Abstract
Background
Patients using cholinesterase inhibitors (ChEIs) have a delay in nursing home (NH) admission compared with those who were not using the medication. There are no long-term studies of the effects of memantine in combination with ChEIs use in Alzheimer disease (AD). This study was conducted to examine the effects of ChEIs and memantine on time to death and time to NH admission.
Methods
Time to NH admission and death was examined in 943 probable AD patients who had at least a 1-year follow-up evaluation. Of these patients, 140 (14.9%) used both ChEIs and memantine, 387 (45.0%) used only ChEIs, and 416 (40.1%) used neither. The mean (SD) follow-up time was 62.3 (35.8) months. The analysis was conducted with multivariable Cox proportional hazard models controlling for critical covariates (ie, age, education level, gender, severity of the dementia, hypertension, diabetes mellitus, heart disease, psychiatric symptoms and use of psychotropic medications).
Results
Compared with those who never used cognitive enhancers, patients who used ChEIs had a significant delay in NH admission (HR: 0.37, 95% CI 0.27 to 0.49); this effect was significantly augmented with the addition of memantine (HR: 0.29, 95% CI 0.11 to 0.72) (memantine+ChEI vs ChEI alone). ChEIs alone, or in combination with memantine had no significant association on time to death.
Conclusions
This observational study revealed that the addition of the NMDA receptor antagonist memantine to the treatment of AD with ChEI significantly altered the treated history of AD by extending time to nursing home admission.
The increased longevity of elderly populations will be accompanied by a growing prevalence of dementia, especially Alzheimer disease (AD).1 2 The annual number of AD cases likely will double over the next 50 years, and its prevalence could quadruple to more than 10 million cases by 2050.3 The progressive nature of AD, leading to severe functional and cognitive deterioration,4 is one of the major determinants of nursing home admission 5 and increased mortality in the older population. 6 The majority of Americans whose cause of death was judged to be dementia (by their death certificate) died in nursing homes,7 and had a larger number of comorbidities compared with non-demented subjects.5 Consequently, the magnitude of this devastating disease has a significant impact on care givers, and healthcare systems and their resources.
One of the most important advances in the field of dementia has been the introduction of symptomatic treatment for AD: cholinesterase inhibitors (ChEIs) (tacrine, donepezil, rivastigmine and galantamine) and N-methyl-d-aspartate (NMDA) receptor modulators (memantine). Clinical trials with ChEIs,8–11 memantine,12 and 1-year open-label studies11 13 14 have shown the efficacy of these compounds. Short-duration combination therapy trials (ChEIs+memantine) have also shown functional and cognitive improvements in AD patients.15 However, AD is a chronic degenerative disease with a clinical syndrome that may extend over many years; only a few studies have explored the long-term response of AD patients to ChEIs.16 17 One study conducted by our group found that AD patients treated with ChEIs had a decreased risk of institutionalisation compared with those who were never exposed to these compounds, with similar survival rates between those with and without treatment.17 This was interpreted as a sign of better functional state and quality of life of the patients, which allowed them to remain at home for a longer period of time.
The introduction of memantine as symptomatic treatment for AD has changed the standard of care of AD patients, from ChEIs alone to combination therapy. However, there are no studies that have explored the long-term effect (>1 year) of the combination treatment. In this observational study, we described the effects of the use of ChEIs alone, or in combination with memantine on time to nursing home (NH) admission and time to death in 943 probable AD patients across a wide range of dementia severity —all the patients had a baseline assessment and at least one follow-up evaluation in our referral research clinic (follow-up time ranged from 0.8 to 18 years).
MATERIAL AND METHODS
All the patients were participants in the Alzheimer’s Research Program (ARP) (1983–1988) or the Alzheimer disease Research Center (ADRC) at the University of Pittsburgh (1985 to present). Subjects received an extensive neuropsychiatric evaluation including medical history and physical examination, neurological history and examination, semistructured psychiatric interview, and neuropsychological assessment.18 The clinical classification of the patients was made initially by a neurologist and a psychiatrist based on their clinical evaluation. The diagnosis was later reviewed by the study team (neurologists, neuroradiologists, neuropsychologists and psychiatrists) at a Consensus Conference. All subjects were examined at the clinic every year, and telephone interviews were conducted every 6 months. For those who did not want to continue participating in the study or were unable to travel to the clinic, annual telephone interviews with the primary care giver were conducted. These interviews included obtaining information on current status (living at home, and date of NH admission or death), hospitalisations, systemic or neurological diseases, and medications. The ARP and ADRC were approved by the local institutional review board.
The inclusion and exclusion criteria for the research centre have been published previously.18 19 Briefly, we excluded subjects with lifetime history of schizophrenia, manic-depressive disorder, or schizoaffective disorder, history of electroconvulsive therapy, alcohol or drug abuse/dependence within 2 years of the onset of the cognitive symptoms, history of cancer within the previous 5 years, and any significant disease or unstable medical condition that could affect the cognitive assessment (eg, chronic renal failure, chronic hepatic disease, severe pulmonary disease). All patients were required to have a reliable informant (or care giver).
Subjects
For the purpose of this report, we examined the data from 943 of the 1539 probable AD20 patients examined at the ADRC of Pittsburgh from April 1983 to December 2004; these were all the patients with probable AD enrolled in the centre who had at least one follow-up evaluation. For those subjects whose initial visit was in 2004, we included those who had their second evaluation through February 2006. The 596 patients who did not continue being followed at the clinic were older and less educated, were more likely to be African Americans, had a longer duration of symptoms (from first cognitive symptom to initial evaluation) and had a worse Mini-Mental State Examination (MMSE),21 Mattis Dementia Rating Scale (MDRS),22 Clinical Dementia Rating (CDR),23 Blessed Dementia Rating Scale for Activities of Daily Living (BDRS for ADL),24 Hamilton Depression Rating Scale (HDRS),25 Hachinski Rating Scale (HRS) for vascular dementia26 and NYU scale for parkinsonism27 scores than those who continued in the study (see table 1).
Table 1.
