Abstract
A total of 195 subjects, including 141 exposed workers and 54 farmers, were recruited in China to evaluate the usefulness of chromium (Cr) in erythrocytes as a biomarker of exposure to CrVI. The levels of Cr in red blood cells (RBC) were remarkably elevated even in a group of workers routinely exposed to CrVI as low as 5–15 μg m−3 and showed a significant exposure–response trend over the exposure range from 0.002 to 1152 μg m−3 (p<0.0001). Multiple linear regression analyses indicated that age and cigarette smoke were not associated with Cr in RBC. However, female subjects had lower Cr in RBC compared with their male counterparts for about the same exposure levels (p<0.05). The genotypes of band III, which encodes for anion transport protein and may regulate across cell membranes, were also identified and included for analysis. The ratios of Cr in RBC to CrVI exposure were higher in subjects with a wild genotype than in those who had heterozygous or homozygous variant alleles. However, the difference was not statistically significant probably due to the limited number of participating subjects. In addition, 15 of the 141 workers were selected for multiple exposure monitoring and blood sample collections to evaluate the inter- and intraindividual variations of Cr in RBC. Compared with the personal exposure levels, Cr in RBC had small intraindividual variations with a reliability coefficient of 0.88. The study suggests that Cr in RBC may serve as a sensitive and reliable biomarker for long-term exposure to CrVI.
Introduction
Chromium (Cr) is a generally abundant element in the earth’s crust and occurs in oxidation states ranging from Cr2+ to Cr6+, but only trivalent (CrIII) and hexavalent (CrVI) forms are of biological significance. CrIII, the more common form, is known to be essential for certain physiological functions and does not show significant health hazard to humans partly due to its poor absorption and inability to cross cell membranes (NRC 1989, Anderson 1994, Costa 1997). In contrast, CrVI is a strong oxidizing agent and poses much greater human health hazards than its trivalent analogue. CrVI has been consistently associated with increased mortality due to respiratory cancer in numerous studies of workers with occupational exposures, and therefore, is recognized as a human carcinogen (IARC 1990, Moulin et al. 1990, Davies et al. 1991, Wang et al. 1994, De Flora 2000). CrVI is present in most of the Superfund toxic waste dump sites and has been known as a widespread environmental contaminant; therefore, its potential toxic and carcinogenic effects at ambient levels on humans have attracted increasing public concern.
Monitoring of Cr in different biological matrices, such as urine, plasma, erythrocytes, lymphocytes and exhaled breath condensate, etc. has been used as a biomarker of Cr exposure (McAughey et al. 1988, Minoia et al. 1988, Lukanova et al. 1996, Miksche & Lewalter 1997, Caglieri et al. 2006, Murgia et al. 2006). Unlike many other metals, CrVI exhibits some chemical properties that significantly affect its bioavailability in cells. As indicated in a recent review, CrVI readily crosses the cell membrane as a tetrahedral divalent anion, which is structurally similar to and anions, and therefore may share with them the same anion transport carrier identified as a band III protein (Chiu et al. 2004). Band III is a member of a widely distributed family of proteins present in many tissues. They play important roles in many cellular processes that involve the exchange transport of anions across membranes (Alper 1991, Bruce & Tanner 1999). Once entering into cells via band III-regulated uptake, CrVI is reduced quickly through several steps into CrIII, which forms octahedral complexes and is trapped inside the cells because it typically crosses cell membranes by simple diffusion at a rate three orders of magnitude lower than CrVI (Cohen et al. 1993, Chiu et al. 2004). Intracellular reduction and trapping of reduced Cr appear to be important determinants of accumulation of chromate in cells, and therefore, intracellular Cr has been proposed as an indicator of CrVI exposure (Cohen et al. 1993, Alexander & Aaseth 1995). The present study was conducted in a Chinese population with a broad range of CrVI exposure to examine the usefulness of Cr in erythrocytes as an exposure biomarker for CrVI. The study also examines the potential effects of band III polymorphism on Cr in erythrocytes.
Materials and methods
The human subject protocol for this study was approved by the IRBs of both the New York University School of Medicine and the Central South University School of Public Health in China. Written informed consent was obtained from all participating subjects.
