Abstract
Type 2 diabetes is responsible for the increased prevalence of ischemic heart disease, generally related to coronary artery disease, which is associated with increased morbidity and death in diabetic patients. Epidermal growth factor receptor (EGFR) tyrosine kinase, one of the many factors involved in cell growth and migration has been shown to be key element in the development of microvessel myogenic tone. In a recent study, we have shown that microvascular dysfunction in type 2 diabetes is dependent on the exacerbation of the EGFR tyrosine kinase phosphorylation. Thus, further elucidation of this EGFR transactivation and down stream signaling will offer a new direction for the investigation of the mechanism of microvascular dysfunction responsible for heart disease that occurs in type 2 diabetes. In this review, we discuss the link between the EGFR transactivation and microvascular dysfunction that occurs in type 2 diabetes.
Type 2 Diabetes, a chronic disease, is a rapidly increasing epidemic in the whole world affecting nearly 20 million individuals and acknowledged as a major health concern worldwide. Type 2 Diabetes is responsible for increased prevalence of associated-vascular complications leading to considerable morbidity and mortality (1). Although microvascular physiopathology that has been studied over the last three decades, the scientific community has yet to reach a definitive consensus about the underlying mechanisms involving the fundamental processes that govern resistance artery physio-pathological function and morphological changes associated with diabetes. It is well established that the effects of type 2 diabetes on microvessels include alteration of endothelial and smooth muscle cell (SMC) function and induction of structural wall remodeling. Both process lead to the altered tissue perfusion.
Studies in both human and experimental type 2 diabetic animal models have shown vascular dysfunction and changes in structural arterial wall. These pathological consequences are regarded as risk factors leading to increasing morbidity and mortality (2). Altered microvascular function in type 2 diabetes may influence critical determinants of circulatory function, including tissue local blood flow. However, the passive mechanical properties of blood vessel walls are also critical determinants of pressure-flow-diameter relationships, speed of pulse waves in vessels and stress distribution in vessel walls (3).
The EGFR is an 1186-amino-acid glycoprotein containing a single transmembrane domain, an extracellular portion involved in ligand binding, and an intracellular portion harboring the tyrosine kinase domain. EGFR can be activated by different ligands such as EGF and heparin-binding EGF-like. More recently angiotensin II and leptin have been shown to transactivate EGFR (4-6). Recently, Schneider and Wolf discussed the EGFR signaling and their roles in the development, the physiology, and the pathology (7). The tyrosine-kinase epidermal growth factor receptor regulates key processes of cell biology, such as proliferation, survival, differentiation during development, tissue homeostasis, and recently has been shown in enhancing the vasculogenesis effect of stem cells (8). There is a wealth of information with regard to the growth-promoting effects of EGFR in cultured VSMCs, but its role in the regulation of vascular function has been recently demonstrated by our group showing that EGFR tyrosine kinase is critical in the development of myogenic tone of resistance artery (5,9).
In a previous work, we showed that mesenteric resistance artery (MRA) myogenic tone development is highly dependent on epidermal growth factor receptor (RGFR) tyrosine kinase trans-activation (5). The increase in intraluminal pressure initiates a fundamental process including metalloproteinases 2 and 9 (MMP2/9) activation and heparin-binding epidermal growth factor-like (HB-EGF) shedding from SMC membranes, which in turn activates EGFR tyrosine kinase and leading to myogenic tone development (Figure 1, published in reference 5). It is noteworthy that the underpinning mechanism(s) by which intraluminal pressure activates MMPs remains largely unsettled. Accordingly, the need for studies that delineate the mechanism dictating the activation of MMPs in response to intraluminal pressure changes is becoming imperative.
Figure 1.