Demographic, clinical, and genetic characteristics of 1539 probable Alzheimer disease (AD) patients
| Probable AD subjects | ||||
|---|---|---|---|---|
| With at least one annual evaluation | Without annual evaluation | t Test/χ2 | p Value | |
| No of subjects | 943 | 596 | ||
| Mean (SD) age at study entry | 73.1 (8.7) | 74.2 (8.1) | 2.53 | 0.01 |
| Gender: women (%) | 635 (67) | 421 (71) | 1.84 | 0.17 |
| Mean (SD) education level (years) | 12.4 (2.9) | 11.7 (2.9) | −4.69 | <0.001 |
| Race: Caucasians (%) | 888 (94) | 546 (92) | 3.75 | 0.05 |
| Mean (SD) MMSE | 18.2 (5.4) | 15.0 (6.3) | −10.5 | <0.001 |
| Mean (SD) MDRS (n=1160) | 109.5 (19.5) | 102.3 (23.6) | −5.65 | <0.001 |
| Mean (SD) CDR | 1.2 (0.59) | 1.5 (0.78) | 9.39 | <0.001 |
| Mean (SD) BDRS for ADL | 5.2 (3.8) | 6.8 (4.6) | 7.25 | <0.001 |
| Mean (SD) NYU scale for parkinsonism | 9.7 (12.0) | 14.0 (12.9) | 6.16 | <0.001 |
| Mean (SD) HDRS | 6.1 (4.3) | 7.4 (5.3) | 4.70 | <0.001 |
| Mean (SD) HRS | 2.4 (1.7) | 2.7 (2.1) | 3.27 | 0.001 |
| Mean (SD) duration (years) of the disease* | 3.8 (2.3) | 4.4 (2.9) | 11.4 | 0.001 |
| APOE-4 allele (n=1067) (%) | 416 (58) | 201 (57) | 0.16 | 0.68 |
| Hypertension†(%) | 377 (40) | 227 (38) | 0.54 | 0.45 |
| Diabetes mellitus‡ (%) | 63 (7) | 47 (8) | 0.79 | 0.37 |
| Heart disease§ (%) | 132 (14) | 88 (11) | 2.16 | 0.14 |
From symptom onset to initial evaluation.
Told by doctor.
Told by doctor and using hypoglycaemic agents.
History of congestive heart failure, angina, or coronary by pass grafting/coronary angioplasty.
BDRS for ADL, Blessed Dementia Rating Scale for Activities of Daily Living; CDR, Clinical Dementia Rating; HDRS, Hamilton Depression Rating Scale; HRS, Hachinski Rating Scale; MDRS, Mattis Dementia Rating Scale; MMSE, Mini-Mental State examination; NYU, New York University.
Psychiatric examination
The evaluations were conducted by geriatric psychiatrists using a semistructured interview,28 and the Consortium to Establish a Registry for Alzheimer disease (CERAD) Behavioral Rating Scale.29 The HDRS and the BDRS interviews were completed by the psychiatrist on the basis of data from each patient and primary care giver. A total of six psychiatrists were involved in the psychiatric diagnosis of these patients from 1983 to 2004. The reliability of each item of the CERAD Behavioral Rating Scale ranged from kappa 0.60 to 0.85 (substantial to near perfect agreement) among psychiatrists.18
Patients were evaluated by a psychiatrist at their annual visit, and the psychiatric diagnosis was made at the Consensus Conference among the ADRC psychiatrists. For the purpose of this analysis, symptoms were recorded as either present or absent. All patients diagnosed as having depression met the DSM-IV criteria for major depressive disorder (in remission, in partial remission, active, recurrent or single episode). Agitation required the presence of signs of emotional distress, with or without increased motor activity. Aggression occurred when patients displayed verbal or physical aggressive behaviour. Delusions were defined in accordance with the DSM- IV criteria,30 and were distinguished from confabulations, disorientation and amnesia by requiring that the false beliefs persisted in spite of evidence to the contrary. Hallucinations were accepted as present if the patient spontaneously reported a sensory perception with no concomitant external stimulus. The diagnostic criteria for major depression have remained stable during the last 20 years,30–32 and the diagnosis of agitation, aggression and psychosis has not been modified in our clinic since its inception.
Neurological examination
The neurological exam included a semistructured interview with the care givers that examined the effects of cognition on the most relevant instrumental and performance in activities of daily living (eg, household chores, driving, job performance, handling finances, hygiene, continence), as well as the actual exam. The examiner also completed the NYU Scale for parkinsonism and the HRS.
Neuropsychological evaluation
Between 1983 and 1988, all of the subjects completed extensive neuropsychological testing designed, in part, to help in the development of an understanding of the differing patterns of presentation and progression in AD and related dementias.33 Beginning in 1988, the battery was shortened and streamlined as the clinical course of AD became better understood. The neuropsychological evaluation included two global measures of cognition: the MMSE (from 1983 to 2004) and the MDRS (from 1983 to 2002). The neuropsychological battery used after 1988 included measures of: Memory—immediate and delayed recall of a word list (from the Alzheimer’s Disease Assessment Scale),34 and of the modified Rey–Osterreith figure;35 Language—Modified Boston Naming Test,36 and verbal (FAS)37 and category fluency33 tests; Visuospatial/Visuoconstructional—Visual discrimination test,38 and copy of the modified Rey–Osterreith;35 and Attention/executive functions—Digit spans,39 and Trail-making test.40
Statistical analysis
The study data were maintained and managed using SPSS for Windows (v12–v15); the analysis was completed using SAS (SAS Institute, Cary, North Carolina). We used t tests to compare continuous variables, and χ2 to compare categorical variables.