Subject recruitment and personal exposure monitoring of CrVI
A total of 195 subjects were recruited through questionnaire interview, physical examination and personal exposure monitoring for CrVI. These included 141 workers from a chromate factory, in which sodium dichromate (Na2Cr2O7) was used as a raw material to produce chromic anhydride (CrO3), and 54 farmers recruited from an area about 90 miles away from the chromate facility in Shandong, China. A full-shift (8 h) personal-exposure sample was collected for each individual subject onto a 0.8 μm mixed cellulose ester (MCE) filter. A small personal pump drew air through the lapel-mounted filter holder at 2 l min−1. For quality control purposes, both blank and spiked monitors (10% of the total samples) were also prepared in the field. At the end of personal-exposure monitoring, all subjects were asked to provide 50 ml urine samples. Each sample was collected in a sterile specimen container and then an aliquot was transferred into a 15 ml cell culture centrifuge tube. These aliquots of urine samples were kept at 4°C after collection in the field and during transportation to a local laboratory. All urine samples were then stored at −20°C until analysed for cotinine and creatinine. In addition, a 5 ml blood sample was collected into a heparinized Vacutainer tube from each of the participating subjects by a local registered nurse.
NIOSH method 7600 was employed to determine CrVI sampled on the filters (Eller & Cassinelli 1994). The recovery rate of CrVI from the filters spiked with K2CrO4 standards was 92.1±3.0%. The limit of detection was 0.06 μg per filter as estimated based on the measurement of 10 blank filters (mean ± SD of blank filters). The concentration (C) of CrVI in air was calculated by the following equation:
where W and B are mass of CrVI in each sample and average blank, respectively. V is the total air volume sampled.
Blood sample preparation and analysis of Cr in red blood cells
Red blood cells (RBC) were separated and prepared according to the method described by Lukanova et al. (1996). Briefly, haematocrit 1 (Ht 1) was first determined soon after collection, using a haematocrit centrifuge. Samples were then left for 30–40 min at room temperature to allow the blood to separate into two fractions: fraction 1, the supernatant, contained the plasma and white blood cells, and fraction 2 the residual, contained predominantly red blood cells. The interphase between the two fractions, containing mainly the lymphocytes, was transferred into cryogenic vials and stored at −70°C for DNA isolation and genotype identification. The second fraction was resuspended with 0.9% sodium chloride up to the initial volume of blood collected, allowed to stand at room temperature for 10 min, and centrifuged for 10 min at 3000 rpm. The same washing procedure was repeated twice more with 0.9% NS. Haematocrit 2 measurement (Ht 2) and RBC counts were made just before centrifuging for the last washing. The erythrocyte pellet after the last wash was diluted to the final volume of the blood sample with Triton X-100 at 0.1%. The lysed RBC preparations were then ready to use for Cr analysis.
Cr levels in erythrocytes were measured by graphite furnace atomic absorption with Zeeman background correction as described by Gao et al. (1993) using a Thermo Elemental Solaar M6 atomic absorption spectrometer. Reference standard solution (potassium dichromate) certified by Fisher was used to make a series standards for calculation. The limit of detection in this study was 0.3 μgl−1. To investigate any error inherent in the procedures of RBC preparation and Cr analysis, we identified one subject who agreed to provide one single donation of 25 ml of blood samples (collected in five separate Vacutainer tubes). These tubes of blood, serving as quality control (QC) samples, were coded to appear like regular study samples so that those performing the RBC preparations and analyses of Cr were not aware of the nature of these QC samples. The coefficient of variation (CV) for Cr levels in erythrocytes of these QC samples was 12.9%.
DNA isolation and genotype identification of band III gene
DNA was isolated from blood samples using the commercial QIAamp DNA Mini kit (Qiagen, Valencia, CA, USA).
Band III polymorphism (band III Memphis) at codon 56
Human erythrocyte band III polymorphism of Lys56Glu was identified by using allele-specific probes in real-time polymerase chain reaction (PCR). Primers and probes were designed using PrimerExpress 3.0 software (Applied Biosystems, Foster City, CA, USA). The primers were: 5′-CACAGACTACCACACCACATCACA-3′ (forward) and 5′-AAAGGCAGCATGGGAAAGAA-3′ (reverse). 5′-VICACCCACGAGGTGAGMGB-3′ and 5′-FAMACCCACAAGGTGAGGMGB-3′ were used as specific probes for A and G alleles, respectively. Reactions were performed using the Applied Biosystems 7300 real-time PCR instrument in 96-well plates with each well containing 20 ng of genomic DNA isolated from blood, 1 × TaqMan master mix, dual-labelled probes (100 nM each) and PCR primers (900 nM each). Each plate contained two control DNA samples for each homozygous genotype and four no template controls. In addition, 10% of samples were repeated for quality control purpose.
