Abstract
Type I collagen forms the main constituent of the extracellular matrix in visceral organs. We reported here that cyclophosphamide (CYP)-induced cystitis significantly increased the production of type I collagen in the inflamed bladder leading to increases in the bladder weight and the thickness of the bladder wall. The endogenous nerve growth factor (NGF) in the urinary bladder regulated type I collagen expression because the neutralizing NGF antibody attenuated cystitis-induced type I collagen up-regulation in the inflamed bladder. Neutralizing NGF antibody also subsequently reversed cystitis-induced increases in bladder weight. Further studies on the intermediate signaling pathways mediating NGF-induced type I collagen expression in the inflamed bladder during cystitis revealed that Akt, JNK, and ERK1/2 activities were increased in the inflamed bladder, whereas p38 MAPK remained unchanged. Suppression of endogenous NGF level with neutralizing NGF antibody significantly blocked the increased activity of Akt, JNK, and ERK1/2 in the inflamed bladder during cystitis. These results indicate that endogenous NGF plays an important role in the activation of Akt and MAPK in the urinary bladder and in bladder hypertrophy during cystitis.
Keywords: NGF, Bladder, Collagen, Cystitis, Hypertrophy, Signal Transduction
Introduction
Numerous inflammatory mediators including cytokines, chemokines, and growth factors are identified to play significant roles in mediating the inflammatory process in visceral organs (1–3). Increases in the inflammatory mediators in the inflamed visceral organs may lead to increases in the excitability of the axonal terminals located in the organ resulting in sensory hypersensitivity (1); the increase in the axonal terminal excitability, in turn, promotes neuropeptide expression in and release from primary afferent neurons at the peripheral terminals (4–7) and increases local blood flow exacerbating the inflammatory process, together leading to the petechial hemorrhages and visceral organ hypertrophy. The growth factors that are elevated in the inflamed or hypertrophied urinary bladder include nerve growth factor (NGF),2 brain-derived neurotrophic factor, basic fibroblast growth factor, and epidermal growth factor, etc (8–12). Up-regulation of these factors may facilitate the intracellular signal transduction pathways and lead to changes in gene expression and cellular growth in the urinary bladder (13).
Increases in collagen deposition play a critical role in organ hypertrophy (14–16). Collagen is the main constituent of the extracellular matrix. The major four types of collagen, types I, II, III, and IV, are made up of 90% of the total collagen in the body. The type I collagen is the most abundant collagen in the body responsible for forming mature tissue and is present in skin, tendon, vascular, ligature, organs, and bone (17). Increases in the production of type I collagen are one of the major factors contributing to organ hypertrophy resulted from diseases or injury (14–15, 18). Factors that are involved in the regulation of type I collagen gene expression include cytokines and growth factors such as tumor necrosis factor-α, interferon-γ, transforming growth factor-β, and fibroblast growth factor through the alteration of one or more transcription factors, which have been reviewed by Ghosh et al. (19). Although several of the transcription factors that regulate type I collagen expression such as Smad and activating protein 1 binding element can also be regulated by NGF (20–23), the role of NGF in the regulation of type I collagen in the urinary bladder is unknown and is investigated in the present study.
Cystitis induced by intraperitoneal injection of cyclophosphamide (CYP) results in significant increases in bladder weight and thickness of the bladder wall (muscular layer). Previous studies showed that CYP cystitis increased the expression level of TrkA and p75NTR in the urinary bladder (24, 25). The increases in the expression of NGF receptors would enhance the responsiveness of the cells to NGF and may contribute to the morphological and cellular changes in the inflamed bladder during cystitis. Upon NGF binding to its receptors, several intracellular signaling pathways are activated. Two major pathways that are involved in gene expression and cellular growth are MAPK pathway and phosphoinositide 3-kinases/Akt pathway (26).
MAPKs are a family of serine/threonine kinases including extracellular signal-regulated kinase (ERK), c-Jun NH2 terminal kinase (JNK), and p38 MAPK. Activation of ERK1/2 either led to decreased expression of type I collagen in human skin fibroblasts (27) or mediate transforming growth factor-β1-induced collagen synthesis in NIH 3T3 fibroblast cells (28). In cardiac fibroblasts, activation of ERK1/2 enhanced while activation of p38 MAPK reduced procollagen mRNA expression (29). In human osteosarcoma cells, selective p38 MAPK inhibitors blocked up-regulation of collagen gene transcription (30). These results indicate cell-type specific effects of MAPK pathways in the regulation of collagen gene expression.
The involvement of phosphoinositide 3-kinase/Akt pathway in collagen expression was recently observed in hepatic fibrosis (31). Although Akt is not the only target of phosphoinositide 3-kinase-induced survival activity, Akt serves as a very important convergence point and targets various survival signals such as Bcl proteins, procaspase, and Forkhead (32–35), which may increase cellular growth and survival and contribute to inflammation-induced visceral organ hypertrophy.
In the present study, we investigated the role of NGF in the regulation of type I collagen gene expression in the urinary bladder and its role in cystitis-induced bladder hypertrophy. Previous studies have shown that the level of NGF was increased in the urine and the urinary bladder of patients with cystitis (11–12, 36). Here, we used a chemically induced cystitis rat model treated with an intraperitoneal injection of CYP, an animal model that exhibits similar symptoms observed in patients with cystitis, and characterized the role of endogenous NGF in the activation of intracellular signaling pathways and the type I collagen gene expression in the urinary bladder and the changes in bladder morphology during cystitis.
