., Sallés J. (2006) Neurochem. Int. 49, 72–79 [DOI] [PubMed] [Google Scholar]
- 11.Ruiz de Azúa I., del Olmo E., Pazos A., Sallés J. (2006) J. Neurosci. Res. 84, 13–26 [DOI] [PubMed] [Google Scholar]
- 12.Chandrashekar J., Hoon M. A., Ryba N. J., Zuker C. S. (2006) Nature 444, 288–294 [DOI] [PubMed] [Google Scholar]
- 13.Wu D., Katz A., Simon M. I. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 5297–5301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S. B., Shin S. H., Hepler J. R., Gilman A. G., Rhee S. G. (1993) J. Biol. Chem. 268, 25952–25957 [PubMed] [Google Scholar]
- 15.Illenberger D., Schwald F., Gierschik P. (1997) Eur. J. Biochem. 246, 71–77 [DOI] [PubMed] [Google Scholar]
- 16.Drin G., Scarlata S. (2007) Cell. Signal. 19, 1383–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder J. T., Singer A. U., Wing M. R., Harden T. K., Sondek J. (2003) J. Biol. Chem. 278, 21099–21104 [DOI] [PubMed] [Google Scholar]
- 18.Jezyk M. R., Snyder J. T., Gershberg S., Worthylake D. K., Harden T. K., Sondek J. (2006) Nat. Struct. Mol. Biol. 13, 1135–1140 [DOI] [PubMed] [Google Scholar]
- 19.Akasaki T., Koga H., Sumimoto H. (1999) J. Biol. Chem. 274, 18055–18059 [DOI] [PubMed] [Google Scholar]
- 20.Benard V., Bohl B. P., Bokoch G. M. (1999) J. Biol. Chem. 274, 13198–13204 [DOI] [PubMed] [Google Scholar]
- 21.Li S., Yamauchi A., Marchal C. C., Molitoris J. K., Quilliam L. A., Dinauer M. C. (2002) J. Immunol. 169, 5043–5051 [DOI] [PubMed] [Google Scholar]
- 22.Rabiet M. J., Tardif M., Braun L., Boulay F. (2002) Blood 100, 1835–1844 [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin S., Wang J., Gambhir A., Murray D. (2002) Annu. Rev. Biophys. Biomol. Struct. 31, 151–175 [DOI] [PubMed] [Google Scholar]
- 24.Di Paolo G., De Camilli P. (2006) Nature 443, 651–657 [DOI] [PubMed] [Google Scholar]
- 25.Wedegaertner P. B., Chu D. H., Wilson P. T., Levis M. J., Bourne H. R. (1993) J. Biol. Chem. 268, 25001–25008 [PubMed] [Google Scholar]
- 26.Katz A., Wu D., Simon M. I. (1992) Nature 360, 686–689 [DOI] [PubMed] [Google Scholar]
- 27.Haugh J. M., Codazzi F., Teruel M., Meyer T. (2000) J. Cell Biol. 151, 1269–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang S., Lifshitz L., Patki-Kamath V., Tuft R., Fogarty K., Czech M. P. (2004) Mol. Cell Biol. 24, 9102–9123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roth M. G. (2004) Physiol. Rev. 84, 699–730 [DOI] [PubMed] [Google Scholar]
- 30.Emoto K., Inadome H., Kanaho Y., Narumiya S., Umeda M. (2005) J. Biol. Chem. 280, 37901–37907 [DOI] [PubMed] [Google Scholar]
- 31.Field S. J., Madson N., Kerr M. L., Galbraith K. A., Kennedy C. E., Tahiliani M., Wilkins A., Cantley L. C. (2005) Curr. Biol. 15, 1407–1412 [DOI] [PubMed] [Google Scholar]
- 32.Golebiewska U., Nyako M., Woturski W., Zaitseva I., McLaughlin S. (2008) Mol. Biol. Cell 19, 1663–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hilgemann D. W. (2007) Pflugers Arch. 455, 55–67 [DOI] [PubMed] [Google Scholar]
- 34.Yaradanakul A., Hilgemann D. W. (2007) J. Membr. Biol. 220, 53–67 [DOI] [PubMed] [Google Scholar]
- 35.Matsuoka S., Iijima M., Watanabe T. M., Kuwayama H., Yanagida T., Devreotes P. N., Ueda M. (2006) J. Cell Sci. 119, 1071–1079 [DOI] [PubMed] [Google Scholar]
- 36.Hammond G. R., Sim Y., Lagnado L., Irvine R. F. (2009) J. Cell Biol. 184, 297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henis Y. I., Rotblat B., Kloog Y. (2006) Methods 40, 183–190 [DOI] [PubMed] [Google Scholar]
- 38.Arkinstall S., Chabert C., Maundrell K., Peitsch M. (1995) FEBS Lett. 364, 45–50 [DOI] [PubMed] [Google Scholar]
- 39.Johnston C. A., Siderovski D. P. (2007) Mol. Pharmacol. 72, 219–230 [DOI] [PubMed] [Google Scholar]