Measurement of urinary cotinine and creatinine
Urinary cotinine, one of the major metabolites of nicotine, was employed to evaluate the smoking status of study subjects and was quantified by cotinine direct enzyme-linked immunosorbent (ELISA) kits (Immunalysis, Pomona, CA, USA). In order to adjust the levels of cotinine in urine samples, urinary creatinine was also determined using commercially available creatinine assay kits according to the standard procedure provided (ThermoDMA, Arlington, TX, USA).
Statistical analyses
For statistical analyses, log transformation was performed on levels of Cr in both RBC and air to improve the normality. Differences in the levels of Cr in RBC among groups were compared by two-sample Student’s t test. The levels of Cr in RBC and CrVI exposure among non-smokers, moderate smokers and heavy smokers in participating farmers were compared using ANOVA. The multiple linear regression analysis was used to evaluate the impact of CrVI exposure on Cr in RBC while controlling potential confounders such as age, gender, cotinine levels and genotypes of band III. The data were analysed using SAS statistical packages (SAS Institute Inc., Cary, NC, USA). All the p values were two-sided.
Results
The demographic characteristics of the participating subjects and the levels of their personal exposure to CrVI monitored on the day of biological sample collections are shown in Table I. Both self-reported data on smoking habits and urinary cotinine levels were initially used to discriminate smokers from non-smokers. It was observed that all self-reported smokers had elevated cotinine levels (≥100 μg g−1 creatinine). On the other hand, almost all non-smokers were found to have cotinine levels far below 100 μg g−1 creatinine, except three farmers with levels slightly higher than 100 μg g−1 creatinine. Therefore, a cotinine level of 100 μg g−1 creatinine was used as criteria to affirm smoking status. As shown in Table I, there were no significant differences in age, sex and smoking status between the two groups. The median exposure levels of CrVI monitored in the participating workers and farmers on the day of biological sample collections were 17.8 μg m−3 and 0.06 μg m−3, respectively. Accordingly, the levels of Cr in RBC in workers were significantly higher than those in the participating farmers (p<0.0001). To evaluate the sensitivity of Cr in RBC as a biomarker for CrVI exposure, the study subjects were further divided into six subgroups according to their exposure levels of CrVI. As shown in Figure 1, the geometric means of Cr in RBC increased significantly in groups of workers exposed to CrVI at or above 5 μg m−3 and showed a significant exposure–response trend over the entire exposure range from 0.002 to 1152 μg m−3 (p<0.0001).
Table I.
Characteristics of the study subjects, exposure levels and Cr in red blood cells (RBC).
| Workers (n=141) |
Farmers (n=54) |
p-Values | |
|---|---|---|---|
| Female, n (%) | 39 (27.7) | 11 (20.4) | 0.30 |
| Smoking (cotinine >100 μg g−1 creatinine), n (%) | 66 (46.8) | 33 (61.1) | 0.076 |
| Age (years), median (1st and 3rd quartiles) | 35 (31, 41) | 34 (30, 39) | 0.23 |
| CrVI exposure (μg m−3), median (1st and 3rd quartiles) |
17.8 (6.8, 46.3) | 0.06 (0.01, 0.13) | 0.001 |
| Cr in RBC (μg l−1), median (1st and 3rd quartiles) | 6.00 (3.75, 11.61) | 2.64 (1.44, 3.55) | <0.0001 |
Figure 1.
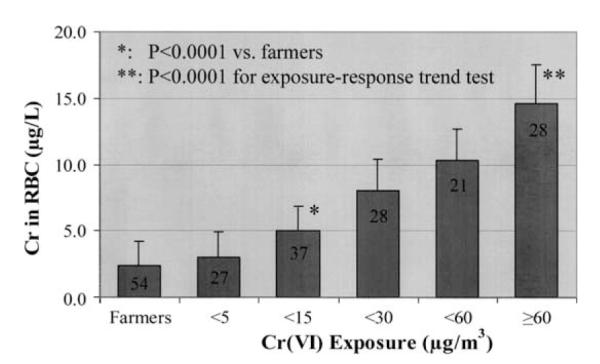
Relationship between CrVI exposure and the levels of Cr in red blood cells (RBC) (geometric mean and SD). The number shown in the columns represents the subject number in corresponding subgroup. *Compared with farmers, p<0.0001; **exposure–response trend test, p<0.0001.