EXPERIMENTAL PROCEDURES
Experimental Animals and Reagents
Adult male rats (150–200 g) from Harlan Sprague-Dawley, Inc. (Indianapolis, IN) were used. All experimental protocols involving animal use were approved by the Institutional Animal Care and Use Committee at the Virginia Commonwealth University. Animal care was in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and National Institutes of Health guidelines. All efforts were made to minimize the potential for animal pain, stress, or distress as well as to reduce the number of animals used. Cyclophosphamide, β-actin antibody, and other chemicals used in this experiment were purchased from Sigma-Aldrich. Antibodies against type I collagen, Akt/phospho-Akt, ERK1/2/phospho-ERK1/2, JNK/phospho-JNK, p38 MAPK/phospho-p38 MAPK, were from Cell Signaling Technology (Danvers, MA). NGF antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Real-time PCR reagents were purchased from Applied Biosystems (Foster City, CA). Western blot secondary antibody was from Pierce Biotechnology (Rockford, IL) and Li-cor Biosciences, Inc. (Lincoln, NE).
Cyclophosphamide-induced Cystitis
CYP cystitis was induced in rats by the technique described previously (37). Briefly, cystitis was induced in rats by injecting CYP intraperitoneally at a single dose of 150 mg/kg for 2, 8, or 48 h. Control rats received volume-matched injections of saline. All injections were performed under isoflurane (2%) anesthesia.
Protein Extraction
The urinary bladder was freshly dissected out and homogenized in T-per buffer (Pierce) supplemented with protease and phosphatase inhibitor cocktails (Sigma). The homogenate was centrifuged at 20,200 × g for 10 min at 4 °C, and the supernatant was removed to a fresh tube for further analysis. The protein concentration was determined using a Bio-Rad DC protein assay kit.
Western Blot
Proteins were separated on a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane. The membrane was blocked with 5% milk in Tris-buffered saline for 1 h and then incubated with a specific primary antibody followed by horseradish peroxidase-conjugated or IRDye secondary antibody. For internal loading control, the same membrane was striped and reprobed with anti-β-actin antiserum or antibody to a nonphosphorylation form of the kinase examined. The bands were visualized with an ODYSSEY infrared imaging system (Li-cor Bioscience) or by enhanced chemiluminescence. Densitometric quantification of immunoreactive bands was performed using the software FluorChem 8800 (Alpha Innotech, San Leabdro, CA). The density of the specific band for phosphoprotein was normalized with the density of the band for the nonphosphorylated form of the protein. The density of nonphosphoproteins was normalized with the density of the bands of β-actin.
RNA Extraction and Quantitative Real-time PCR
Total RNA was extracted using an RNA extraction kit RNAqueous (Ambion, TX). RNA concentration was determined spectrophotometrically. cDNA was synthesized using Cloned AMV First-Strand synthesis kit (Invitrogen) with random hexamers. Following reverse transcription, quantitative real-time PCR was performed for type I collagen with a Taqman probe mixed with PCR Master-Mix for 40 cycles (95 °C for 15 s and 60 °C for 1 min) on a 7300 real-time PCR system (Applied Biosystems). Quantitative real-time PCR of the same sample was performed for β-actin or GAPDH expression as internal control. The level of type I collagen mRNA was normalized against β-actin or GAPDH expression in the same sample that was calculated with ΔCt method. The expression level of type I collagen mRNA in control animal from each independent experiment was considered as 1, and the relative expression level of type I collagen mRNA in experimental animals was adjusted as a ratio to its control in each independent experiment and expressed as fold changes (2ΔΔCt-fold).
Statistical Analysis
At least three independent experiments were done in each group of study. Comparison between control and experimental groups was made by using one-way analysis of variance followed by Dunnett's test. Differences between means at a level of p ≤ 0.05 were considered to be significant.
RESULTS
Cystitis-induced Bladder Hypertrophy Involves Increased Production of Type I Collagen
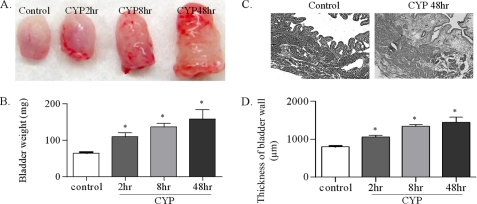
Intraperitoneal injection of CYP induced a significant increase in the weight of the urinary bladder (Fig. 1B) with increased petechial hemorrhages (Fig. 1A). The bladder weight showed consistent increment post-CYP injection as that: 63 ± 6.7 mg in control animals, 109 ± 12.7 mg at 2 h post-CYP injection, 135 ± 15.9 mg at 8 h, and 158 ± 34.4 mg at 48 h. Further examination of the microscopic structure of the bladder wall with hematoxylin and eosin staining showed that cystitis induced significant increases (p < 0.05) in the thickness of the bladder wall and substantial edema and damage of the urothelium (Fig. 1, C and D).
FIGURE 1.
Cystitis increases the urinary bladder weight and the thickness of the bladder wall. After CYP treatment for 2, 8 or 48 h, the urinary bladder was dissected out, and the weight was measured. The paraformaldehyde-fixed bladder was sectioned at a thickness of 10 μm and stained by hematoxylin and eosin. Cystitis caused significant increases in petechial hemorrhages (A) and in the weight (B) of the urinary bladder starting from 2 h following CYP treatment. Hematoxylin and eosin staining shows edema, infiltration and considerable damage of the urothelium (C). The thickness of the bladder wall was significantly increased from 2 h post-CYP injection (D). Results were presented as mean ± S.E. from 4–5 animals at each time point. *, p < 0.05 versus control. Bar, 500 μm.
