Abstract
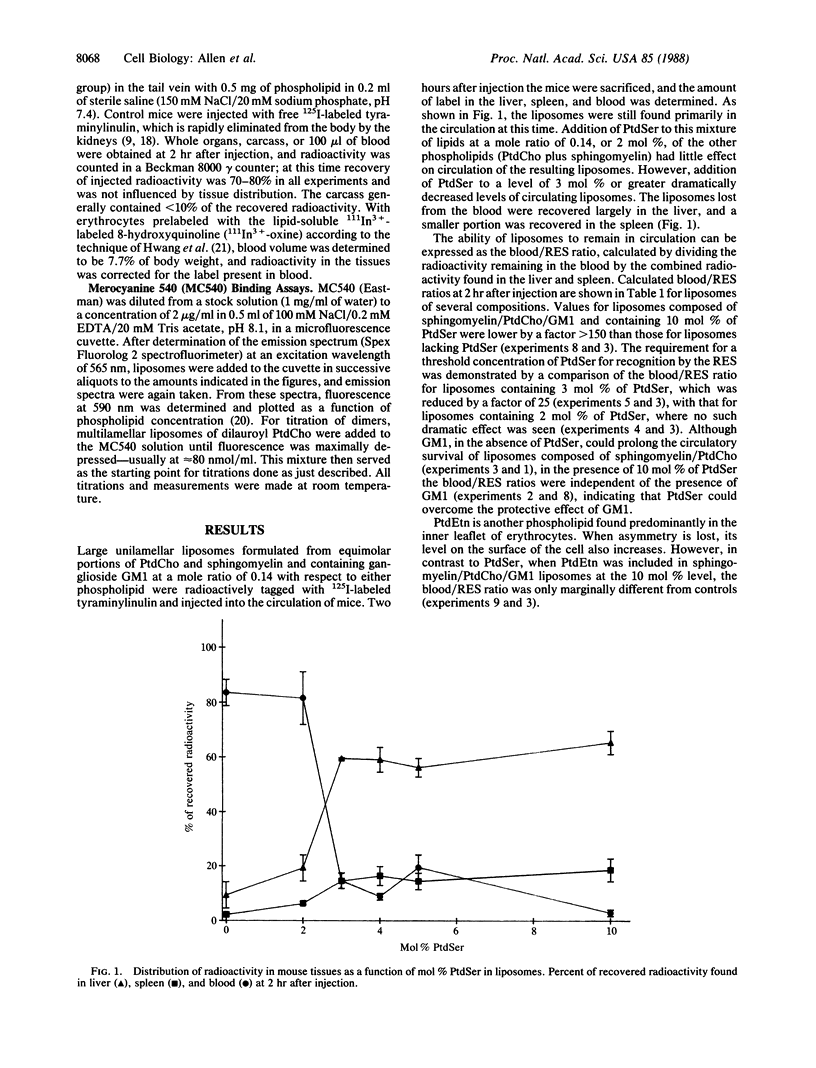
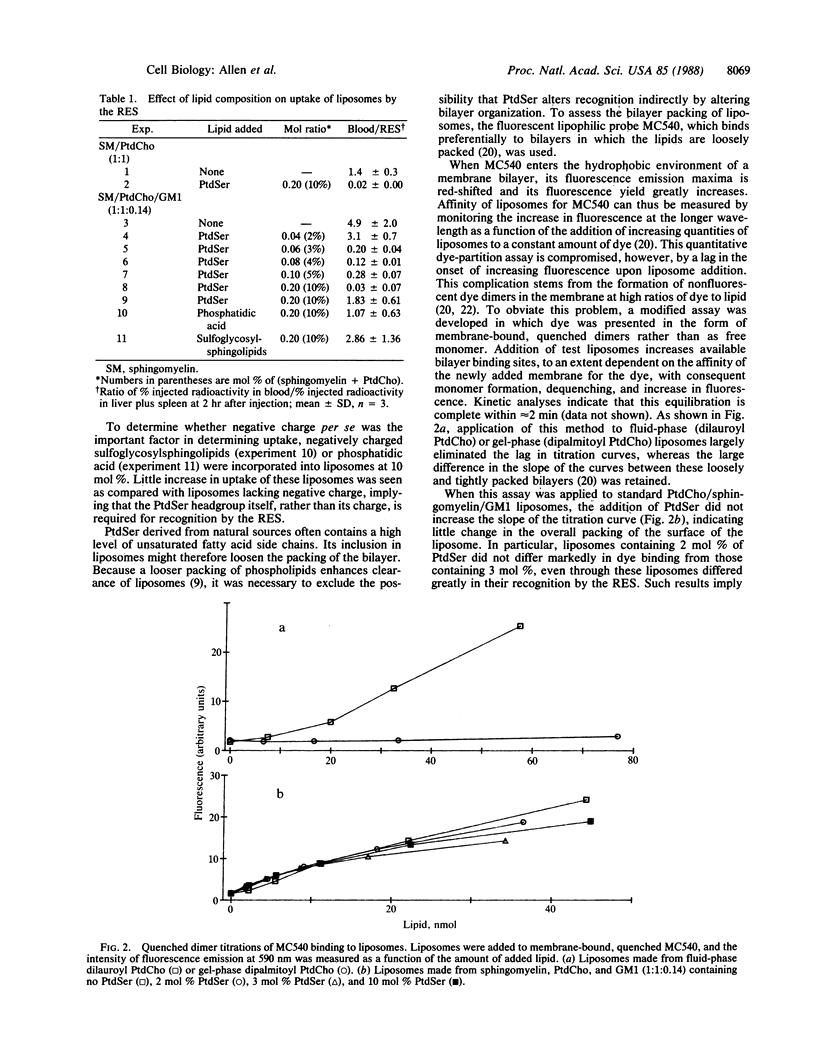
Liposomes formulated to resemble the outer leaflet of the erythrocyte membrane were found to substantially avoid recognition and clearance by the reticuloendothelial system. When these models of the erythrocyte surface were modified by the incorporation of greater than 2 mol % of phosphatidylserine (PtdSer), their ability to remain in the circulation of mice was greatly reduced. To examine whether this altered behavior was the consequence of an alteration in bilayer organization induced by PtdSer, a method utilizing the fluorescent dye merocyanine 540 was used to assess the packing of external phospholipids. No significant difference in overall membrane lipid organization was detected between liposomes containing 2 or 3 mol % of PtdSer, at which dramatic differences in recognition and clearance occurred. These results exclude alterations in phospholipid packing as an indirect cause of increased clearance of PtdSer-containing liposomes and implicate PtdSer directly in recognition by the reticuloendothelial system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. M., Chonn A. Large unilamellar liposomes with low uptake into the reticuloendothelial system. FEBS Lett. 1987 Oct 19;223(1):42–46. doi: 10.1016/0014-5793(87)80506-9. [DOI] [PubMed] [Google Scholar]
- Allen T. M., Everest J. M. Effect of liposome size and drug release properties on pharmacokinetics of encapsulated drug in rats. J Pharmacol Exp Ther. 1983 Aug;226(2):539–544. [PubMed] [Google Scholar]
- Allen T. M., Murray L., MacKeigan S., Shah M. Chronic liposome administration in mice: effects on reticuloendothelial function and tissue distribution. J Pharmacol Exp Ther. 1984 Apr;229(1):267–275. [PubMed] [Google Scholar]
- Durocher J. R., Payne R. C., Conrad M. E. Role of sialic acid in erythrocyte survival. Blood. 1975 Jan;45(1):11–20. [PubMed] [Google Scholar]