In order to examine the effects of confounding factors including smoking and other demographic factors, such as age and sex, on the levels of Cr in RBC, an initial analysis was first conducted to compare the levels of Cr in RBC among non-smokers, moderate smokers and heavy smokers in the recruited farmers. As shown in Table II, no significant difference was detected in the levels of Cr in RBC between smokers and non-smokers. Furthermore, multiple regression analyses of Cr in RBC were conducted on CrVI exposure in all recruited subjects, controlling for age, gender and smoking as possible confounders. The results indicated that age and cigarette smoke were not among the confounders. However, female subjects may have lower Cr in RBC compared with male counterparts at about the same exposure levels (Table III, p<0.05).
Table II.
Effects of cigarette smoking intensity on the levels of Cr in red blood cells (RBC). Values are median (1st quartile, 3rd quartile).
| Groupsa | No. of subjects | Cr exposure (μg m−3) | Cr in RBC (μg l−1) |
|---|---|---|---|
| Non-smokers | 21 | 0.06 (0.02, 0.14) | 2.70 (1.76, 3.15) |
| Moderate smokers | 10 | 0.05 (0.01, 0.08) | 3.36 (2.41, 4.30) |
| Heavy smokers | 23 | 0.05 (0.01, 0.14) | 1.84 (1.25, 3.45) |
Grouped according to urinary cotinine levels: non-smokers, <100 μg g−1 creatinine; moderate smokers, 100 to <1000 μg g−1 creatinine; heavy smokers, ≥1000 μg g−1 creatinine.
Table III.
Least squares regression analysis relating Cr in red blood cells (RBC) to Cr exposure levels with or without adjustment by sex, smoking, age and band III.
| β (SE) | p-Values | |
|---|---|---|
| Cr exposure only without adjustment | ||
| Ln Cr exposure | 0.16 (0.013) | <0.0001 |
| Adjusted for sex | ||
| Ln Cr exposure | 0.15 (0.013) | <0.0001 |
| Sex, female | −0.11 (0.054) | 0.042 |
| Adjusted for sex, smoking and age | ||
| Ln Cr exposure | 0.15 (0.013) | <0.0001 |
| Sex, female | −0.14 (0.063) | 0.0309 |
| Log Cotinine | −0.05 (0.054) | 0.4127 |
| Age | −0.003 (0.004) | 0.4952 |
| Adjusted for sex, smoking, age and band III | ||
| Ln Cr exposure | 0.15 (0.013) | <0.0001 |
| Sex, female | −0.13 (0.065) | 0.0486 |
| Log Cotinine | −0.014 (0.027) | 0.6088 |
| Age | −0.003 (0.004) | 0.5150 |
| Band III polymorphism | 0.003 (0.060) | 0.9551 |
To evaluate whether or not genetic variations contribute to the difference in individuals’ accumulation of Cr in RBC due to CrVI exposure, the band III Memphis polymorphisms were identified using the real-time PCR technique. The genotype identification demonstrated that there were only three subjects who had homozygous variant alleles. Therefore, the data obtained from subjects who had either heterozygous or homozygous variant alleles were merged together and compared with that from those who had a wild genotype. Because the exposure levels of CrVI were not comparable between the two genotype groups, it is not appropriate to compare Cr levels in RBC directly. Alternatively, the ratios of Cr in RBC to CrVI exposure (μg l−1 per μg m−3 of CrVI) were calculated and analysed. They were found to be higher in subjects with a wild genotype than that in those with either heterozygous or homozygous variant alleles. However, the differences were not statistically significant in either occupationally or non-occupationally exposed subjects probably due to the limited number of participating subjects (Figure 2). A multiple regression analysis further suggested that Band III Memphis polymorphism may not significantly affect the transport of CrVI across membranes of erythrocytes (Table III).
Figure 2.
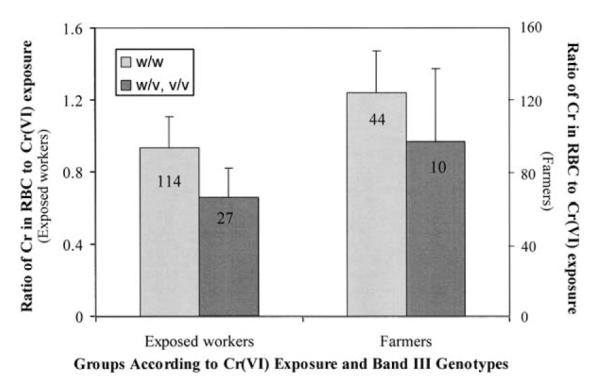
Effects of band III polymorphism on Cr levels in red blood cells (RBC) (μg l−1 per μg m−3 of CrVI exposure). The number shown in the columns represents the subject number in corresponding subgroup.