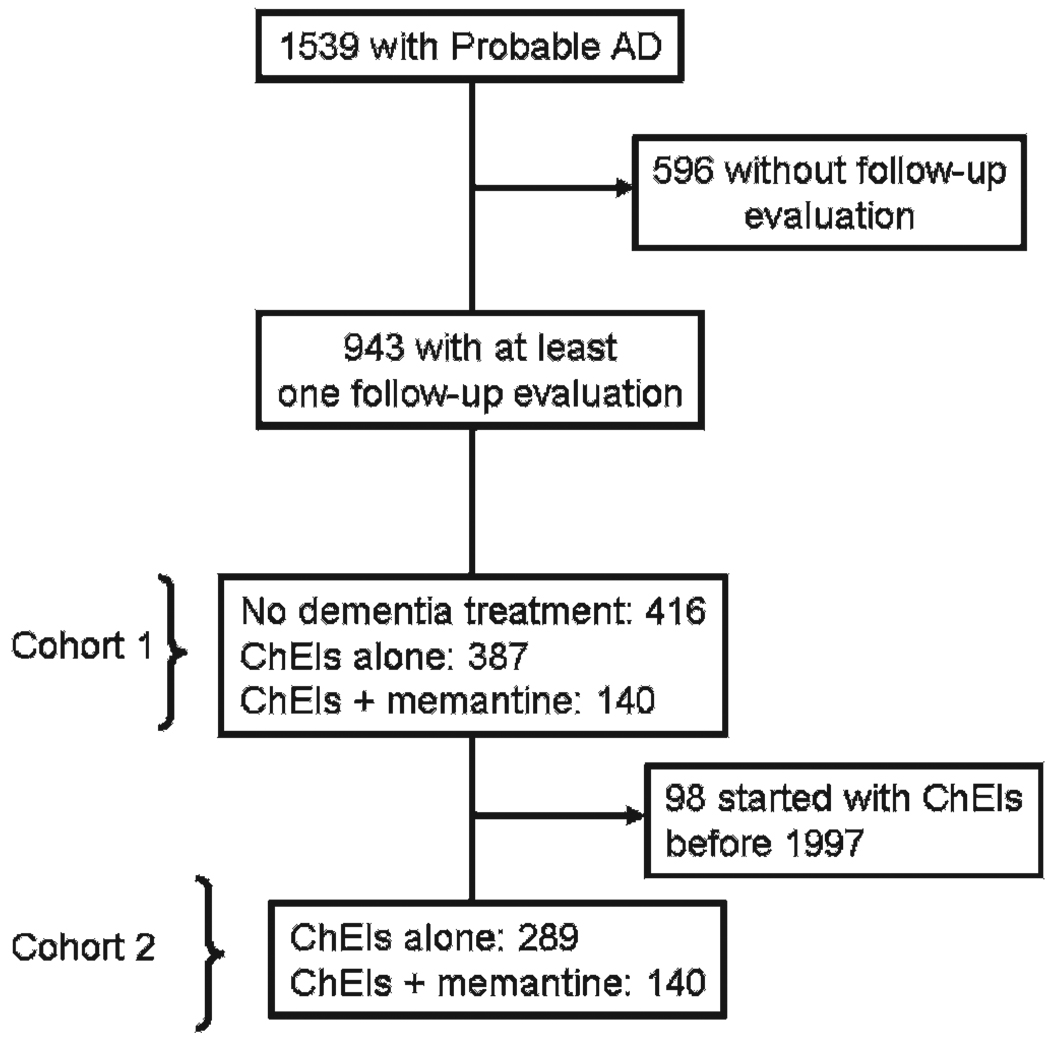
Of the 1539 subjects in the entire cohort, three used memantine alone and were excluded from the analyses. Consequently, 1536 were entered in the analyses; 144 (9.4%) used memantine+ChEIs, 526 (34.2%) used ChEIs only, and 866 (56.4%) used neither. However, when we limit the cohort to those who had at least 1 year of follow-up, the numbers are 140 (14.9%), 387 (45.0%), 416 (40.1%), respectively, yielding a total of 943 patients, which we refer to as Cohort 1 (see fig 1). For all the patients who took medication, the mean time on ChEIs was 38.4 (22.2) months, and on memantine 19.2 (9.6) months. The first patient on ChEIs began taking the medication on 9 July 1990 (as part of a controlled trial). The patient with the longest time in the ADRC to receive ChEI was entered in the study on 4 January 1984 and began taking medication on 9 October 1997.
Figure 1.
Flow chart of the patients examined from 1983 to 2004, and the number of patients in each cohort. AD, Alzheimer disease; ChEIs, cholinesterase inhibitors.
The first use of memantine was recorded for a patient on 28 January 2002 (this patient was enrolled into the study on 29 July 1997). Therefore, we had to create a second cohort of patients to account for any differences that could be related to the year of entry into the study. A subcohort of 443 patients was identified who enrolled on or after 29 July 1997, and had at least 1 year of follow-up. However, 14 of these patients did not use any medication, resulting in 429 patients available for analysis; 140 patients used memantine+ChEI, and 289 used only ChEIs. We refer to this subgroup of 429 patients as Cohort 2. Twenty-four subjects used memantine before its FDA approval in the USA; three participated in the memantine trial, and 21 purchased their medication overseas.
For the survival analysis, Cox proportional hazard models treated medication use as a time-dependent covariate. In addition, age, education level, baseline MMSE scores, duration of symptoms, APOE-4 allele, hypertension, diabetes mellitus, heart disease, major depression, psychosis, aggression, agitation and psychiatric medication (antidepressants, antipsychotic, sedatives/anxiolytics and hypnotics) were considered as time-dependent covariates in the fully adjusted model. The time to NH was also entered as a covariate in the analysis of time to death.
We used a stepwise method for variable selection in the proportional hazard model to identify covariates for both end-points. In order to account for the selection bias due to the inclusion of the subjects with a 1-year follow-up, we repeated the analysis assigning each subject a weight equal to the reciprocal of the probability of having a 1-year follow-up, which is estimated using the covariates. The results from weighted and unweighted analysis were similar, so only the unweighted results are shown.
RESULTS
Table 2 shows the baseline characteristics of the subjects in Cohort 1. The patients who were never exposed to any dementia treatment had a lower prevalence of hypertension, heart disease, lower MMSE and MDRS scores, and a shorter overall follow-up time compared with the other two groups. The patients using ChEIs only were older, and had higher HRS scores than the other two groups. The patients using ChEIs+memantine were better educated and had a lower NYU scale score. The three groups of patients were different from each other in terms of BDRS-ADL and frequency of deceased and institutionalised patients.
Table 2.
Demographic characteristics by dementia medication of the subjects who had at least 1 year of follow-up (Cohort 1)
| No medication | ChEI | ChEI+memantine | F ratio/χ2 | p Value | |
|---|---|---|---|---|---|
| No of subjects | 416 | 387 | 140 | ||
| Mean (SD) overall follow-up (months), range | 62.3 (35.8), 9.3 to 202.0 | 44.6 (31.0)*, 9.4 to 216.2 | 40.4 (19.7)†, 10.8 to 90.7 | 41.4 (a) | <0.001 |
| Mean (SD) age at study entry | 71.8 (8.1) | 74.6 (8.5) | 72.8 (10.2) | 10.3 (b) | <0.001 |
| Gender: women (%) | 285 (68.5) | 261 (67) | 89 (64) | 1.16 | 0.55 |
| Mean (SD) education level | 12.1 (2.9) | 12.4 (2.9) | 13.3 (3.1) | 9.14 (c) | <0.001 |
| Race: Caucasians (%) | 392 (94) | 364 (94) | 132 (94) | 0.015 | 0.99 |
| Mean (SD) MMSE | 17.4 (5.6) | 18.9 (5.1) | 18.6 (5.1) | 8.10 (a) | <0.001 |
| Mean (SD) MDRS (n=650) | 105.4 (22.4) | 113.3 (15.4) | 114.0 (15.3) | 15.0 (a) | <0.001 |
| Mean (SD) CDR | 1.2 (0.59) | 1.1 (0.58) | 1.1 (0.62) | 2.88 | 0.06 |
| Mean (SD) BDRS for ADL | 6.4 (4.1) | 4.6 (3.3) | 3.4 (2.7) | 42.8 (d) | <0.001 |
| Mean (SD) NYU scale for parkinsonism | 10.3 (12.0) | 9.8 (12.4) | 4.9 (8.0) | 4.17 (c) | 0.01 |
| Mean (SD) HDRS | 6.1 (3.9) | 6.1 (4.3) | 6.1 (5.6) | 0.001 | 0.99 |
| Mean (SD) HRS | 2.2 (1.6) | 2.7 (1.9) | 2.1 (1.3) | 9.51 (b) | <0.001 |
| Mean (SD) duration (years) of the disease‡ | 3.8 (2.4) | 3.9 (2.3) | 3.6 (1.8) | 0.77 | 0.46 |
| APOE-4 allele (n=714) (%) | 120 (55) | 222 (60) | 74 (58) | 1.47 | 0.47 |
| Hypertension§ (%) | 109 (26) | 201 (52) | 67 (48) | 59.5 (a) | <0.001 |
| Diabetes mellitus¶ (%) | 27 (6.5) | 30 (8) | 6 (4) | 2.02 | 0.36 |
| Heart disease**(%) | 35 (8) | 66 (17) | 31 (22) | 21.4 (a) | <0.001 |
| Deceased (%) | 250 (60) | 126 (33) | 20 (14) | 114.2 (d) | 0.001 |
| Nursing home admission (%) | 203 (49) | 83 (21) | 7 (5) | 122.7 (d) | <0.001 |
ANOVA/χ2 a, no medication different from the other two groups; b, cholinesterase inhibitor (ChEI) different from the other two groups; c, ChEI+memantine different from the other two groups; d, the three groups are different from each other.