- Hwang K. J., Luk K. F., Beaumier P. L. Hepatic uptake and degradation of unilamellar sphingomyelin/cholesterol liposomes: a kinetic study. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4030–4034. doi: 10.1073/pnas.77.7.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy L., Williamson P., Schlegel R. A. Membrane phospholipid asymmetry as a determinant of erythrocyte recognition by macrophages. Proc Natl Acad Sci U S A. 1986 May;83(10):3311–3315. doi: 10.1073/pnas.83.10.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrot G., Cribier S., Devaux P. F., Geldwerth D., Davoust J., Bureau J. F., Fellmann P., Herve P., Frilley B. Asymmetric lateral mobility of phospholipids in the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6863–6867. doi: 10.1073/pnas.83.18.6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op den Kamp J. A. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- Patel H. M., Tuzel N. S., Ryman B. E. Inhibitory effect of cholesterol on the uptake of liposomes by liver and spleen. Biochim Biophys Acta. 1983 Dec 13;761(2):142–151. doi: 10.1016/0304-4165(83)90223-4. [DOI] [PubMed] [Google Scholar]
- Proffitt R. T., Williams L. E., Presant C. A., Tin G. W., Uliana J. A., Gamble R. C., Baldeschwieler J. D. Liposomal blockade of the reticuloendothelial system: improved tumor imaging with small unilamellar vesicles. Science. 1983 Apr 29;220(4596):502–505. doi: 10.1126/science.6836294. [DOI] [PubMed] [Google Scholar]
- Schlegel R. A., Prendergast T. W., Williamson P. Membrane phospholipid asymmetry as a factor in erythrocyte-endothelial cell interactions. J Cell Physiol. 1985 May;123(2):215–218. doi: 10.1002/jcp.1041230210. [DOI] [PubMed] [Google Scholar]
- Schlegel R. A., Williamson P. Membrane phospholipid organization as a determinant of blood cell-reticuloendothelial cell interactions. J Cell Physiol. 1987 Aug;132(2):381–384. doi: 10.1002/jcp.1041320229. [DOI] [PubMed] [Google Scholar]
- Schroit A. J., Madsen J. W., Tanaka Y. In vivo recognition and clearance of red blood cells containing phosphatidylserine in their plasma membranes. J Biol Chem. 1985 Apr 25;260(8):5131–5138. [PubMed] [Google Scholar]
