Abstract
Development of the nervous system in the amphibian embryo is initiated during gastrulation by an inductive interaction between chordamesoderm and dorsal ectoderm. The induced ectoderm forms the neural plate while uninduced ectoderm generates epidermis. We screened for genes activated during gastrulation and expressed specifically in the nervous system of Xenopus laevis in the expectation that clones representing such genes will constitute useful markers for the study of early neurogenesis. Probes were prepared from adult brain RNA by subtraction with RNA from ovary and from different combinations of adult kidney, muscle, and skin; cDNA libraries prepared from early to late neurula embryo RNA were screened with these probes. Six clones were chosen for further study. Three of these clones are not represented in the maternal RNA population but are activated at the late gastrula stage; the other three increase from a maternal base. Expression of five of the genes is restricted to the neural plate during embryogenesis, and all six are restricted to the central nervous system in premetamorphic tadpoles and adults. One of the clones encodes an apparently neurospecific isoform of beta-tubulin; the identity of the other clones is unknown. Expression of all six genes is suppressed in axis-deficient embryos that lack dorsal structures including the brain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akers R. M., Phillips C. R., Wessells N. K. Expression of an epidermal antigen used to study tissue induction in the early Xenopus laevis embryo. Science. 1986 Feb 7;231(4738):613–616. doi: 10.1126/science.3945801. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Baker N. E. Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. EMBO J. 1987 Jun;6(6):1765–1773. doi: 10.1002/j.1460-2075.1987.tb02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balak K., Jacobson M., Sunshine J., Rutishauser U. Neural cell adhesion molecule expression in Xenopus embryos. Dev Biol. 1987 Feb;119(2):540–550. doi: 10.1016/0012-1606(87)90057-1. [DOI] [PubMed] [Google Scholar]
- Boucaut J. C., Darribere T., Li S. D., Boulekbache H., Yamada K. M., Thiery J. P. Evidence for the role of fibronectin in amphibian gastrulation. J Embryol Exp Morphol. 1985 Nov;89 (Suppl):211–227. [PubMed] [Google Scholar]
- Cabrera C. V., Alonso M. C., Johnston P., Phillips R. G., Lawrence P. A. Phenocopies induced with antisense RNA identify the wingless gene. Cell. 1987 Aug 14;50(4):659–663. doi: 10.1016/0092-8674(87)90039-0. [DOI] [PubMed] [Google Scholar]
- Carrasco A. E., Malacinski G. M. Localization of Xenopus homoeo-box gene transcripts during embryogenesis and in the adult nervous system. Dev Biol. 1987 May;121(1):69–81. doi: 10.1016/0012-1606(87)90139-4. [DOI] [PubMed] [Google Scholar]
- Chowdhury K., Dressler G., Breier G., Deutsch U., Gruss P. The primary structure of the murine multifinger gene mKr2 and its specific expression in developing and adult neurons. EMBO J. 1988 May;7(5):1345–1353. doi: 10.1002/j.1460-2075.1988.tb02950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darribère T., Yamada K. M., Johnson K. E., Boucaut J. C. The 140-kDa fibronectin receptor complex is required for mesodermal cell adhesion during gastrulation in the amphibian Pleurodeles waltlii. Dev Biol. 1988 Mar;126(1):182–194. doi: 10.1016/0012-1606(88)90252-7. [DOI] [PubMed] [Google Scholar]
- Davis C. A., Noble-Topham S. E., Rossant J., Joyner A. L. Expression of the homeo box-containing gene En-2 delineates a specific region of the developing mouse brain. Genes Dev. 1988 Mar;2(3):361–371. doi: 10.1101/gad.2.3.361. [DOI] [PubMed] [Google Scholar]
- Dawid I. B., Sargent T. D. Xenopus laevis in developmental and molecular biology. Science. 1988 Jun 10;240(4858):1443–1448. doi: 10.1126/science.3287620. [DOI] [PubMed] [Google Scholar]
- DiCicco-Bloom E., Black I. B. Insulin growth factors regulate the mitotic cycle in cultured rat sympathetic neuroblasts. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4066–4070. doi: 10.1073/pnas.85.11.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe C. Q., Hiromi Y., Gehring W. J., Goodman C. S. Expression and function of the segmentation gene fushi tarazu during Drosophila neurogenesis. Science. 1988 Jan 8;239(4836):170–175. doi: 10.1126/science.2892267. [DOI] [PubMed] [Google Scholar]
