Abstract
Background
Implantable cardioverter defibrillator (ICD) therapy significantly prolongs life in patients at increased risk of sudden cardiac death from depressed left ventricular function. However, it is unclear whether this increased longevity is accompanied by deterioration in quality of life.
Methods
The Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) compared ICD therapy or amiodarone versus state-of-the-art medical therapy alone in 2521 stable heart failure patients with depressed left ventricular function. Quality of life, a secondary end point of the trial, was prospectively measured at baseline, 3, 12, and 30 months and was 93% to 98% complete. The Duke Activity Status Index (which measures cardiac physical functioning) and the SF-36 Mental Health Inventory (which measures psychological well-being or distress) were prespecified principal quality-of-life outcomes. Multiple additional quality-of-life outcomes were also examined.
Results
Compared with medical therapy alone, psychological well-being in the ICD arm significantly improved at 3 months (p=0.01) and 12 months (p=0.004) but not at 30 months. No clinically or statistically significant differences in physical functioning by treatment were observed. Some other quality-of-life measures improved in the ICD arm at 3 and/or 12 months but none differed significantly at 30 months. ICD shocks within the month preceding a scheduled assessment were associated with decreased quality of life in multiple domains. Amiodarone had no significant effects on the principal quality-of-life outcomes.
Conclusions
In a large primary prevention population with moderately symptomatic heart failure, single lead ICD therapy was not associated with any detectable adverse quality-of-life effects over 30 months of follow-up.
Keywords: Sudden cardiac death, congestive heart failure, implantable cardioverter-defibrillator, quality of life
INTRODUCTION
Implantable cardioverter-defibrillators (ICDs) significantly extend survival in patients who are at high risk for sudden cardiac death due to the severity of their underlying heart disease.1,2 Concerns have emerged, however, about the effects of ICD therapy on quality of life. One concern is that use of ICD therapy could trade a quick, relatively painless (albeit premature) death for a more unpleasant death due to progressive deterioration of the underlying heart disease or comorbidity.3 Further, in some previous studies, receipt of multiple ICD shocks has been associated with worse quality of life, although the causality of this relationship is unclear.
To date, only two secondary prevention trials (in which an ICD is implanted after a life-threatening arrhythmia, to prevent future events) and one primary prevention trial (in which an ICD is implanted in patients who are at increased risk but have not previously had a life-threatening arrhythmia) have reported quality of life outcomes.4–6 While these data have not shown any consistent evidence of worse quality of life with ICD therapy, the conclusions derivable from these studies are limited by methodological problems and relatively short follow-up.
The issue of long-term quality of life is particularly important in a primary prevention setting, where the willingness to accept a potentially unpleasant therapy for uncertain future benefit may be low. We therefore examined the effects of primary prevention ICD therapy on health-related quality of life in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Although amiodarone did not improve survival in SCD-HeFT, earlier work suggested it might improve functional status.7,8 Thus, we also report on the quality-of-life outcomes from the amiodarone versus placebo comparison in this trial.
METHODS
Patient Population and Study Overview
Between September 16, 1997 and July 18, 2001, SCD-HeFT enrolled 2521 patients ≥18 years of age with New York Heart Association (NYHA) Class II or III chronic stable congestive heart failure and a left ventricular ejection fraction ≤35 percent. Complete inclusion and exclusion criteria have been previously published.1 The etiology of heart failure was ischemic in 52 percent and nonischemic in 48 percent. Ninety percent of the study patients were enrolled in the U.S. with the remainder in Canada and New Zealand.
Study subjects were randomly assigned to state-of-the-art medical therapy plus either an amiodarone placebo, amiodarone (Cordarone, Wyeth-Ayerst Pharmaceuticals), or a conservatively programmed, single-chamber ICD (Medtronic, model 7223). Therapy was initiated in the outpatient setting by protocol. After a median follow-up of 45.5 months (range, 24 to 72 months), ICD therapy was associated with a 23% reduction in mortality compared to medical therapy.
Quality-of-Life Study
The quality-of-life portion of the trial was funded by the National Heart, Lung, and Blood Institute. Study design, data collection, data analysis and interpretation, and preparation of this manuscript were all performed by the authors of this paper. The corporate sponsors who contributed to the support of the trial (Wyeth-Ayerst, Knoll, and Medtronic) had no role in the design, analysis, or interpretation of the quality-of-life study.
All patients provided written informed consent, and the study was conducted in cooperation with the National Heart, Lung, and Blood Institute. Study protocol approval was obtained from each site’s institutional review board or ethics committee.