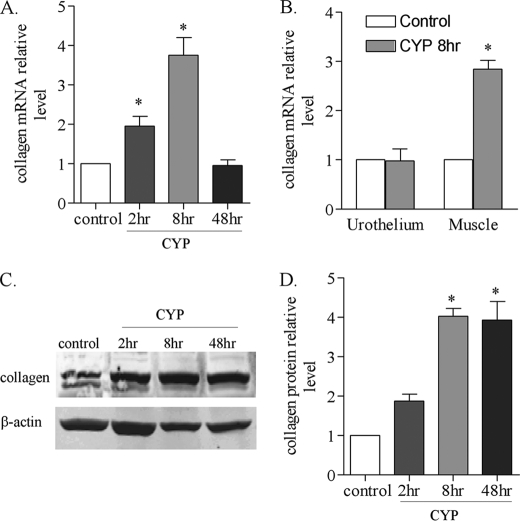
To examine whether cystitis-induced bladder hypertrophy is attributable to, at least in part, the changes in the level of extracellular matrix, we examined the production of type I collagen in the urinary bladder from control animal and animals treated with CYP. Real-time PCR results showed that the level of type I collagen mRNA was increased at 2 and 8 h post-CYP injection, but was not changed at 48 h post-CYP injection (Fig. 2A). To identify the specific bladder tissue that produced type I collagen in the urinary bladder during cystitis, we separated and examined the urothelium and the detrusor smooth muscle layer from control animals and animals treated with CYP for 8 h. We found that cystitis increased the level of type I collagen mRNA in the smooth muscle but not in the urothelium (Fig. 2B).
FIGURE 2.
Increases in the type I collagen mRNA and protein expression levels in the urinary bladder following CYP-induced cystitis. After CYP treatment, the urinary bladder was homogenized for RNA or protein extraction. The urothelium and smooth muscle layers were also separated and homogenized for RNA extraction. Real-time PCR results showed that the type I collagen mRNA level was increased at 2–8 h post-CYP treatment and returned to normal level at 48 h post-CYP treatment (A). At 8 h of cystitis, the level of type I collagen mRNA was increased in the smooth muscle but not urothelium of the inflamed bladder (B). Western blot (C) showed that the type I collagen protein level was increased at 8 h and 48 h post CYP treatment (D). Results were presented as mean ± S.E. from 3–5 animals at each time point. *, p < 0.05 versus control.
Western blot results showed that collagen protein level was also increased in the inflamed bladder following CYP treatment (Fig. 2C). The level of type I collagen protein was significantly increased (p < 0.05) at 8 and 48 h post-CYP injection (Fig. 2D).
Activation of Intracellular Signaling Kinases in the Urinary Bladder during Cystitis
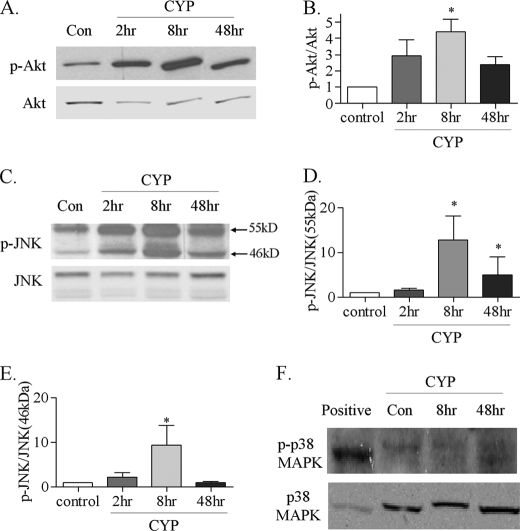
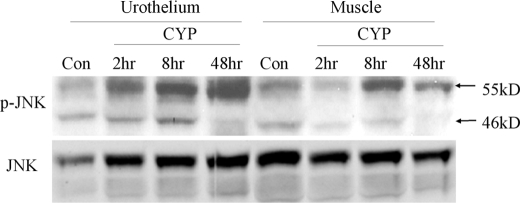
In addition to the increased activation of ERK1/2 in the urinary bladder during cystitis (37), the phosphorylation levels of Akt and JNK were also increased in the inflamed bladder (Fig. 3). We previously reported that the ERK1/2 activity was increased in the urinary bladder at 2 h post-CYP injection (37). The present study showed that the Akt activity was increased at 8 h (Fig. 3A, B), and JNK at 8–48 h (Fig. 3C), post-CYP injection. Both two isoforms of JNK, 55 kDa and the 46 kDa, were activated in the inflamed bladder with cystitis (Fig. 3D, E), which were mainly expressed in the urothelium (Fig. 4). In contrast, the phosphorylation level of p38 MAPK was unchanged in the inflamed urinary bladder when compared with control (Fig. 3F).
FIGURE 3.
Changes in Akt, JNK, and p38 MAPK phosphorylation levels in the urinary bladder during cystitis. Following CYP treatment, the Akt phosphorylation level was examined by Western blot (A) and showed a significant increase in the inflamed bladder at 8 h (B). The JNK phosphorylation level was also increased (C, D, and E) in the inflamed bladder as that the 55-kDa isoform of JNK1 was increased at 8–48 h post-CYP treatment (D), while the 46-kDa isoform of JNK1 was increased at 8 h post-CYP treatment (E). Western blot (F) showed that the phosphorylation level of p38 MAPK was not changed in the inflamed bladder. Results were presented as mean ± S.E. from 3–6 animals at each time point. *, p < 0.05 versus control.