- Doe C. Q., Scott M. P. Segmentation and homeotic gene function in the developing nervous system of Drosophila. Trends Neurosci. 1988 Mar;11(3):101–106. doi: 10.1016/0166-2236(88)90154-3. [DOI] [PubMed] [Google Scholar]
- Doe C. Q., Smouse D., Goodman C. S. Control of neuronal fate by the Drosophila segmentation gene even-skipped. Nature. 1988 May 26;333(6171):376–378. doi: 10.1038/333376a0. [DOI] [PubMed] [Google Scholar]
- Dworkin-Rastl E., Kelley D. B., Dworkin M. B. Localization of specific mRNA sequences in Xenopus laevis embryos by in situ hybridization. J Embryol Exp Morphol. 1986 Feb;91:153–168. [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules in the regulation of animal form and tissue pattern. Annu Rev Cell Biol. 1986;2:81–116. doi: 10.1146/annurev.cb.02.110186.000501. [DOI] [PubMed] [Google Scholar]
- Fainsod A., Awgulewitsch A., Ruddle F. H. Expression of the murine homeo box gene Hox 1.5 during embryogenesis. Dev Biol. 1987 Nov;124(1):125–133. doi: 10.1016/0012-1606(87)90465-9. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gaunt S. J. Homoeobox gene Hox-1.5 expression in mouse embryos: earliest detection by in situ hybridization is during gastrulation. Development. 1987 Sep;101(1):51–60. [PubMed] [Google Scholar]
- Gimlich R. L., Cooke J. Cell lineage and the induction of second nervous systems in amphibian development. Nature. 1983 Dec 1;306(5942):471–473. doi: 10.1038/306471a0. [DOI] [PubMed] [Google Scholar]
- Godsave S. F., Anderton B. H., Wylie C. C. The appearance and distribution of intermediate filament proteins during differentiation of the central nervous system, skin and notochord of Xenopus laevis. J Embryol Exp Morphol. 1986 Sep;97:201–223. [PubMed] [Google Scholar]
- Grant P., Wacaster J. F. The amphibian gray crescent region--a site of developmental information? Dev Biol. 1972 Jul;28(3):454–471. doi: 10.1016/0012-1606(72)90029-2. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Embryonic induction--molecular prospects. Development. 1987 Mar;99(3):285–306. doi: 10.1242/dev.99.3.285. [DOI] [PubMed] [Google Scholar]
- Jacobson M., Huang S. Neurite outgrowth traced by means of horseradish peroxidase inherited from neuronal ancestral cells in frog embryos. Dev Biol. 1985 Jul;110(1):102–113. doi: 10.1016/0012-1606(85)90068-5. [DOI] [PubMed] [Google Scholar]
- Jacobson M., Rutishauser U. Induction of neural cell adhesion molecule (NCAM) in Xenopus embryos. Dev Biol. 1986 Aug;116(2):524–531. doi: 10.1016/0012-1606(86)90153-3. [DOI] [PubMed] [Google Scholar]
- Jamrich M., Sargent T. D., Dawid I. B. Cell-type-specific expression of epidermal cytokeratin genes during gastrulation of Xenopus laevis. Genes Dev. 1987 Apr;1(2):124–132. doi: 10.1101/gad.1.2.124. [DOI] [PubMed] [Google Scholar]
- Kalcheim C., Barde Y. A., Thoenen H., Le Douarin N. M. In vivo effect of brain-derived neurotrophic factor on the survival of developing dorsal root ganglion cells. EMBO J. 1987 Oct;6(10):2871–2873. doi: 10.1002/j.1460-2075.1987.tb02589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay B. K., Schwartz L. M., Rutishauser U., Qiu T. H., Peng H. B. Patterns of N-CAM expression during myogenesis in Xenopus laevis. Development. 1988 Jul;103(3):463–471. doi: 10.1242/dev.103.3.463. [DOI] [PubMed] [Google Scholar]
- Kelley M. R., Kidd S., Deutsch W. A., Young M. W. Mutations altering the structure of epidermal growth factor-like coding sequences at the Drosophila Notch locus. Cell. 1987 Nov 20;51(4):539–548. doi: 10.1016/0092-8674(87)90123-1. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell. 1987 Dec 4;51(5):869–877. doi: 10.1016/0092-8674(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Kintner C. R., Melton D. A. Expression of Xenopus N-CAM RNA in ectoderm is an early response to neural induction. Development. 1987 Mar;99(3):311–325. doi: 10.1242/dev.99.3.311. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987 Sep 4;237(4819):1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Pringle N., Mosley M. J., Westermark B., Dubois-Dalcq M. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell. 1988 Apr 22;53(2):309–319. doi: 10.1016/0092-8674(88)90392-3. [DOI] [PubMed] [Google Scholar]