Mean (SD) time on cholinesterase inhibitors: 38.4 (22.4) months
Mean (SD) time on memantine: 19.2 (9.6) months.
From symptom onset to initial evaluation.
Told by doctor.
Told by doctor and using hypoglycaemic agents.
History of congestive heart failure, angina, or coronary by pass grafting/coronary angioplasty.
BDRS for ADL, Blessed Dementia Rating Scale for Activities of Daily Living; CDR, Clinical Dementia Rating; HDRS, Hamilton Depression Rating Scale; HRS, Hachinski Rating Scale; MDRS, Mattis Dementia Rating Scale; MMSE, Mini-Mental State examination; NYU, New York University.
Table 3 shows the total number of psychiatric symptoms in the three groups in Cohort 1. Patients who never used medication were less likely to have had major depression, psychosis or agitation compared with those who did use ChEIs and/or memantine. Subjects using ChEIs only had a greater frequency of aggression compared with those who never used cognitive-enhancing medication. Patients who were never exposed to dementia medication used more antipsychotics, and fewer antidepressants, multivitamins, vitamin E, aspirin or estrogens (women only) than the medicated patients. However, they used more tricyclics/MAOI and fewer SSRI/third-generation antidepressants than the other two groups. Subjects taking ChEIs+memantine used fewer sedatives/anxiolytics and hypnotics than the other two groups.
Table 3.
Psychiatric symptoms and medication use at any time (baseline+follow-up) in subjects who had at least 1 year of follow-up (Cohort 1)
| No medication | ChEI | ChEI+memantine | χ2 | p Value | |
|---|---|---|---|---|---|
| No of subjects | 416 | 387 | 140 | ||
| Psychiatric symptoms | |||||
| Major depression (%) | 52 (12.5) | 83 (21) | 28 (20) | 12.0 (a) | 0.002 |
| Psychosis (%) | 207 (50) | 255 (66) | 88 (63) | 22.4 (a) | <0.001 |
| Aggression (%) | 92 (22) | 115 (30) | 39 (28) | 6.41 (c) | 0.04 |
| Agitation (%) | 249 (60) | 327 (85) | 123 (88.5) | 82.1 (a) | <0.001 |
| Psychiatric medication | |||||
| Antidepressants (%) | 98 (24) | 200 (52) | 71 (51) | 75.8 (a) | <0.001 |
| Tricyclics/MAOI (%) | 37 (9) | 4 (1) | 2 (1) | 134.4 (a) | <0.001 |
| SSRI/third generation (%) | 59 (14) | 186 (48) | 69 (46) | ||
| Antipsychotics (%) | 142 (34) | 75 (19) | 23 (15) | 30.0 (a) | <0.001 |
| Typical (%) | 129 (31) | 10 (3) | 0 (0) | 181.7 (a) | <0.001 |
| Atypical (%) | 13 (3) | 65 (17) | 23 (16) | ||
| Sedatives, anxiolytics and hypnotics (%) | 70 (17) | 72 (18) | 11 (8) | 8.93 (d) | 0.01 |
| Other treatments | |||||
| Multivitamins (%) | 37 (9) | 169 (44) | 68 (49) | 147.9 (a) | <0.001 |
| Vitamin E≥400 IU (%) | 15 (4) | 105 (27) | 53 (38) | 116.0 (b) | <0.001 |
| Lipid-lowering agents (%) | 1 (1) | 75 (19) | 59 (42) | 163.6 (b) | <0.001 |
| ASA ≥81 mg (%) | 85 (20) | 121 (31) | 40 (28) | 12.7 (a) | 0.002 |
| Estrogens (n=635) (%) | 23 (8) | 55 (21) | 16 (18) | 19.0 (a) | <0.001 |
χ2 a, no medication different from the other two groups; b, the three groups are different from each other; c, cholinesterase inhibitor (ChEI) alone different from no medication; d, ChEI+memantine different from the other two groups.
ASA, acid acetylsalicylic; MAOI, monoamine oxidase inhibitors; SSRI, selective serotonin-reuptake inhibitors.
Table 4 shows the baseline characteristics of Cohort 2. Patients using ChEIs alone were older and had a lower education level and higher scores on the BDRS, NYU scale and the HRS compared with those using ChEIs+memantine. A greater proportion of the patients using ChEIs alone died or were admitted to nursing homes during follow-up than those with combination therapy. Table 5 shows the psychiatric symptoms and psychotropic and other medication use. A greater proportion of the patients using ChEIs alone also used sedatives/anxiolytics, hypnotics and aspirin compared with the combination therapy group. A greater proportion of patients on ChEIs+memantine used more lipid-lowering agents than the group taking ChEIs alone.
Table 4.