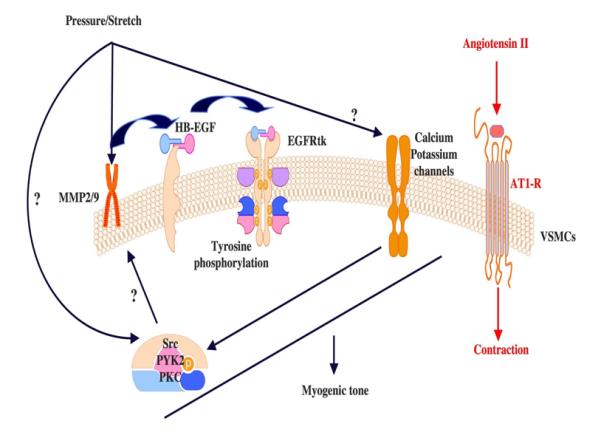
The EGFR tyrosine kinase is not well characterized in term of its role in microvascular physio-pathology. In addition, the mechanisms linking EGFR tyrosine kinase phosphorylation to microvascular endothelial and SMC dysfunction are yet to be determined in type 2 diabetes. Recently, it has been shown that leptin signaling pathway is mediated through the EGFR tyrosine kinase transactivation (6). Leptin level is elevated in type 2 diabetes and this could explain the exacerbation of EGFR tyrosine kinase phosphorylation. In a previous study we have shown that ERK1/2 MAP-Kinase is critical in myogenic tone development (10). Our recent findings indicate that EGFR tyrosine kinase activation leads to ERK1/2 MAP-kinase activation and this could be a potential link between EGFR tyrosine kinase and vascular contraction. We also showed that myogenic tone of coronary arteriole is dependent on the down stream signaling (JAK-STAT) of the EGFR tyrosine kinase (unpublished data).
A rather limited number of studies that addressed the relationship between hyperglycemia and altered vascular responsiveness have been conducted in the microvasculature from diabetic models with conflicting results and conclusions. For instance, skeletal muscle arterioles of streptozotocin-treated rats exhibit enhanced pressure-induced myogenic responsiveness that is endothelium-independent but requires increased activation of L-type Ca2+ channels and protein kinase C (11). Lagaud et al (12) demonstrated increased myogenic tone (MT) in MRA from type 2 diabetic mice compared to control mice, which was insensitive to L-NAME treatment or removal of the endothelium. Bagi et al (13), however, showed no significant increase in MT in coronary arterioles from type 2 diabetic mice. Small arteries (65-230 μm) from gluteal fat biopsies from patients with type 2 diabetes demonstrated decreased myogenic responsiveness (14). Although the reasons behind these discrepancies remains unclear, they may represent differences in vascular beds, type 1 vs. type 2 diabetes, as well as differences in species and animal genetic background.
Our recent study, using the mesenteric resistance artery and coronary arteriole from type 2 diabetic mice, revealed an increase in myogenic tone and an alteration in endothelial cells function assessed with reduced relaxation in response to dose-response of acetylcholine and shear stress (15) (Figure 2). Western blot analysis and immunostaining showed an increase in EGFR tyrosine kinase phsophorylation in smooth muscle cells and endothelial cells of mesenteric resistance artery and coronary arteriole from type 2 diabetic mice compared to control (Figure 2). The increase in EGFR tyrosine kinase phosphorylation could be the result of different factors, such as hyperglycemia as was previously demonstrated (15,16). Interestingly, the in vivo treatment of type 2 diabetic mice with EGFR tyrosine kinase inhibitor for two weeks reduced EGFR tyrosine kinase phosphorylation, significantly improved the endothelial function and lowered the MT potentiation in mesenteric resistance artery and coronary arteriole.
Figure 2.
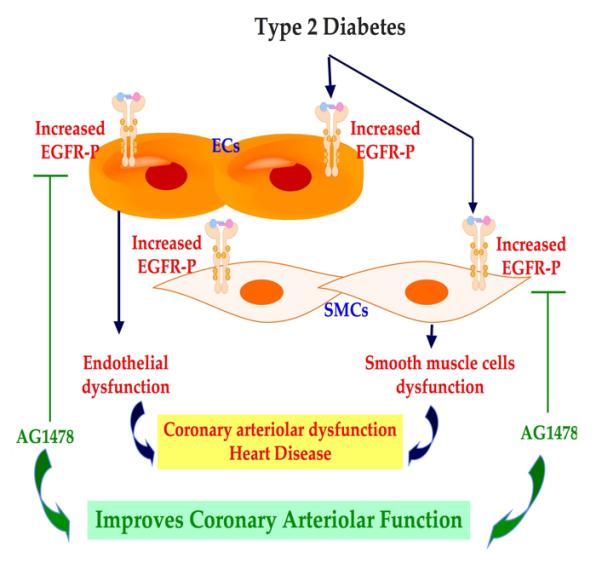
Additionally, we showed an induction of structural wall remodeling (eutrophic remodeling) of mesenteric resistance artery (Figure 3) and coronary arteriole, which was improved with EGFR tyrosine kinase inhibition (17). These data provide evidence that EGFR tyrosine kinase represents a key element in microvascular physiopathology and could be a therapeutic target. Interestingly, Benter et al., have shown that the early inhibition of EGFR tyrosine kinase prevents the induction of multiple signaling pathways involved in diabetes-induced vascular dysfunction (18). These data clearly demonstrate the key mechanism of EGFR tyrosine kinase in cardiovascular complication in type 2 diabetes.