- Richter K., Egger R., Kreil G. Sequence of preprocaerulein cDNAs cloned from skin of Xenopus laevis. A small family of precursors containing one, three, or four copies of the final product. J Biol Chem. 1986 Mar 15;261(8):3676–3680. [PubMed] [Google Scholar]
- Rijsewijk F., Schuermann M., Wagenaar E., Parren P., Weigel D., Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987 Aug 14;50(4):649–657. doi: 10.1016/0092-8674(87)90038-9. [DOI] [PubMed] [Google Scholar]
- Rosa F., Roberts A. B., Danielpour D., Dart L. L., Sporn M. B., Dawid I. B. Mesoderm induction in amphibians: the role of TGF-beta 2-like factors. Science. 1988 Feb 12;239(4841 Pt 1):783–785. doi: 10.1126/science.3422517. [DOI] [PubMed] [Google Scholar]
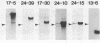
- Sargent T. D., Dawid I. B. Differential gene expression in the gastrula of Xenopus laevis. Science. 1983 Oct 14;222(4620):135–139. doi: 10.1126/science.6688681. [DOI] [PubMed] [Google Scholar]
- Sargent T. D., Jamrich M., Dawid I. B. Cell interactions and the control of gene activity during early development of Xenopus laevis. Dev Biol. 1986 Mar;114(1):238–246. doi: 10.1016/0012-1606(86)90399-4. [DOI] [PubMed] [Google Scholar]
- Scharf S. R., Gerhart J. C. Axis determination in eggs of Xenopus laevis: a critical period before first cleavage, identified by the common effects of cold, pressure and ultraviolet irradiation. Dev Biol. 1983 Sep;99(1):75–87. doi: 10.1016/0012-1606(83)90255-5. [DOI] [PubMed] [Google Scholar]
- Shackleford G. M., Varmus H. E. Expression of the proto-oncogene int-1 is restricted to postmeiotic male germ cells and the neural tube of mid-gestational embryos. Cell. 1987 Jul 3;50(1):89–95. doi: 10.1016/0092-8674(87)90665-9. [DOI] [PubMed] [Google Scholar]
- Sharpe C. R. Developmental expression of a neurofilament-M and two vimentin-like genes in Xenopus laevis. Development. 1988 Jun;103(2):269–277. doi: 10.1242/dev.103.2.269. [DOI] [PubMed] [Google Scholar]
- Sharpe C. R., Fritz A., De Robertis E. M., Gurdon J. B. A homeobox-containing marker of posterior neural differentiation shows the importance of predetermination in neural induction. Cell. 1987 Aug 28;50(5):749–758. doi: 10.1016/0092-8674(87)90333-3. [DOI] [PubMed] [Google Scholar]
- Slack J. M., Darlington B. G., Heath J. K., Godsave S. F. Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature. 1987 Mar 12;326(6109):197–200. doi: 10.1038/326197a0. [DOI] [PubMed] [Google Scholar]
- Slack J. M. Peanut lectin receptors in the early amphibian embryo: regional markers for the study of embryonic induction. Cell. 1985 May;41(1):237–247. doi: 10.1016/0092-8674(85)90077-7. [DOI] [PubMed] [Google Scholar]
- Smith J. C. A mesoderm-inducing factor is produced by Xenopus cell line. Development. 1987 Jan;99(1):3–14. doi: 10.1242/dev.99.1.3. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Dale L., Slack J. M. Cell lineage labels and region-specific markers in the analysis of inductive interactions. J Embryol Exp Morphol. 1985 Nov;89 (Suppl):317–331. [PubMed] [Google Scholar]
- Sullivan K. F., Cleveland D. W. Identification of conserved isotype-defining variable region sequences for four vertebrate beta tubulin polypeptide classes. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4327–4331. doi: 10.1073/pnas.83.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks D. L., Melton D. A. A maternal mRNA localized to the vegetal hemisphere in Xenopus eggs codes for a growth factor related to TGF-beta. Cell. 1987 Dec 4;51(5):861–867. doi: 10.1016/0092-8674(87)90109-7. [DOI] [PubMed] [Google Scholar]
- Wharton K. A., Johansen K. M., Xu T., Artavanis-Tsakonas S. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell. 1985 Dec;43(3 Pt 2):567–581. doi: 10.1016/0092-8674(85)90229-6. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bailes J. A., McMahon A. P. Expression of the proto-oncogene int-1 is restricted to specific neural cells in the developing mouse embryo. Cell. 1987 Jul 3;50(1):79–88. doi: 10.1016/0092-8674(87)90664-7. [DOI] [PubMed] [Google Scholar]