Demographic characteristics by dementia medication of the subjects who had at least 1 year of follow-up (Cohort 2)
| ChEI | ChEI+memantine | F ratio/χ2 | p Value | |
|---|---|---|---|---|
| No of subjects | 289 | 140 | ||
| Mean (SD) overall follow-up (months), range | 35.9 (19.7), 9.4 to 102.0 | 40.4 (19.7)*, 10.8 to 90.7 | −2.20 | 0.02 |
| Mean (SD) age at study entry | 75.6 (8.3) | 72.8 (10.2) | 3.80 | 0.002 |
| Gender: women (%) | 198 (68.5) | 89 (64) | 1.04 | 0.30 |
| Mean (SD) education level | 12.4 (2.8) | 13.3 (3.1) | −2.93 | 0.004 |
| Race: Caucasians (%) | 268 (93) | 132 (94) | 0.36 | 0.54 |
| Mean (SD) MMSE | 18.7 (5.1) | 18.6 (5.1) | 0.30 | 0.75 |
| Mean (SD) MDRS (n=650) | 113.4 (15.9) | 114.0 (15.3) | −1.11 | 0.26 |
| Mean (SD) CDR | 1.2 (0.61) | 1.1 (0.62) | 0.89 | 0.37 |
| Mean (SD) BDRS for ADL | 4.6 (3.4) | 3.4 (2.7) | 3.51 | <0.001 |
| Mean (SD) NYU scale for parkinsonism | 9.6 (12.4) | 4.9 (8.0) | 2.56 | 0.01 |
| Mean (SD) HDRS | 6.3 (4.4) | 6.1 (5.6) | 0.40 | 0.68 |
| Mean (SD) HRS | 2.8 (2.0) | 2.1 (1.3) | 3.54 | <0.001 |
| Mean (SD) duration (years) of the disease† | 3.8 (2.3) | 3.6 (1.8) | 0.91 | 0.36 |
| APOE-4 allele (n=401) (%) | 161 (59) | 74 (58) | 0.009 | 0.92 |
| Hypertension‡ (%) | 163 (56) | 67 (48) | 2.76 | 0.09 |
| Diabetes mellitus§ (%) | 26 (9) | 6 (4) | 3.07 | 0.08 |
| Heart disease¶ (%) | 55 (19) | 31 (22) | 0.57 | 0.45 |
| Deceased (%) | 80 (28) | 20 (14) | 9.46 | 0.002 |
| Nursing home admission (%) | 51 (18) | 7 (5) | 12.9 | <0.001 |
Mean (SD) time on memantine: 19.2 (9.6) months.
From symptom onset to initial evaluation.
Told by doctor.
Told by doctor and using hypoglycaemic agents.
History of congestive heart failure, angina, or coronary by pass grafting/coronary angioplasty.
BDRS for ADL, Blessed Dementia Rating Scale for Activities of Daily Living; CDR, Clinical Dementia Rating; ChEI, cholinesterase inhibitor; HDRS, Hamilton Depression Rating Scale; HRS, Hachinski Rating Scale; MDRS, Mattis Dementia Rating Scale; MMSE, Mini-Mental State examination; NYU, New York University.
Table 5.
Psychiatric symptoms and medication use at any time (baseline+follow-up) in subjects who had at least 1 year of follow-up (Cohort 2)
| ChEI | ChEI+memantine | χ2 | p Value | |
|---|---|---|---|---|
| No of subjects | 289 | 140 | ||
| Psychiatric symptoms | ||||
| Major depression (%) | 60 (21) | 28 (20) | 0.12 | 0.72 |
| Psychosis (%) | 194 (67) | 88 (63) | 0.76 | 0.38 |
| Aggression (%) | 89 (31) | 39 (28) | 0.38 | 0.53 |
| Agitation (%) | 249 (86) | 123 (88.5) | 0.25 | 0.61 |
| Psychiatric medication | ||||
| Antidepressants (%) | 156 (54) | 71 (51) | 0.40 | 0.52 |
| Tricyclics/MAOI (%) | 4 (1) | 2 (1) | 5.23 | 0.26 |
| SSRI/third generation (%) | 152 (53) | 69 (46) | ||
| Antipsychotics (%) | 56 (19) | 23 (15) | 0.54 | 0.46 |
| Typical (%) | 9 (3) | 0 | 5.25 | 0.26 |
| Atypical (%) | 47 (16) | 23 (16) | ||
| Sedatives, anxiolytics and hypnotics (%) | 52 (18) | 11 (8) | 7.73 | 0.005 |
| Other treatments | ||||
| Multivitamins (%) | 135 (47) | 68 (49) | 0.13 | 0.71 |
| Vitamin E≥400 IU (%) | 168 (58) | 53 (38) | 0.87 | 0.35 |
| Lipid-lowering agents (%) | 71 (25) | 59 (42) | 13.7 | <0.001 |
| ASA ≥81 mg (%) | 95 (33) | 40 (28) | 0.20 | 0.002 |
| Estrogens (n=635) (%) | 35 (18) | 16 (18) | 0.04 | 0.83 |
ASA, acid acetylsalicylic; ChEI, cholinesterase inhibitor; MAOI, monoamine oxidase inhibitors; SSRI, selective serotonin-reuptake inhibitors.
Survival analysis
The principal finding from this analysis is that the addition of memantine to a regime including ChEIs further delays nursing home admission from that time afforded by ChEIs alone. Neither single nor combination therapy affected time to death.
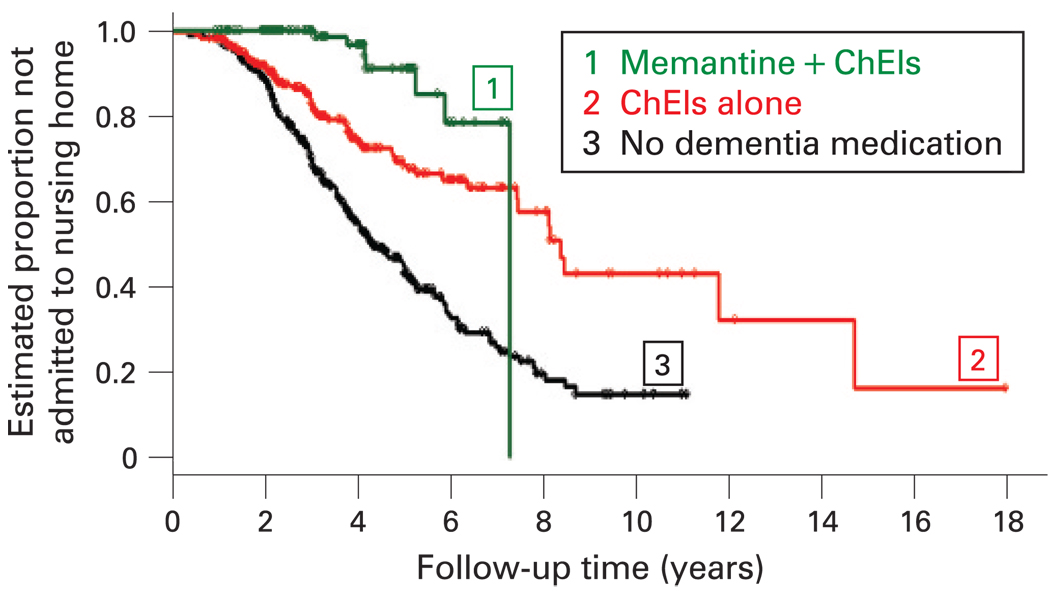
The analysis of Cohort 1 used the untreated patients as the reference group (see fig 2). The adjusted analysis showed that patients on ChEIs only (Relative Hazard (RH): 0.37 (95% CI 0.27 to 0.49)) and those taking both (ChEIs+memantine) (RH: 0.29 (95% CI 0.11 to 0.72)) were less likely to be admitted to a nursing home during follow-up than the untreated patients. The RH of ChEIs+memantine versus ChEI was 0.29 (95% CI 0.11 to 0.72), showing that the risk of NH admission in the combination therapy group was reduced by a factor of 3.4 relative to the group taking only ChEIs. There was no association with medication use and time to death; ChEIs versus untreated (RH: 1.1 (95%CI 0.88 to 1.38), and ChEIs+memantine versus untreated (RH: 0.92 (95% CI 0.56 to 1.49)).