- Schroit A. J., Tanaka Y., Madsen J., Fidler I. J. The recognition of red blood cells by macrophages: role of phosphatidylserine and possible implications of membrane phospholipid asymmetry. Biol Cell. 1984;51(2):227–238. doi: 10.1111/j.1768-322x.1984.tb00303.x. [DOI] [PubMed] [Google Scholar]
- Seigneuret M., Zachowski A., Hermann A., Devaux P. F. Asymmetric lipid fluidity in human erythrocyte membrane: new spin-label evidence. Biochemistry. 1984 Sep 11;23(19):4271–4275. doi: 10.1021/bi00314a002. [DOI] [PubMed] [Google Scholar]
- Sommerman E. F., Pritchard P. H., Cullis P. R. 125I labelled inulin: a convenient marker for deposition of liposomal contents in vivo. Biochem Biophys Res Commun. 1984 Jul 18;122(1):319–324. doi: 10.1016/0006-291x(84)90477-7. [DOI] [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K. I., Ohnishi S. Heterogeneity in the fluidity of intact erythrocyte membrane and its homogenization upon hemolysis. Biochim Biophys Acta. 1976 Mar 5;426(2):218–231. doi: 10.1016/0005-2736(76)90333-3. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Schroit A. J. Insertion of fluorescent phosphatidylserine into the plasma membrane of red blood cells. Recognition by autologous macrophages. J Biol Chem. 1983 Sep 25;258(18):11335–11343. [PubMed] [Google Scholar]
- Williamson P., Bateman J., Kozarsky K., Mattocks K., Hermanowicz N., Choe H. R., Schlegel R. A. Involvement of spectrin in the maintenance of phase-state asymmetry in the erythrocyte membrane. Cell. 1982 Oct;30(3):725–733. doi: 10.1016/0092-8674(82)90277-x. [DOI] [PubMed] [Google Scholar]
- Williamson P., Mattocks K., Schlegel R. A. Merocyanine 540, a fluorescent probe sensitive to lipid packing. Biochim Biophys Acta. 1983 Jul 27;732(2):387–393. doi: 10.1016/0005-2736(83)90055-x. [DOI] [PubMed] [Google Scholar]
- van Oss C. J. Phagocytosis as a surface phenomenon. Annu Rev Microbiol. 1978;32:19–39. doi: 10.1146/annurev.mi.32.100178.000315. [DOI] [PubMed] [Google Scholar]