In order to examine further the reliability of Cr in RBC to index CrVI exposure, 15 out of the 141 exposed workers were selected for multiple personal exposure monitoring and blood sample collections on three consecutive Mondays to evaluate the inter- and intraindividual variations of Cr in RBC. As shown in Table IV, the variations in personal exposure levels of CrVI were substantially larger within subjects relative to between subjects. Compared with the personal exposure levels, Cr in RBC had relatively small intraindividual variations with reliability coefficient of 0.88, suggesting that Cr in RBC may serve as a sensitive and reliable biomarker for long-term CrVI exposure (Table IV).
Table IV.
Intra- and interindividual variations of Cr in red blood cells (RBC).
| Variance Components |
|||
|---|---|---|---|
| Between subjects | Within subjects | Reliability coefficient (R)a |
|
| Ln Cr in RBC | 0.556 | 0.077 | 0.88 |
| Ln CrVI exposure | 0.641 | 0.666 | 0.49 |
Reliability coefficient (R)=VarianceBetween/(VarianceBetween+VarianceWithin).
Discussion
As shown in Table I, Cr levels in RBC were significantly higher in exposed workers than in participating farmers (p<0.0001); however, the difference appears to be relatively small compared with the extremely different exposure levels between the two groups. Because Cr may be lost during the process of erythrocyte preparation the validity of the results needs to be addressed. According to the literature, the background levels of Cr in RBC are 2.5±1.5 μg l−1 (mean±SD) and 4.5 μg l−1 (mean plus 1 standard error) in control subjects (Kerger et al. 1996, Lukanova et al. 1996). Cr in RBC measured in the participating farmers in the present study is comparable to these reference levels with a median of 2.64 μg l−1. This may suggest that there was no significant loss or contamination of Cr in samples collected in farmers due to the procedure of RBC preparation. Furthermore, the results of QC sample analyses in this study suggested that there was no significant error inherent in the procedures of RBC preparation and Cr analysis. Any loss of Cr in RBC during preparation should be randomly distributed into samples collected from either exposed or unexposed subjects. It is not likely that a potential loss of Cr due to preparation procedures will contribute only to the measurement of samples collected from exposed subjects. Therefore, based on the outcomes of QC sample analyses and background levels in farmers the Cr levels of RBC in exposed workers should be reliable. This is further supported by the excellent exposure–response relationship as shown in Figure 1.
Cr in RBC has long been recognized as a biomarker to index an integrated CrVI exposure over a lifespan of erythrocytes (approximately 120 days) (Wiegand et al. 1988, Lukanova et al. 1996, Miksche & Lewalter 1997). However, very few studies have been conducted so far to evaluate how well and reliable a single measurement of Cr in RBC reflects the integrated exposure level of CrVI within an individual. For this purpose, the analyses of inter- and intraindividual variations over time with regard to levels of CrVI exposure and Cr in RBC were performed in this study on 15 subjects with data gathered on three consecutive Mondays. As shown in Table IV, the results revealed that the personal exposure levels to CrVI fluctuated greatly within individuals over time and the intraindividual variations were even greater than interindividual variations. Unlike personal exposure monitoring, the levels of Cr in RBC were relatively stable within an individual over time. The within-individual variations of Cr in RBC were small relative to the between-individual variations with reliability coefficient of 0.88. These suggest that a single measurement of Cr in RBC is representative of the average of multiple sample analyses of Cr in RBC, and therefore, it is appropriate and reliable to use one sample measurement per subject to index his/her integrated CrVI exposure.
It has been shown that CrVI is not stable in the bloodstream after absorption due to the presence of various reductants in plasma, such as ascorbic acid and glutathione. As indicated in a number of studies, following exposure, CrVI can reach the bloodstream and is subject to further reduction within plasma, which may prevent CrVI uptake by erythrocytes (Harzdorf & Lewalter 1997, Miksche & Lewalter 1997, Corbett et al. 1998). Harzdorf & Lewalter (1997) investigated an in vitro reduction rate of CrVI in plasma and found that CrVI concentration faded gradually after a single exposure and decreased by about 20% within the initial 60 min. This finding may suggest that plasma reductive capacity is not sufficiently strong to prevent CrVI from penetrating into cells, including erythrocytes. Other factors should also be considered as potential confounders when examining confounding effects for Cr uptake by erythrocytes.