FIGURE 4.
Distribution of phospho-JNK in the inflamed bladder during cystitis. The JNK phosphorylation level was examined from the urothelium and muscle layer of the urinary bladder. The urothelium expressed more phospho-JNK than the muscle layer did after bladder inflammation.
Endogenous NGF Activates ERK1/2, Akt and JNK in the Inflamed Bladder
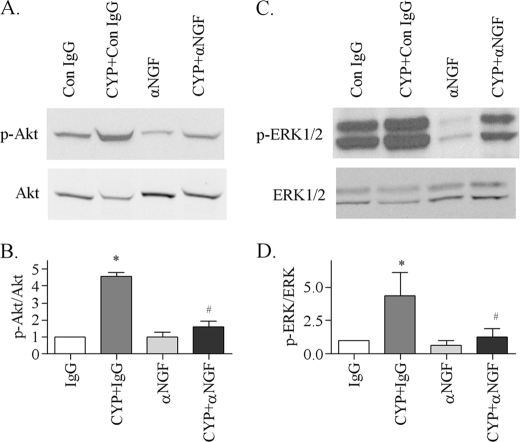
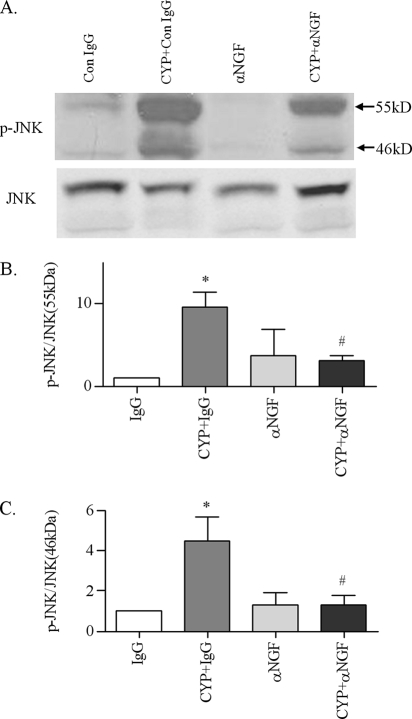
To examine whether cystitis-induced elevation of NGF system in the inflamed bladder (8, 11–12) has a role in the activation of ERK1/2, Akt, and JNK, we injected the NGF neutralizing antibody intraperitoneally at a dose of 30 μg/kg according to a previously published method (38). Animals that received control IgG served as control to the antibody. One injection was made immediately after the CYP injection, and the animals were sacrificed 8 h after the CYP treatment, because at this time point, the phosphorylation levels of ERK1/2, Akt, and JNK were also increased when compared with noninflamed animals. The results showed that cystitis-induced up-regulation of phospho-Akt in the urinary bladder were inhibited by anti-NGF when compared with that treated with CYP and control IgG (Fig. 5, A and B). Consistently, anti-NGF also attenuated the up-regulation of phospho-ERK1/2 (Fig. 5, C and D), and both isoforms of JNK (Fig. 6) in the urinary bladder. It is noted that in most animals studied, blockade of endogenous NGF in noninflamed animals decreased the basal phosphorylation level of ERK1/2 and JNK when normal IgG was used as control.
FIGURE 5.
Attenuation of cystitis-induced Akt and ERK1/2 phosphorylation in the urinary bladder by NGF antibody. In the control IgG-treated animals, cystitis increased the phosphorylation levels of Akt (A and B) and ERK1/2 (C and D) in the inflamed bladder. Administration of NGF neutralizing antibody had no effects on the basal level of phospho-Akt and phospho-ERK1/2 in the urinary bladder but significantly blocked cystitis-induced up-regulation of phospho-Akt and phospho-ERK1/2 in the inflamed bladder. Results were presented as mean ± S.E. from 3 animals at each time point. *, p < 0.05 versus IgG; #, p < 0.05 versus CYP+IgG.
FIGURE 6.
Attenuation of cystitis-induced JNK phosphorylation in the urinary bladder by NGF antibody. In the control IgG-treated animals, cystitis increased the phosphorylation levels of JNK 55 kDa (A and B) and 46 kDa (A and C) in the inflamed bladder. Administration of NGF neutralizing antibody had no effects on the basal level of phospho-JNK in the urinary bladder but significantly blocked cystitis-induced up-regulation of phospho-JNK in the inflamed bladder. Results were presented as mean ± S.E. from 3 animals at each time point. *, p < 0.05 versus IgG; #, p < 0.05 versus CYP+IgG.