Figure 2.
Time to nursing home admission in Cohort 1. ChEIs, cholinesterase inhibitors.
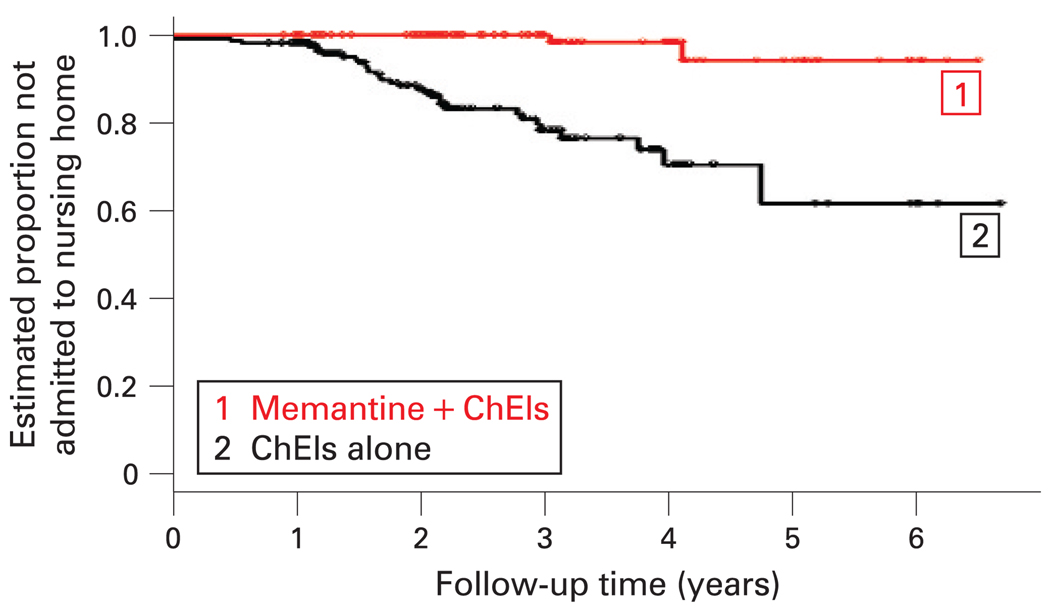
The analysis of Cohort 2 used the patients on ChEIs monotherapy as the reference group (see fig 3). In the fully adjusted model, patients using ChEIs+memantine were more than seven times less likely to go to a nursing home (RH: 0.13 (95% CI 0.03 to 0.56)), and no association was found with time to death during the observation period. In Cohort 2, nursing home admission was a predictor of time to death (RH: 1.94 (95% CI 1.17 to 3.24)).
Figure 3.
Time to nursing home admission in Cohort 2. ChEIs, cholinesterase inhibitors.
DISCUSSION
This observational study is the first to report that the addition of an NMDA receptor modulator to a regimen of ChEIs augments their effects on the treated history of AD. The addition of memantine to the ChEIs regimen provides benefits above those of ChEI alone by extending the time to NH admission but does so without affecting the time to death.17
Although all of the medicated patients had a decreased risk of NH admission compared with untreated AD subjects, there was more than a threefold decrease in risk in the ChEIs+memantine group compared with ChEIs alone. These findings extend the results of the short-term trials (ie, 12–24 weeks) conducted in moderate to severe AD patients who showed additional cognitive and functional benefits in subjects receiving combination therapy.41 Other factors (ie, psychiatric symptoms) that are known to increase the likelihood of nursing home admission appear to be less severe in patients with combination therapy.42 All of this suggests that the patients using combination therapy were more easily cared for by their care givers, and consequently remained at home for a longer period of time.
Previous studies of both pharmacological and non-pharmacological treatment in AD can be interpreted in the context of compression of morbidity.43 While therapy may improve cognition and behaviour, and result in a longer time at home prior to residential care, none of the studies reported extended survival.17 We propose that the augmentation of the effects of ChEIs by memantine may be due to changes in the communications skills of the doubly treated patients. This suggestion is consistent with previous observations of improved cognition, function and behaviour in institutionalised patients treated with ChEIs44 45 or memantine.46 Language, praxis and visuospatial functions, as well as specific basic activities of daily living (ie, getting dressed, bowel/bladder control), improved with ChEIs in a nursing home population.45 Therefore, it is possible that combination therapy may enhance these effects, allowing subjects to maintain better communication skills, and enhancing physical health prior to the institutionalisation period.
This study has several strengths. First, it includes a large cohort of AD patients across the whole spectrum of dementia severity that was characterised carefully using standardised assessments. Longitudinal follow-up was intensive and included interim telephone contact with the patients’ care givers. Second, the detailed clinical information available from each patient allowed us to control for the major determinants of institutionalisation (eg, psychosis, disruptive behaviours, antipsychotic use)4 47–52 and death (eg, sedative use).4 53 Third, the requirement that each patient have a reliable care giver maximised the likelihood that medication compliance was optimal, and that medical management was optimised. This latter point, however, also raises the possibility that patients enrolled in this study may overestimate the benefits of ChEIs and memantine in that medical management is likely better (due to persistent contact with the ADRC staff) than that received by the “average” AD patient without access to such expertise.
The most clinically meaningful effects of the medications currently used to treat AD occur with long follow-up intervals, usually 1 year or more. Unfortunately, these critical outcomes, time to institutionalisation, death or rate of change cannot be reasonably evaluated in the standard 24- or 48-week clinical trial. Further, because ChEIs are the standard of care in AD, placebo-controlled trials are ethically difficult. Thus, observational studies such as this one may be one of the only ways to evaluate the long-term effects of these medications. It should be noted that our findings cannot be easily compared with those of the AD 2000 Collaborative Study.54 That study recruited only individuals with AD and vascular dementia whose treating physicians were uncertain about the benefits of the treatment. Furthermore, unlike even the untreated subjects in our study, the majority of the critical events in the AD 2000 study occurred within the first 48 weeks of the study (58% of the NH admissions and 75% of the deaths), suggesting that those patients had multiple and severe comorbidities.