Band III is a member of a widely distributed family of proteins present in many tissues, which play important roles in many cellular processes that involve the exchange transport of anions across membranes (Alper 1991, Bruce & Tanner 1999]. It was, therefore, proposed that band III proteins may also be important in regulating CrVI transport across membranes of erythrocytes due to the similarity of with and . The role of band III proteins in modulating CrVI uptake by erythrocytes was first investigated using 51CrVI in human erythrocytes independently by two groups of researchers (Ottenwaelder et al. 1988, Alexander & Aaseth 1995). Both studies demonstrated that the uptake of 51CrVI by erythrocytes was fast and efficient, and could be significantly inhibited by the addition of 4,4′-diisothiocyana-tostilbene-2,2′-disulfonic acid, an established band III protein inhibitor. These findings suggest that CrVI uptake by erythrocytes is indeed dependent on band III activity. However, there has been no study conducted so far to evaluate if the genetic variations of band III account for the observed interindividual difference in cellular uptake of CrVI.
The gene encoding band III protein has been found to be polymorphic. The most common polymorphism is band III Memphis and represents a widespread polymorphism, being detected with a prevalence ranging from 5% to 24% in virtually all ethnic groups with slightly reduced anion transport activity (Palatnik et al. 1990, Jarolim et al. 1992, Bruce et al. 1994, Bruce & Tanner 1999). The present study examined, for the first time, the potential effects of band III Memphis polymorphism on the relationship between Cr in RBC and CrVI exposure in humans. Our data indicate that the ratios of Cr in RBC to CrVI exposure were higher in subjects with the wild genotype than that in those with heterozygous or homozygous variant alleles. However, the differences were not statistically significant probably for two reasons. First, the anion transport activity is only slightly reduced in subjects with variant alleles compared with those with wild genotype. As a result, detection of the weak effect of variant genotype may need a relatively large number of subjects. In fact, the number of subjects who have either heterozygous or homozygous alleles is only 27 and 10 in exposed workers and farmers, respectively. Obviously, a further study with relatively large number of subjects, especially those who have homozygous alleles, is needed in order to reach a firm conclusion.
The monitoring of Cr in urine provides a useful measure of internal Cr exposure and has been used in the workplace to assess short-term high-level exposures to CrVI. However, urinary Cr mainly reflects the most current exposure and is not predictive for integrated or cumulative exposures since the biological half-life of Cr in urine is very short (Paustenbach et al. 1997). Most importantly, urinary Cr levels may only represent the total Cr excretion in urine and do not reflect the real exposure or uptake of CrVI due to a number of factors which govern the distribution and excretion of CrVI in the body. In the bloodstream, the absorbed CrVI easily enters into blood cells where CrVI is rapidly reduced into CrIII and trapped inside the cells (Ottenwaelder et al. 1988, Coogan et al. 1991, Cohen et al. 1993, Corbett et al. 1998). Therefore, the processes of cellular uptake and intracelluar reduction prevent the absorbed CrVI from being excreted in urine except for a portion which has been reduced in plasma before entering the cells. On the other hand, CrIII in blood, whether absorbed as such or resulting from CrVI reduction in plasma, can bind to plasma protein and the unbound CrIII is rapidly excreted in urine as cell membranes are impermeable to CrIII (Christensen 1995, Miksche & Lewalter 1997). Accordingly, urinary Cr seems to only provide information of total extracellular CrIII burden with no indication of CrVI uptake. Considering the fact that in cases of CrVI exposure, the CrIII excreted in urine is actually the detoxified part, its usefulness for hazard evaluation is limited. For all these reasons, the measurement of urinary Cr was not included in this study.
In conclusion, Cr in RBC may serve as a sensitive and reliable biomarker for long-term occupational, but not environmental, exposure to CrVI. Statistical analyses indicate that age and cigarette smoke are not among the confounders. However, female subjects may have lower Cr in RBC compared with male counterparts at about the same exposure levels. The band III genotype study may suggest a potential downregulation of CrVI uptake by erythrocytes in subjects who have either heterozygous or homozygous variant alleles. This potential effect, however, needs to be confirmed.