Contribution of NGF to the Type I Collagen Expression and Urinary Bladder Hypertrophy
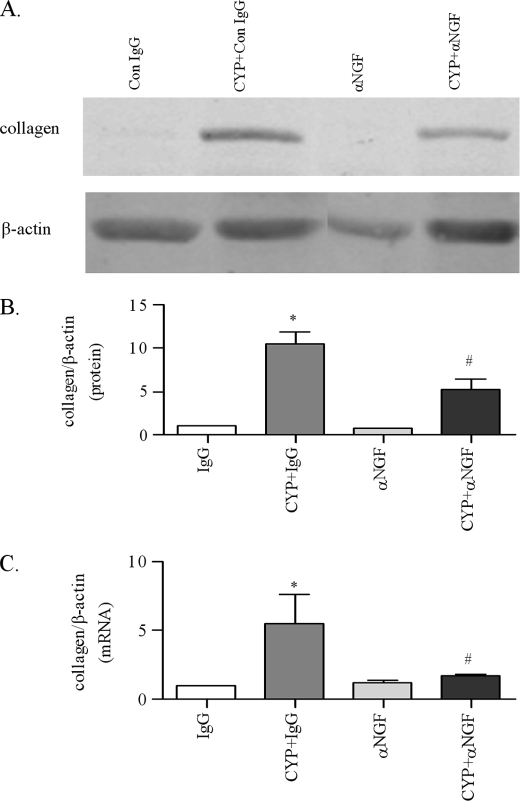
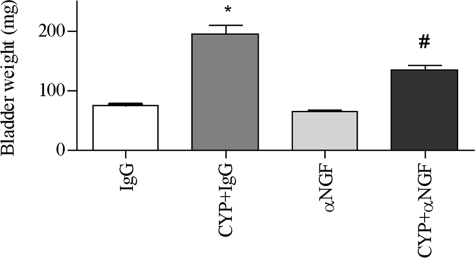
At 8 h post-CYP and anti-NGF treatment, cystitis-induced increases in the type I collagen expression was significantly attenuated by anti-NGF (Fig. 7). Anti-NGF treatment blocked type I collagen mRNA synthesis (Fig. 7C) and reduced the collagen protein level in the urinary bladder by 40% when compared with that from animals treated with CYP and control IgG (Fig. 7B). The cystitis-induced increases in the weight of the urinary bladder was also reduced by anti-NGF to 133.3 ± 20.7 mg when compared with that from animals receiving CYP and control IgG, which was 193.3 ± 41.3 mg (Fig. 8).
FIGURE 7.
Attenuation of cystitis-induced type I collagen expression in the urinary bladder by NGF antibody. In the control IgG-treated animals, cystitis increased the type I collagen protein (A and B) and gene expression (C) level in the inflamed bladder. Administration of NGF neutralizing antibody had no effects on the basal level of type I collagen mRNA and protein in the urinary bladder but significantly blocked cystitis-induced up-regulation of type I collagen expression in the inflamed bladder. Results were presented as mean ± S.E. from 3 animals at each time point. *, p < 0.05 versus IgG; #, p < 0.05 versus CYP+IgG.
FIGURE 8.
Attenuation of cystitis-induced bladder hypertrophy by NGF antibody. In the control IgG-treated animals, cystitis increased the weight of the urinary bladder. Administration of NGF neutralizing antibody alone had no effects on the urinary bladder weight when compared with control IgG treatment but significantly blocked cystitis-induced increases in the bladder weight. Results were presented as mean ± S.E. from 3 animals at each time point. *, p < 0.05 versus IgG; #, p < 0.05 versus CYP+IgG.
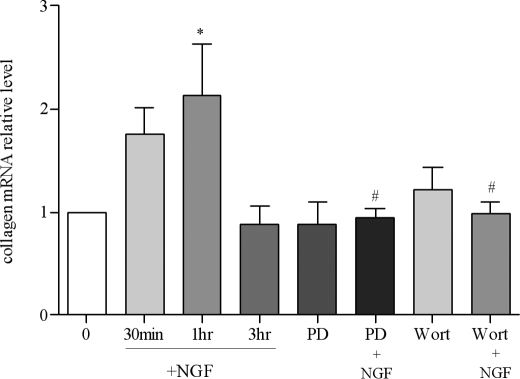
To examine whether NGF acted directly on the bladder smooth muscle to induce the production of type I collagen, we isolated the urinary bladder from naive animals and cultured the bladder smooth muscle explants acutely and treated them with NGF (100 ng/ml) for 30 min, 1 h, and 3 h. We used naive animals to minimize the effects of neuronal mediators secreted from the nerve terminals during cystitis. We found that type I collagen mRNA level was significantly increased at 1 h post-NGF stimulation (Fig. 9). Pretreatment of the culture with PD98059 (1 μm, an inhibitor for the MEK/ERK pathway) or wortmannin (0.5 μm, an inhibitor for phosphoinositide 3-kinase/Akt pathway) significantly attenuated NGF-induced type I collagen production (Fig. 9).
FIGURE 9.
Up-regulation of type I collagen expression in the urinary bladder smooth muscle by NGF. In cultured detrusor smooth muscle, the level of type I collagen mRNA was significantly increased by NGF treatment (100 ng/ml) for 1 h. Pretreatment for 30 min with PD98059 (1 μm) or wortmannin (0.5 μm) attenuated NGF-induced type I collagen expression. PD98059 or wortmannin alone had no effects on the level of type I collagen mRNA expression. The results were from 3 independent experiments and presented as mean ± S.E. *, p < 0.05 versus 0 treatment; #, p < 0.05 versus NGF treatment for 1 h.
DISCUSSION
The present study demonstrated that cystitis induced significant increases in the weight of the urinary bladder and increases in the thickness of the bladder wall reflecting bladder hypertrophy. The bladder weight and the thickness of the bladder wall were immediately increased at 2 h post-CYP injection and continued increasing until 48 h as examined. There are several possible mechanisms and pathways involved in visceral organ hypertrophy, including increases in the production of extracellular matrix (13, 39, 40) and increases in muscular growth (13, 41). In the current study, we examined the expression level of type I collagen, the main constituent of the extracellular matrix, in the inflamed bladder and found that the mRNA level of type I collagen was significantly increased at 2 and 8 h post-CYP injection and was back to normal level at 48 h post-CYP injection. The level of type I collagen protein was increased at 8 and 48 h in the urinary bladder following CYP treatment, suggesting that cystitis increased the synthesis and deposition of type I collagen in the inflamed bladder.