Figure 3.
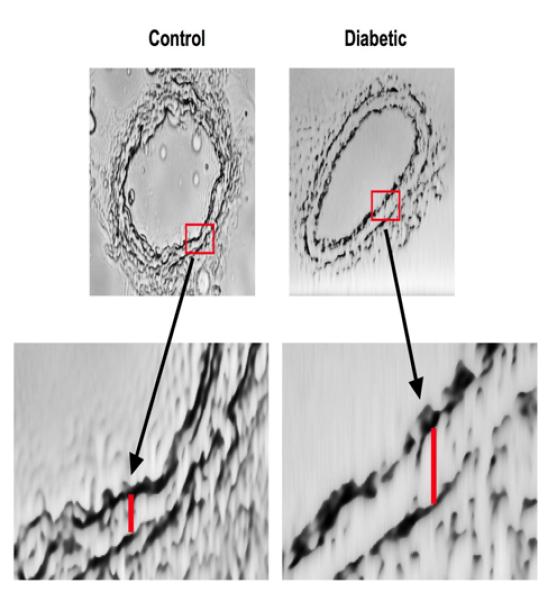
We strongly consider that EGFR tyrosine kinase pathway as a new and important direction for the investigation of the overall mechanisms leading to microvascular dysfunction in type 2 diabetes. We believe that such focus will significantly facilitate our understanding of the vulnerability of microvascular complications in type 2 diabetes and the development of therapies to treat this disorder.
Acknowledgments
Sources of Funding We acknowledge grant support from the National Institutes of Health (P20RR017659, HL26371 NCRR)
Footnotes
Disclosures None.
References
- 1.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001 Sep;44(Suppl 2):S14–21. doi: 10.1007/pl00002934. [DOI] [PubMed] [Google Scholar]
- 2.Mahmud A, Feely J. Arterial stiffness and the renin-angiotensin-aldosterone system. J Renin Angiotensin Aldosterone Syst. 2004 Sep;5(3):102–108. doi: 10.3317/jraas.2004.025. [DOI] [PubMed] [Google Scholar]
- 3.Kassab GS, Molloi S. Cross-sectional area and volume compliance of porcine left coronary arteries. Am J Physiol Heart Circ Physiol. 2001 Aug;281(2):H623–H628. doi: 10.1152/ajpheart.2001.281.2.H623. [DOI] [PubMed] [Google Scholar]; Hoffman JI, Spaan JA. Pressure-flow relations in coronary circulation. Physiol Rev. 1990 Apr;70(2):331–390. doi: 10.1152/physrev.1990.70.2.331. [DOI] [PubMed] [Google Scholar]
- 4.Zhai P, Galeotti J, Liu J, Holle E, Yu X, Wagner T, Sadoshima J. An angiotensin II type 1 receptor mutant lacking epidermal growth factor receptor transactivation does not induce angiotensin II-mediated cardiac hypertrophy. Circ Res. 2006 Sep 1;99(5):528–536. doi: 10.1161/01.RES.0000240147.49390.61. [DOI] [PubMed] [Google Scholar]
- 5.Lucchesi PA, Sabri A, Belmadani S, Matrougui K. Involvement of metalloproteinases 2/9 in epidermal growth factor receptor transactivation in pressure-induced myogenic tone in mouse mesenteric resistance arteries. Circulation. 2004 Dec 7;110(23):3587–3593. doi: 10.1161/01.CIR.0000148780.36121.47. [DOI] [PubMed] [Google Scholar]
- 6.Shida D, Kitayama J, Mori K, Watanabe T, Nagawa H. Transactivation of epidermal growth factor receptor is involved in leptin-induced activation of janus-activated kinase 2 and extracellular signal-regulated kinase 1/2 in human gastric cancer cells. Cancer Res. 2005 Oct 15;65(20):9159–9163. doi: 10.1158/0008-5472.CAN-05-0598. [DOI] [PubMed] [Google Scholar]
- 7.Schneider MR, Wolf E. The epidermal growth factor receptor ligands at a glance. J Cell Physiol. 2009 Mar;218(3):460–466. doi: 10.1002/jcp.21635. [DOI] [PubMed] [Google Scholar]