Acknowledgements
The authors would like to thank all our Chinese colleagues who participated in this study for their excellent work, and are grateful for the participation of the workers, and the cooperation of management in the Jinan Yuxing Chemical Plant in China. This work was supported by grants R830682 from EPA, ES00260 and ES10344 from NIEHS.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alexander J, Aaseth J. Uptake of chromate in human red blood cells and isolated rat liver cells: the role of the anion carrier. Analyst. 1995;120:931–933. doi: 10.1039/an9952000931. [DOI] [PubMed] [Google Scholar]
- Alper SL. The band 3-related anion exchanger (AE) gene family. Annual Review of Physiology. 1991;53:549–564. doi: 10.1146/annurev.ph.53.030191.003001. [DOI] [PubMed] [Google Scholar]
- Anderson RA. Nutritional and toxicologic aspects of chromium intake: an overview. In: Mertz W, Abernathy CO, Olin SS, editors. Assessment of essential elements. ILSI Press; Washington, DC: 1994. pp. 187–196. [Google Scholar]
- Bruce LJ, Anstee DJ, Spring FA, Tanner MJ. Band 3 memphis variant II. Altered stilbene disulfonate binding and the diego (dia) blood group antigen are associated with the human erythrocyte band 3 mutation Pro854–>Leu. Journal of Biological Chemistry. 1994;269:16155–16158. [PubMed] [Google Scholar]
- Bruce LJ, Tanner MJ. Erythroid band 3 variants and disease. Bailliéres Best Practice & Research. Clinical Haematology. 1999;12:637–654. doi: 10.1053/beha.1999.0046. [DOI] [PubMed] [Google Scholar]
- Caglieri A, Goldoni M, Acampa O, Andreoli R, Vettori MV, Corradi M, Apostoli P, Mutti A. The effect of inhaled chromium on different exhaled breath condensate biomarkers among chrome-plating workers. Environmental Health Perspectives. 2006;114:542–456. doi: 10.1289/ehp.8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu A, Katz AJ, Beaubier J, Chiu N, Shi X. Genetic and cellular mechanisms in chromium and nickel carcinogenesis considering epidemiologic findings. Molecular & Cellular Biochemistry. 2004;255:181–194. doi: 10.1023/b:mcbi.0000007274.25052.82. [DOI] [PubMed] [Google Scholar]
- Christensen JM. Human exposure to toxic metals: factors influencing interpretation of biomonitoring results. The Science of the Total Environment. 1995;166:89–135. doi: 10.1016/0048-9697(95)04478-j. [DOI] [PubMed] [Google Scholar]
- Cohen MD, Kargacin B, Klein CB, Costa M. Mechanisms of chromium carcinogenicity and toxicity. Critical Reviews in Toxicology. 1993;23:255–281. doi: 10.3109/10408449309105012. [DOI] [PubMed] [Google Scholar]
- Coogan T, Ssquibb K, Motz J, Kinney P, Costa M. Distribution of chromium within cells of the blood. Toxicology and Applied Pharmacology. 1991;108:157–166. doi: 10.1016/0041-008x(91)90279-n. [DOI] [PubMed] [Google Scholar]
- Corbett GE, Dodge DG, O’Flaherty E, Liang J, Throop L, Finley BL, Kerger BD. In vitro reduction kinetics of hexavalent chromium in human blood. Environmental Research. 1998;78:7–11. doi: 10.1006/enrs.1998.3840. [DOI] [PubMed] [Google Scholar]
- Costa M. Toxicity and carcinogenicity of cr(VI) in animal models and humans. Critical Reviews in Toxicology. 1997;27:431–442. doi: 10.3109/10408449709078442. [DOI] [PubMed] [Google Scholar]
- Davies JM, Easton DF, Bidstrup PL. Mortality from respiratory cancer and other causes in united kingdom chromate production workers. British Journal of Industrial Medicine. 1991;48:299–313. doi: 10.1136/oem.48.5.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Flora S. Threshold mechanisms and site specificity in chromium(VI) carcinogenesis. Carcinogenesis. 2000;21:533–541. doi: 10.1093/carcin/21.4.533. [DOI] [PubMed] [Google Scholar]
- Eller PM, Cassinelli ME. NIOSH manual of analytical methods. 4th ed US Department of Health and Human Services, Public Health Service, Centers for Diseases Control and Prevention, National Institute for Occupational Safety and Health; Cincinnati, OH: 1994. [Google Scholar]
- Gao M, Levy LS, Braithwaite RA, Brown SS. Monitoring of total chromium in rat fluids and lymphocytes following intratracheal administration of soluble trivalent or hexavalent chromium compounds. Human & Experimental Toxicology. 1993;12:377–382. doi: 10.1177/096032719301200506. [DOI] [PubMed] [Google Scholar]