- 40.Cole C., Barber J. D., Barton G. J. (2008) Nucleic Acids Res. 36, W197–W201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conklin B. R., Farfel Z., Lustig K. D., Julius D., Bourne H. R. (1993) Nature 363, 274–276 [DOI] [PubMed] [Google Scholar]
- 42.Tesmer V. M., Kawano T., Shankaranarayanan A., Kozasa T., Tesmer J. J. (2005) Science 310, 1686–1690 [DOI] [PubMed] [Google Scholar]
- 43.Lutz S., Shankaranarayanan A., Coco C., Ridilla M., Nance M. R., Vettel C., Baltus D., Evelyn C. R., Neubig R. R., Wieland T., Tesmer J. J. (2007) Science 318, 1923–1927 [DOI] [PubMed] [Google Scholar]
- 44.Hollins B., Kuravi S., Digby G. J., Lambert N. A. (2009) Cell. Signal. 21, 1015–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marcus S. L., Knodler L. A., Finlay B. B. (2002) Cell. Microbiol. 4, 435–446 [DOI] [PubMed] [Google Scholar]
- 46.Seed B., Aruffo A. (1987) Proc. Natl. Acad. Sci. U.S.A. 84, 3365–3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Axelrod D., Koppel D. E., Schlessinger J., Elson E. L., Webb W. W. (1976) Biophys. J. 16, 1055–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koppel D. E., Axelrod D., Schlessinger J., Elson E. L., Webb W. W. (1976) Biophys. J. 16, 1315–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petersen N. O., Felder S., Elson E. L. (1986) in Handbook of Experimental Immunology ( Weir D. M., Herzenberg L. A., Blackwell C. C., Herzenberg L. A. eds) pp. 24.21–24.23, Blackwell Scientific Publications, Edinburgh [Google Scholar]
- 50.Efron B., Tibshirani R. (1993) in An Introduction to Bootstrap ( Cox D. R., Hinkley D. V., Reid N., Rubin D. B., Silverman B. W. eds) pp. 124–130, Chapman & Hall, London [Google Scholar]
- 51.Offermanns S., Simon M. I. (1995) J. Biol. Chem. 270, 15175–15180 [DOI] [PubMed] [Google Scholar]
- 52.Camps M., Hou C. F., Jakobs K. H., Gierschik P. (1990) Biochem. J. 271, 743–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bradford M. M. (1976) Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 54.Hepler J. R., Kozasa T., Smrcka A. V., Simon M. I., Rhee S. G., Sternweis P. C., Gilman A. G. (1993) J. Biol. Chem. 268, 14367–14375 [PubMed] [Google Scholar]
- 55.Jiang H., Kuang Y., Wu Y., Smrcka A., Simon M. I., Wu D. (1996) J. Biol. Chem. 271, 13430–13434 [DOI] [PubMed] [Google Scholar]
- 56.Shvartsman D. E., Donaldson J. C., Diaz B., Gutman O., Martin G. S., Henis Y. I. (2007) J. Cell Biol. 178, 675–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bulinski J. C., Odde D. J., Howell B. J., Salmon T. D., Waterman-Storer C. M. (2001) J. Cell Sci. 114, 3885–3897 [DOI] [PubMed] [Google Scholar]
- 58.Wolfenson H., Lubelski A., Regev T., Klafter J., Henis Y. I., Geiger B. (2009) PLoS ONE 4, e4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teruel M. N., Meyer T. (2000) Cell 103, 181–184 [DOI] [PubMed] [Google Scholar]
- 60.Terebiznik M. R., Vieira O. V., Marcus S. L., Slade A., Yip C. M., Trimble W. S., Meyer T., Finlay B. B., Grinstein S. (2002) Nat. Cell Biol. 4, 766–773 [DOI] [PubMed] [Google Scholar]
- 61.Mason D., Mallo G. V., Terebiznik M. R., Payrastre B., Finlay B. B., Brumell J. H., Rameh L., Grinstein S. (2007) J. Gen. Physiol. 129, 267–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Digby G. J., Lober R. M., Sethi P. R., Lambert N. A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 17789–17794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lober R. M., Pereira M. A., Lambert N. A. (2006) J. Neurosci. 26, 12602–12608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Camps M., Hou C., Sidiropoulos D., Stock J. B., Jakobs K. H., Gierschik P. (1992) Eur. J. Biochem. 206, 821–831 [DOI] [PubMed] [Google Scholar]
- 65.Paterson A., Boyer J. L., Watts V. J., Morris A. J., Price E. M., Harden T. K. (1995) Cell. Signal. 7, 709–720 [DOI] [PubMed] [Google Scholar]