Our cohort reflects the multiple transformations in the care and treatment of AD patients over the last 20 years, as well as the type of patients recruited through referral clinics. The ADRC initially enrolled AD patients with few comorbidities, since the Centre was focused on understanding the clinical and biological characterisation of the syndrome. This explains the relatively few cases of heart disease and hypertension in subjects who were never exposed to dementia medication. At the same time, there was a change in the treatment of disruptive behaviours (eg, agitation, aggression) from using antipsychotic medications to using antidepressants.55 Similarly, the use of lipid-lowering agents became more widespread when statins were introduced in the mid-1990s. The use of time-dependent covariates took into account the majority of the factors that were associated with progression in AD. Nevertheless, this is an observational study that can be influenced by the temporal variation of the factors that can influence survival. In order to address this issue, we created Cohort 2, which included only the patients entered in the study after 1997, the entry date of the first patient treated with memantine. Even in this subset of patients, we were able to detect significant protective effects of combination therapy, above and beyond those conferred by ChEIs alone.
Although there are two types of medication for the treatment of AD, there are no guidelines for the use of dual therapy, and their use may vary from one country to another. Combination therapy is widely available to all AD patients in the US, but there is heterogeneity among other countries in the use of this strategy. Our findings suggest that memantine can augment the effects of ChEIs on the treated history of AD. Both ChEIs and memantine are symptomatic treatments for AD, and they seem to achieve their purpose of slowing down the apparent clinical progression of the disease, and their benefits are most evident over the long term.
Acknowledgments
Funding: This study was fully supported by grants AG03705, AG05133, AG16976, AG20098 and AG027224 from the National Institute on Aging, and by VISN 4 Mental Illness Research Education and Clinical Center (MIRECC), VA Pittsburgh Health Care System, Pittsburgh, Pennsylvania.
Footnotes
To order reprints of this article go to: http://jnnp.bmj.com/cgi/reprintform
Competing interests: None.
Ethics approval: Ethics approval was provided by the University of Pittsburgh Institutional Review Board.
Patient consent: Obtained.
REFERENCES
- 1.Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer’s disease in the US population. Prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology. 1998;51:728–733. doi: 10.1212/wnl.51.3.728. [DOI] [PubMed] [Google Scholar]
- 3.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez OL, Wisniewski SR, Becker JT, et al. Psychiatric medication and abnormal behavior as predictors of progression in probable Alzheimer’s disease. Arch Neurol. 1999;56:1266–1272. doi: 10.1001/archneur.56.10.1266. [DOI] [PubMed] [Google Scholar]
- 5.Eaker ED, Vierkant RA, Mickel SF. Predictors of nursing home admission and/or death in incident Alzheimer’s disease and other dementia cases compared to controls: a population-based study. J Clin Epidemiol. 2002;55:462–468. doi: 10.1016/s0895-4356(01)00498-x. [DOI] [PubMed] [Google Scholar]
- 6.Fitzpatrick A, Kuller L, Lopez OL, et al. Survival following dementia onset: Alzheime’s disease and vascular dementia. J Neurol Sci. 2005;229–230:43–49. doi: 10.1016/j.jns.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell SL, Teno JM, Miller SC, et al. A national study of the location of death for older persons with dementia. J Am Geriatr Soc. 2005;53:299–305. doi: 10.1111/j.1532-5415.2005.53118.x. [DOI] [PubMed] [Google Scholar]
- 8.Farlow M, Gracon SI, Hershey LA, et al. A controlled trial of tacrine in Alzheimer’s disease. The Tacrine Study Group. JAMA. 1992;268:2523–2529. [PubMed] [Google Scholar]
- 9.Rogers SL, Friedhoff LT. The efficacy and safety of donepezil in patients with Alzheimer’s disease: results of a US multicentre, randomized, double-blind, placebo-controlled trual. The Donepezil Study Group. Dementia. 1996;7:293–303. doi: 10.1159/000106895. [DOI] [PubMed] [Google Scholar]
- 10.Rosler M, Anand R, Cicin-Sain A, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ. 1999;318:633–638. doi: 10.1136/bmj.318.7184.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raskind MA, Peskind ER, Wessel T, et al. Galantamine in AD. A six-month, randomized, placebo-controlled trial with a six-month extension. Neurology. 2000;54:2261–2268. doi: 10.1212/wnl.54.12.2261. [DOI] [PubMed] [Google Scholar]
- 12.Reisberg B, Doody R, Stoffler A, et al. A 24-week open-label extension study of memantine in moderate to severe Alzheimer disease. Arch Neurol. 2006:49–54. doi: 10.1001/archneur.63.1.49. [DOI] [PubMed] [Google Scholar]
- 13.Doody RS, Geldmacher DS, Gordon B, et al. Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Arch Neurol. 2001;58:427–433. doi: 10.1001/archneur.58.3.427. [DOI] [PubMed] [Google Scholar]
- 14.Farlow M, Avand R, Messina J, et al. A 52-week study of the efficacy of rivastigmine in patients with mild to moderately severe Alzheimer’s disease. Eur Neurol. 2000;44:236–241. doi: 10.1159/000008243. [DOI] [PubMed] [Google Scholar]
- 15.Areosa SA, Sherriff F. Memantine for dementia. Cochrane Database Syst Rev. 2003;(3) doi: 10.1002/14651858.CD003154. CD003154. [DOI] [PubMed] [Google Scholar]
- 16.Geldmacher DS, Provenzano G, McRae T, et al. Donepezil is associated with delayed nursing home placement in patients with Alzheimer’s disease. J Am Geriatr Soc. 2003;51:937–944. doi: 10.1046/j.1365-2389.2003.51306.x. [DOI] [PubMed] [Google Scholar]
- 17.Lopez OL, Becker JT, Wisniewski S, et al. Cholinesterase inhibitor treatment alters the natural history of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2002;72:310. doi: 10.1136/jnnp.72.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez OL, Becker JT, Klunk W, et al. Research evaluation and diagnosis of possible Alzheimer’s disease over the last two decades: II. Neurology. 2000;55:1863–1869. doi: 10.1212/wnl.55.12.1863. [DOI] [PubMed] [Google Scholar]
- 19.Lopez OL, Becker JT, Klunk W, et al. Research evaluation and diagnosis of probable Alzheimer’s disease over the last two decades: I. Neurology. 2000;55:1854–1862. doi: 10.1212/wnl.55.12.1854. [DOI] [PubMed] [Google Scholar]