- Harzdorf C, Lewalter J. Analytical methodology for biological monitoring of chromium. Regulatory Toxicology & Pharmacology. 1997;26:S86–S93. doi: 10.1006/rtph.1997.1145. [DOI] [PubMed] [Google Scholar]
- IARC . IARC monographs on the evaluation of carcinogenic risks to humans. Vol. 49. IARC; Lyon: 1990. Chromium, nickel and welding. [PMC free article] [PubMed] [Google Scholar]
- Jarolim P, Rubin HL, Zhai S, Sahr KE, Liu SC, Mueller TJ, Palek J. Band 3 memphis: a widespread polymorphism with abnormal electrophoretic mobility of erythrocyte band 3 protein caused by substitution AAG----GAG (lys----glu) in codon 56. Blood. 1992;80:1592–1598. [PubMed] [Google Scholar]
- Kerger BD, Paustenbach DJ, Corbett GE, Finley BL. Absorption and elimination of trivalent and hexavalent chromium in humans following ingestion of a bolus dose in drinking water. Toxicology & Applied Pharmacology. 1996;141:145–158. doi: 10.1006/taap.1996.0271. [DOI] [PubMed] [Google Scholar]
- Lukanova A, Toniolo P, Zhitkovich A, Nikolova V, Panev T, Popov T, Taioli E, Costa M. Occupational exposure to cr(VI): comparison between chromium levels in lymphocytes, erythrocytes, and urine. International Archives of Occupational & Environmental Health. 1996;69:39–44. doi: 10.1007/BF02630737. [DOI] [PubMed] [Google Scholar]
- McAughey JJ, Samuel AM, Baxter PJ, Smith NJ. Biological monitoring of occupational exposure in the chromate pigment production industry. Science of the Total Environment. 1988;71:317–322. doi: 10.1016/0048-9697(88)90203-3. [DOI] [PubMed] [Google Scholar]
- Miksche LW, Lewalter J. Health surveillance and biological effect monitoring for chromium-exposed workers. Regulatory Toxicology & Pharmacology. 1997;26:S94–S99. doi: 10.1006/rtph.1997.1146. [DOI] [PubMed] [Google Scholar]
- Minoia C, Apostoli P, Maranelli G, Baldi C, Pozzoli L, Capodaglio E. Urinary chromium levels in subjects living in two north Italy regions. Science of the Total Environment. 1988;71:527–531. doi: 10.1016/0048-9697(88)90228-8. [DOI] [PubMed] [Google Scholar]
- Moulin JJ, Portefaix P, Wild P, Mur JM, Smagghe G, Mantout B. Mortality study among workers producing ferroalloys and stainless steel in France. British Journal of Industrial Medicine. 1990;47:537–543. doi: 10.1136/oem.47.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgia N, Muzi G, Dell’Omo M, Montuschi P, Melchiorri D, Ciabattoni G, Abbritti EP, Orazi N, Sapia IE, Abbritti G. Induced sputum, exhaled breath condensate and nasal lavage fluid in electroplating workers exposed to chromium. International Journal of Immunopathology & Pharmacology. 2006;19(4 Suppl):67–71. [PubMed] [Google Scholar]
- NRC . Recommended dietary allowances. 10th ed. National Academy Press; Washington, DC: 1989. National Research Council, Subcommittee on the Tenth Edition of the RDAs, Committee on Dietary Allowances. [Google Scholar]
- Ottenwaelder H, Wiegand HJ, Bolt HM. Uptake of 51Cr(VI) by human erythrocytes: evidence for a carrier-mediated transport mechanism. Science of the Total Environment. 1988;71:561–566. doi: 10.1016/0048-9697(88)90237-9. [DOI] [PubMed] [Google Scholar]
- Palatnik M, Simoes ML, Alves ZM, Laranjeira NS. The 60 and 63 kDa proteolytic peptides of the red cell membrane band-3 protein: their prevalence in human and non-human primates. Human Genetics. 1990;86:126–130. doi: 10.1007/BF00197692. [DOI] [PubMed] [Google Scholar]
- Paustenbach DJ, Panko JM, Fredrick MM, Finley BL, Proctor DM. Urinary chromium as a biological marker of environmental exposure: what are the limitations? Regulatory Toxicology & Pharmacology. 1997;261:S23–S34. doi: 10.1006/rtph.1997.1135. [DOI] [PubMed] [Google Scholar]
- Wang X, Qin Q, Xu X, Xu J, Wang J, Zhou J, Huang S, Zhai W, Zhou H, Chen J. Chromium-induced early changes in renal function among ferrochromium-producing workers. Toxicology. 1994;90:93–101. doi: 10.1016/0300-483x(94)90208-9. [DOI] [PubMed] [Google Scholar]
- Wiegand HJ, Ottenwalder H, Bolt HM. Recent advances in biological monitoring of hexavalent chromium compounds. Science of the Total Environment. 1988;71:309–315. doi: 10.1016/0048-9697(88)90202-1. [DOI] [PubMed] [Google Scholar]