To identify the potential signaling pathways that contribute to type I collagen up-regulation in the inflamed bladder during cystitis, we examined the activity (phosphorylation level) of the MAPKs and Akt in the inflamed bladder. The reason for choosing these pathways is that activation of these pathways led to either increases or decreases in the type I collagen gene expression (27–31). Our results showed that following a time course of cystitis the phosphorylation level of ERK1/2 was the highest at 2 h post-CYP injection (37); the phosphorylation level of Akt was the highest at 8 h post-CYP injection; the phosphorylation level of JNK was the highest at 8–48 h post-CYP injection; while the phosphorylation level of p38 MAPK was not changed. To characterize the role of NGF in the activation of above kinases and in the regulation of type I collagen expression, we injected NGF-neutralizing antibody intraperitoneally with or without a combination of CYP injection. We found that blockade of NGF activity with the antiserum reversed the activation of ERK1/2, JNK and Akt induced by cystitis in the urinary bladder. Neutralizing NGF antibody also attenuated cystitis-induced type I collagen up-regulation and increases in the bladder mass. Although previous studies reported that the type I collagen gene expression was regulated by other factors such as transforming growth factor-β involving the activation of ERK1/2 (28, 42), the role of the endogenous NGF in cystitis-induced type I collagen synthesis remains to be elucidated. The current study demonstrates that NGF not only plays a key role in the activation of MAPK and Akt pathways in the inflamed bladder but also contributes significantly to bladder hypertrophy.
Cellular responses mediated by NGF require ligand-binding and subsequent activation of specific receptor tyrosine kinases (Trks), which are embedded in the plasma membrane (43, 44). Neurotrophin general receptor p75NTR, when it dimerizes with Trk, increases the specificity and affinity of Trk for particular ligands (45). Previous studies showed that both TrkA and p75NTR expression were increased in the inflamed urinary bladder following cystitis induced by CYP injection (24, 25). Localization study demonstrated the presence of TrkA immunoreactivity in the bladder smooth muscle cells (25) indicating the ability of these cells in response to NGF, which may lead to the increased type I collagen transcript in the detrusor muscle of the inflamed bladder. Following NGF binding to TrkA and/or p75NTR, a cascade of signal transduction is initiated, which ultimately leads to changes in gene expression. The signaling molecules that we examined in the inflamed bladder during cystitis include ERK1/2, a key molecule in the MEK/MAPK pathway (37), and Akt, a substrate of phosphoinositide 3-kinase. We showed that Akt activity (phosphorylation level) was increased at a relatively later time versus the activation of ERK1/2 (8 h versus 2 h post CYP injection). So far, we don't have evidence about the cross-talk between these two pathways. However, it is apparent that both pathways are downstream of NGF action because NGF neutralizing antibody blocked the activation of these pathways in the inflamed bladder. Blockade of one of these two pathways also attenuated NGF-induced collagen expression in cultured detrusor smooth muscle, suggesting involvement of both pathways in type I collagen production.
CYP-induced cystitis causes dramatic increases in the bladder weight and the thickness of the bladder wall. One of the possible factors contributing to visceral organ hypertrophy involves the increased collagen deposition. Elevated expression of collagen in the urinary bladder has been identified to play a significant role in bladder hypertrophy caused by bladder outlet obstruction (13). Our current study shows that cystitis-induced bladder hypertrophy involves type I collagen up-regulation. It is not surprising that our study demonstrated a partial reduction (40%) of cystitis-induced type I collagen expression by NGF neutralization. The regulation of collagen gene expression is complicated and influenced by many factors, including fibroblast growth factor (13), some cytokines (19) and transforming growth factor-β (19) that were also increased in the urinary bladder with inflammation (12, 46, 47 and supplemental data). The interactive effects of NGF and other growth factors in influencing cellular function have been reported (48, 49); thus, it could be possible that NGF-induced type I collagen expression was also influenced by other growth factors produced in the urinary bladder during cystitis. In our other studies, we found that cystitis increased the secretion of brain-derived neurotrophic factor from the axonal nerve terminals that innervate the urinary bladder (data not shown). The neuronal mediators that are secreted from the nerve terminals during cystitis may also have roles independently from or interactively with NGF in inducing type I collagen expression in the inflamed bladder.
Other MAPK family members, the JNK and p38 MAPK, are more likely involved in stress and cytokines signaling. The present study shows that both 55-kDa and 46-kDa isoforms of JNK (50) were activated in the inflamed bladder; however, p38 MAPK was not, indicating a specific activation of JNK. Although our experiments showed that NGF neutralization blocked JNK activity induced by bladder inflammation, it is reported that JNK is more likely activated by proneurotrophins binding to p75NTR (51). The NGF antibody used in this study (Santa Cruz Biotechnology) does not distinguish the pro-form and mature form NGF, and it could block the activity of both. It would be interesting to examine the level of pro-NGF in the inflamed bladder and the role of pro-NGF in JNK activation in the future studies. When we separated the urothelium and the muscle layers of the urinary bladder, cystitis-induced JNK activation was shown more in the urothelium. This may be due to the damage and cell death of the urothelium during cystitis. CYP treatment of patients and the laboratory animals resulted cystitis (52–54) is largely due to the urotoxicity effects of CYP metabolite, acrolein (55), which causes considerable damage of the urothelium layer. The general neurotrophin receptor p75NTR was found in both urothelium and muscle cells (24) and may play distinct roles specific to different cell type.