- 20.McKhann G, Drachman DA, Folstein MF, et al. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method grading the cognitive state of patients for the clinician. Psychiat Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Mattis S. Mental status examination for organic mental syndrome in the elderly patient. In: Bellak L, Karuso TB, editors. Geriatric psychiatry. New York: Grune & Stratton; 1976. pp. 77–121. [Google Scholar]
- 23.Hughes CP, Berg L, Danzinger WL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 24.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and senile changes in the cerebral white matter of elderly subjects. Br J Psychiat. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hachinski VC, Iliff LD, Zihka E, et al. Cerebral blood flow in dementia. Arch Neurol. 1975;32:632–637. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- 27.Hoehm M, Yahr M. Parkinsonism: Onset, progression, and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 28.Mezzich JE, Dow JT, Cottman GA. Developing an information system for a comprehensive psychiatric institute, I. Principles, design, and organization. Behav Res Meth Instrument. 1981;13:459–463. [Google Scholar]
- 29.Tariot PN, Mack JL, Patterson MB, et al. The behavior rating scale for dementia of the Consortium to Establish a Registry for Alzheimer’s Disease. Am J Psychiatry. 1995;152:1349–1357. doi: 10.1176/ajp.152.9.1349. [DOI] [PubMed] [Google Scholar]
- 30.APA. DSM-IV: Diagnostic and statistic manual of mental disorders. 4th edn. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 31.APA. Diagnostic and statistical manual of mental disorders. 3rd edn. New York: APA; 1980. [Google Scholar]
- 32.APA. Diagnostic and statistical manual on mental disorders—Revised (DSM-III-R) 3rd edn. Washington, DC: American Psychiatric Press; 1987. [Google Scholar]
- 33.Becker JT, Boller F, Lopez OL, et al. The natural history of Alzheimer’s disease: Description of study cohort and accuracy of diagnosis. Arch Neurol. 1994;51:585–594. doi: 10.1001/archneur.1994.00540180063015. [DOI] [PubMed] [Google Scholar]
- 34.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 35.Saxton JA, Becker JT, Wisniewski S. The ROCF and dementia. In: Knight JA, editor. The handbook of Rey–Osterrieth complex figure usage: clinical and research applications. Lutz, FL: Psychological Assessment Resources; 2003. pp. 569–582. [Google Scholar]
- 36.Huff MJ, Mack L, Mahlmann J, et al. A comparison of lexical-semantic impairments in left-hemisphere stroke and Alzheimer’s disease. Brain Lang. 1988;34:262–268. doi: 10.1016/0093-934x(88)90138-1. [DOI] [PubMed] [Google Scholar]
- 37.Benton AL. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- 38.Benton AL, Van Allen MW. Impairment in facial recognition in patients with cerebral disease. Cortex. 1968;4:344–358. [PubMed] [Google Scholar]
- 39.Wechsler D. Wechsler adult intelligence scale—revised. New York: The Psychological Corporation; 1981. [Google Scholar]
- 40.Reitan RM. Validity of the Trail Making test as an indicator of organic brain damage. Percep Mot Skills. 1958;8:271–276. [Google Scholar]
- 41.Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer’s disease already receiving donepezil. JAMA. 2004;291:317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 42.Cummings JL, Schneider E, Tariot PN, et al. Behavioral effects of memantine in Alzheimer disease patients receiving donepezil treatment. Neurology. 2006;67:57–63. doi: 10.1212/01.wnl.0000223333.42368.f1. [DOI] [PubMed] [Google Scholar]
- 43.Becker JT, Mestre LT, Ziolko S, et al. Gene–environment interactions with cognition in late life and compression of morbidity. Am J Psychiatry. 2007;64:849–852. doi: 10.1176/ajp.2007.164.6.849. [DOI] [PubMed] [Google Scholar]
- 44.Cummings JL, Koumaras B, Chen M, et al. Effects of rivastigmine treatment on the neuropsychiatric and behavioral disturbances of nursing home residents with moderate to severe probable Alzheimer’s disease: a 26-week, multicenter, open-label study. Am J Geriatr Pharmacother. 2005;3:137–148. doi: 10.1016/s1543-5946(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 45.Winblad B, Kilander L, Eriksson S, et al. Donepezil in patients with severe Alzheimer’s disease: double blind, parallel-group, placebo-controlled study. Lancet. 2006;367:1057–1065. doi: 10.1016/S0140-6736(06)68350-5. [DOI] [PubMed] [Google Scholar]
- 46.Winblad B, Poritis N. Memantine in severe dementia: results of the 9M-Best Study (Benefit and efficacy in severly demented patients during treatment with memantine) Int J Geriatr Psychiatry. 1999;14:135–146. doi: 10.1002/(sici)1099-1166(199902)14:2<135::aid-gps906>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 47.Hamel M, Gold DP, Andres D, et al. Predictors and consequences of aggressive behavior by community-based dementia patients. Gerontologist. 1990;30:206–211. doi: 10.1093/geront/30.2.206. [DOI] [PubMed] [Google Scholar]
- 48.Ryden MB. Aggressive behavior in persons with dementia who live in the community. Alzheimer Dis Assoc Disord. 1988;2:342–355. doi: 10.1097/00002093-198802040-00003. [DOI] [PubMed] [Google Scholar]
- 49.Lieberman MA, Kramer JH. Factors affecting decisions to institutionalize demented elderly. Gerontologist. 1991;31:371–374. doi: 10.1093/geront/31.3.371. [DOI] [PubMed] [Google Scholar]
- 50.Gilley DW, Bienias IL, Wilson RS, et al. Influence of behavioral symptoms on rates of institutionalization for persons with Alzheimer’s disease. Psychol Med. 2004;34:1129–1135. doi: 10.1017/s0033291703001831. [DOI] [PubMed] [Google Scholar]
- 51.Ray WA. Psychotropic drugs and injuries among the elderly: A review. J Clin Psychopharmacol. 1992;12:386–396. [PubMed] [Google Scholar]
- 52.Wysowski DK, Baum C, Ferguson WJ, et al. Sedative-hypnotic drugs and the risk of hip fracture. J Clin Epidemiol. 1996;49:111–113. doi: 10.1016/0895-4356(95)00057-7. [DOI] [PubMed] [Google Scholar]
- 53.Bass E, French DD, Bradham DD, et al. Risk-adjusted mortality rates of elderly veterans with hip fractures. Ann Epidemiol. 2007;17:514–519. doi: 10.1016/j.annepidem.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Courtney C, Farrell D, Gray R, et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomized double-blind trial. Lancet. 2004;363:2105–2115. doi: 10.1016/S0140-6736(04)16499-4. [DOI] [PubMed] [Google Scholar]
- 55.Lopez OL, Becker JT, Sweet RA, et al. Patterns of change in the treatment of psychiatric symptoms in patients with probable Alzheimer’s disease from 1983 to 2000. J Neuropsychiatry Clin Neurosci. 2003;15:67–73. doi: 10.1176/jnp.15.1.67. [DOI] [PubMed] [Google Scholar]