- 8.Belmadani S, Matrougui K, Kolz C, Pung YF, Palen D, Prockop DJ, Chilian WM. Amplification of coronary arteriogenic capacity of multipotent stromal cells by epidermal growth factor. Arterioscler Thromb Vasc Biol. 2009 Jun;29(6):802–808. doi: 10.1161/ATVBAHA.109.186189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belmadani S, Palen DI, Gonzalez-Villalobos RA, Boulares HA, Matrougui K. Elevated epidermal growth factor receptor phosphorylation induces resistance artery dysfunction in diabetic db/db mice. Diabetes. 2008 Jun;57(6):1629–1637. doi: 10.2337/db07-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palen DI, Belmadani S, Lucchesi PA, Matrougui K. Role of SHP-1, Kv.1.2, and cGMP in nitric oxide-induced ERK1/2 MAP kinase dephosphorylation in rat vascular smooth muscle cells. Cardiovasc Res. 2005 Nov 1;68(2):268–277. doi: 10.1016/j.cardiores.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 11.Ungvari Z, Pacher P, Kecskemeti V, Papp G, Szollar L, Koller A. Increased myogenic tone in skeletal muscle arterioles of diabetic rats. Possible role of increased activity of smooth muscle Ca2+ channels and protein kinase C. Cardiovasc Res. 1999 Sep;43(4):1018–1028. doi: 10.1016/s0008-6363(99)00106-6. [DOI] [PubMed] [Google Scholar]
- 12.Lagaud GJ, Masih-Khan E, Kai S, van Breemen C, Dube GP. Influence of type II diabetes on arterial tone and endothelial function in murine mesenteric resistance arteries. J Vasc Res. 2001 Nov-Dec;38(6):578–589. doi: 10.1159/000051094. [DOI] [PubMed] [Google Scholar]
- 13.Bagi Z, Koller A, Kaley G. Superoxide-NO interaction decreases flow- and agonist-induced dilations of coronary arterioles in Type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol. 2003 Oct;285(4):H1404–H1410. doi: 10.1152/ajpheart.00235.2003. [DOI] [PubMed] [Google Scholar]
- 14.Schofield I, Malik R, Izzard A, Austin C, Heagerty A. Vascular structural and functional changes in type 2 diabetes mellitus: evidence for the roles of abnormal myogenic responsiveness and dyslipidemia. Circulation. 2002 Dec 10;106(24):3037–3043. doi: 10.1161/01.cir.0000041432.80615.a5. [DOI] [PubMed] [Google Scholar]
- 15.Belmadani S, Palen DI, Gonzalez-Villalobos RA, Boulares HA, Matrougui K. Elevated Epidermal Growth Factor Receptor Phosphorylation Induces Resistance Artery Dysfunction in Diabetic db/db mice. Diabetes. 2008 Mar 4; doi: 10.2337/db07-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konishi A, Berk BC. Epidermal growth factor receptor transactivation is regulated by glucose in vascular smooth muscle cells. J Biol Chem. 2003 Sep 12;278(37):35049–35056. doi: 10.1074/jbc.M304913200. [DOI] [PubMed] [Google Scholar]
- 17.Palen DI, Matrougui K. Role of elevated EGFR phosphorylation in the induction of structural remodelling and altered mechanical properties of resistance artery from type 2 diabetic mice. Diabetes Metab Res Rev. 2008 Nov-Dec;24(8):651–656. doi: 10.1002/dmrr.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benter IF, Benboubetra M, Hollins AJ, Yousif MH, Canatan H, Akhtar S. Early inhibition of EGFR signaling prevents diabetes-induced up-regulation of multiple gene pathways in the mesenteric vasculature. Vascul Pharmacol. 2009 Jul 2; doi: 10.1016/j.vph.2009.06.008. [DOI] [PubMed] [Google Scholar]