Studies from interstitial cystitis in patients and CYP-induced cystitis in animals show that NGF is one of the major inflammatory mediators in bladder inflammation (8, 10, 11). NGF is produced in and released from the epithelial cells and mast cells during visceral inflammation, where it acts in a paracrine manner to regulate the cytological changes and the sensitivity of the visceral organ (36, 56, 57). Elevated activation of ERK1/2 and Akt by the endogenous NGF in the inflamed urinary bladder may lead to changes in gene expression and increases in cellular proliferation and growth. Reduction of type I collagen up-regulation and bladder weight by NGF neutralizing antibody suggest that NGF analogs might have potential therapeutic use in the treatment of cystitis-induced bladder abnormality.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant DK077917 (to L.-Y. Q.). This work was also supported by a grant from the Interstitial Cystitis Association (to L.-Y. Q.).

The on-line version of this article (available at http://www.jbc.org) contains a supplemental figure.
- NGF
- nerve growth factor
- CYP
- cyclophosphamide
- JNK
- c-Jun N-terminal kinase
- ERK
- extracellular signal-regulated kinase
- MAPK
- mitogen-activated protein kinase
- MEK
- mitogen-activated protein kinase/extracellular signal-regulated kinase kinase.
REFERENCES
- 1.Nazif O., Teichman J. M., Gebhart G. F. (2007) Urology 69, 24–33 [DOI] [PubMed] [Google Scholar]
- 2.Saini R., Gonzalez R. R., Te A. E. (2008) Curr. Urol. Rep. 9, 314–319 [DOI] [PubMed] [Google Scholar]
- 3.Theiss A. L., Fruchtman S., Lund P. K. (2004) Inflamm. Bowel Dis. 10, 871–880 [DOI] [PubMed] [Google Scholar]
- 4.Donnerer J., Schuligoi R., Stein C. (1992) Neuroscience 49, 693–698 [DOI] [PubMed] [Google Scholar]
- 5.Donnerer J., Stein C. (1992) Ann. N.Y. Acad. Sci. 657, 505–506 [DOI] [PubMed] [Google Scholar]
- 6.Roza C., Reeh P. W. (2001) Pain 93, 213–219 [DOI] [PubMed] [Google Scholar]
- 7.Tonra J. R., Curtis R., Wong V., Cliffer K. D., Park J. S., Timmes A., Nguyen T., Lindsay R. M., Acheson A., DiStefano P. S. (1998) J. Neurosci. 18, 4374–4383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vizzard M. A. (2000) Exp. Neurol. 161, 273–284 [DOI] [PubMed] [Google Scholar]
- 9.Tuttle J. B., Steers W. D., Albo M., Nataluk E. (1994) J. Auton. Nerv. Syst. 49, 147–158 [DOI] [PubMed] [Google Scholar]
- 10.Oddiah D., Anand P., McMahon S. B., Rattray M. (1998) Neuroreport 9, 1455–1458 [DOI] [PubMed] [Google Scholar]
- 11.Okragly A. J., Niles A. L., Saban R., Schmidt D., Hoffman R. L., Warner T. F., Moon T. D., Uehling D. T., Haak-Frendscho M. (1999) J. Urol. 161, 438–442 [PubMed] [Google Scholar]
- 12.Chen M. W., Levin R. M., Buttyan R. (1995) World J. Urol. 13, 344–348 [DOI] [PubMed] [Google Scholar]
- 13.Imamura M., Kanematsu A., Yamamoto S., Kimura Y., Kanatani I., Ito N., Tabata Y., Ogawa O. (2007) Am. J. Physiol. Renal Physiol. 293, F1007–1017 [DOI] [PubMed] [Google Scholar]
- 14.Tagaya E., Tamaoki J. (2007) Allergol Int. 56, 331–340 [DOI] [PubMed] [Google Scholar]
- 15.Lee S. D., Akbal C., Miseeri R., Jung C., Rink R., Kaefer M. (2006) J. Pediatr. Urol. 2, 225–232 [DOI] [PubMed] [Google Scholar]
- 16.Tekgul S., Yoshino K., Bagli D., Carr M. C., Mitchell M. E., Yao L. Y. (1996) J. Urol. 156, 582–586 [DOI] [PubMed] [Google Scholar]
- 17.Fleischmajer R., Perlish J. S., Burgeson R. E., Shaikh-Bahai F., Timpl R. (1990) Ann. N.Y. Acad. Sci. 580, 161–175 [DOI] [PubMed] [Google Scholar]
- 18.Oka M., Fukui T., Ueda M., Tagaya M., Oyama T., Tanaka M. (2009) J. Urol. 182, 382–390 [DOI] [PubMed] [Google Scholar]
- 19.Ghosh A. K. (2002) Exp. Biol. Med. 227, 301–314 [DOI] [PubMed] [Google Scholar]
- 20.Lutz M., Krieglstein K., Schmitt S., ten Dijke P., Sebald W., Wizenmann A., Knaus P. (2004) Eur. J. Biochem. 271, 920–931 [DOI] [PubMed] [Google Scholar]
- 21.Yoon J. K., Lau L. F. (1994) Mol. Cell. Biol. 14, 7731–7743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pellegrino M. J., Stork P. J. (2006) J. Neurochem. 99, 1480–1493 [DOI] [PubMed] [Google Scholar]
- 23.Wu B. Y., Fodor E. J., Edwards R. H., Rutter W. J. (1989) J. Biol. Chem. 264, 9000–9003 [PubMed] [Google Scholar]
- 24.Klinger M. B., Vizzard M. A. (2008) Am. J. Physiol. Renal Physiol. 295, F1778–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray E., Malley S. E., Qiao L. Y., Hu V. Y., Vizzard M. A. (2004) J. Urol. 172, 2434–2439 [DOI] [PubMed] [Google Scholar]
- 26.Segal R. A. (2003) Ann. Rev. Neurosci. 26, 299–330 [DOI] [PubMed] [Google Scholar]
- 27.Reunanen N., Foschi M., Han J., Kahari V. M. (2000) J. Biol. Chem. 275, 34634–34639 [DOI] [PubMed] [Google Scholar]
- 28.Li F., Zeng B., Chai Y., Cai P., Fan C., Cheng T. (2009) Biochem. Biophys. Res. Commun. 386, 289–293 [DOI] [PubMed] [Google Scholar]
- 29.Papakrivopoulou J., Lindahl G. E., Bishop J. E., Laurent G. J. (2004) Cardiovasc. Res. 61, 736–744 [DOI] [PubMed] [Google Scholar]
- 30.Ivaska J., Reunanen H., Westermarck J., Koivisto L., Kähäri V. M., Heino J. (1999) J. Cell Biol. 147, 401–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun G., Hines I. N., Lindquist J., Schrum L. W., Rippe R. A. (2009) Hepatology 50, 1512–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amaravadi R., Thompson C. B. (2005) J. Clin. Invest. 115, 2618–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fayard E., Tintignac L. A., Baudry A., Hemmings B. A. (2005) J. Cell Sci. 118, 5675–5678 [DOI] [PubMed] [Google Scholar]
- 34.Vaillant A. R., Mazzoni I., Tudan C., Boudreau M., Kaplan D. R., Miller F. D. (1999) J. Cell Biol. 146, 955–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manning B. D., Cantley L. C. (2007) Cell 129, 1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowe E. M., Anand P., Terenghi G., Williams-Chestnut R. E., Sinicropi D. V., Osborne J. L. (1997) Br. J. Urol. 79, 572–577 [DOI] [PubMed] [Google Scholar]
- 37.Qiao L. Y., Gulick M. A. (2007) Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1368–1375 [DOI] [PubMed] [Google Scholar]
- 38.Delafoy L., Gelot A., Ardid D., Eschalier A., Bertrand C., Doherty A. M., Diop L. (2006) Gut. 55, 940–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Majumdar P., Chen S., George B., Sen S., Karmazyn M., Chakrabarti S. (2009) Diabetes Metab. Res. Rev. 25, 452–463 [DOI] [PubMed] [Google Scholar]
- 40.Howard P. S., Kucich U., Coplen D. E., He Y. (2005) Urology 66, 1349–1353 [DOI] [PubMed] [Google Scholar]
- 41.Gabella G. (1990) Anat. Embryol. 182, 409–424 [DOI] [PubMed] [Google Scholar]
- 42.Rodríguez-Barbero A., Obreo J., Alvarez-Munoz P., Pandiella A., Bernabéu C., López-Novoa J. M. (2006) Cell Physiol. Biochem. 18, 135–142 [DOI] [PubMed] [Google Scholar]
- 43.Kaplan D. R., Stephens R. M. (1994) J. Neurobiol. 25, 1404–1417 [DOI] [PubMed] [Google Scholar]
- 44.Kaplan D. R., Miller F. D. (1997) Curr. Opin. Cell Biol. 9, 213–221 [DOI] [PubMed] [Google Scholar]
- 45.Barker P. A. (2007) Neuron 53, 1–4 [DOI] [PubMed] [Google Scholar]
- 46.Batler R. A., Sengupta S., Forrestal S. G., Schaeffer A. J., Klumpp D. J. (2002) J. Urol. 168, 819–825 [PubMed] [Google Scholar]
- 47.Gomes T. N., Santos C. C., Souza-Filho M. V., Cunha F. Q., Ribeiro R. A. (1995) Braz. J. Med. Biol. Res. 28, 1103–1108 [PubMed] [Google Scholar]
- 48.Jones D. M., Tucker B. A., Rahimtula M., Mearow K. M. (2003) J. Neurochem. 86, 1116–1128 [DOI] [PubMed] [Google Scholar]
- 49.Madduri S., Papaloïzos M., Gander B. (2009) Neurosci. Res. 65, 88–97 [DOI] [PubMed] [Google Scholar]
- 50.Hibi M., Lin A., Smeal T., Minden A., Karin M. (1993) Genes Dev. 7, 2135–2148 [DOI] [PubMed] [Google Scholar]
- 51.Volosin M., Song W., Almeida R. D., Kaplan D. R., Hempstead B. L., Friedman W. J. (2006) J. Neurosci. 26, 7756–7766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.deVries C. R., Freiha F. S. (1990) J. Urol. 143, 1–9 [DOI] [PubMed] [Google Scholar]
- 53.Stillwell T. J., Benson R. C., Jr. (1988) Cancer 61, 451–457 [DOI] [PubMed] [Google Scholar]
- 54.Levine L. A., Richie J. P. (1989) J. Urol. 141, 1063–1069 [DOI] [PubMed] [Google Scholar]
- 55.Cox P. J. (1979) Biochem. Pharmacol. 28, 2045–2049 [DOI] [PubMed] [Google Scholar]
- 56.Stanzel R. D., Lourenssen S., Blennerhassett M. G. (2008) Exp. Neurol. 211, 203–213 [DOI] [PubMed] [Google Scholar]
- 57.Skaper S. D., Pollock M., Facci L. (2001) Brain Res. Mol. Brain Res. 97, 177–185 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.