Quality-of-Life Data Collection Methods
Quality of life was measured by structured interview at baseline, 3 months, 12 months, and 30 months or end of study follow-up. Interviews were conducted by each site’s study coordinator at the time of a scheduled clinic visit, or by telephone if a clinic visit was missed. Specific training was provided to each site coordinator to ensure standardization of data collection. By protocol, baseline quality-of-life assessments were to be conducted after obtaining informed consent and prior to randomization. Follow-up quality-of-life assessments were to be performed within 1 month of the scheduled contact. For patients who were too ill to complete the full questionnaire, had a language barrier, or were otherwise unable to participate in the full interview, a short proxy form was collected.
Quality-of-Life Measures
Two measures were prespecified as principal end points for the quality-of-life portion of the trial: the Duke Activity Status Index (DASI), reflecting cardiac-specific physical functioning, and the Short Form (SF)-36 Mental Health Inventory (MHI-5), reflecting psychological well-being. DASI was constructed to be a questionnaire-based analog of the maximal exercise stress test used for cardiac patients and is scored from 0 (worst) to 58 (best) with a difference of 4 points or more being considered clinically significant.9 The SF-36 Mental Health Inventory (MHI-5) was used to assess psychological well-being/distress and is scored from 0 (worst) to 100 (best).10 A clinically significant difference in the MHI-5 has not been formally defined but can be approximated by one quarter of a standard deviation (5 points in this study).
Other scales from the SF-36 were used to assess role functioning (both physical- and emotional-related limitations), general health perceptions, bodily pain, social functioning, and vitality. Like the MHI-5, these are also scored from 0 (worst) to 100 (best) with one quarter of a standard deviation representing a reasonable guide to a clinically significant difference.
The quality-of-life interviews also collected information on total numbers of “bed days” (defined as the number of days out of the last 42 days in which the patient was at home in bed for all or most of the day because of their health) and “disability days” (defined as the number of days out of the last 42 days (not counting “bed days”) in which the patient had to cut down on usual activities because of their health), as well as an indication of whether the subject could currently drive a car (yes or no) and manage money independently (yes or no). Employment details were obtained using an abbreviated series of questions adapted from the Bypass Angioplasty Revascularization Investigation Substudy on Economics and Quality of Life.11 Assessment of heart failure-specific quality of life was based on the Minnesota Living with Heart Failure scale.12 This scale is scored from 0 (best) to 105 (worst), and a clinically significant difference is considered to be approximately 5 points.13
Patient-specific utilities, which indicate the relative desirability of each patients health state on a scale from 0 (death) to 1 (excellent health), were assessed using the time trade-off technique.14 Patients were asked to assume that they would have a life expectancy of five years in their current state of health, and then asked in a series of questions to decide how much of those five years they would be willing to trade to live the remaining time in excellent health. As a second, more intuitive global measure, patients were also asked to rate their health on a 0 to 100 scale, where 100 was assigned to excellent health and 0 to a state of health equivalent to being dead. A 5-point difference in this scale (one quarter of a standard deviation) approximates clinical significance.
Statistical Analyses
We used means and standard deviations, medians and 25th to 75th percentiles, or both to describe the distributions of continuous variables. Percentages were used to describe categorical variables. Univariate comparisons were performed using the Pearson Chi-Square Test for categorical variables and the Wilcoxon Rank Sum Test for continuous variables. Each active treatment was compared pair-wise with the placebo group.
Patients with an ICD who received a device shock within the month preceding a scheduled quality-of-life assessment were compared with ICD patients not receiving a shock in the same time period. These comparisons were based on the Wilcoxon rank sum test of the change scores from the most recent pre-shock quality-of-life measurements. This analysis was repeated using 2-month and 12-month time frames.
The primary end point of the trial was all-cause mortality, and a statistically significant difference was observed between ICD and placebo. This mortality difference resulted in a non-random subgroup of respondents surviving for quality-of-life comparisons. To account for this potential bias, we applied an estimator for the survival average causal effect (SACE) as a sensitivity analysis.15,16 These estimates are based on weighted averages of the observed quality-of-life data multiplied by treatment group-specific survival estimates, with p-values and 95% confidence intervals for the SACE estimates based on a non-parametric bootstrap procedure.17
All reported p-values are 2-sided. No adjustments were made for multiple testing.
RESULTS
Patient Population and Baseline Characteristics
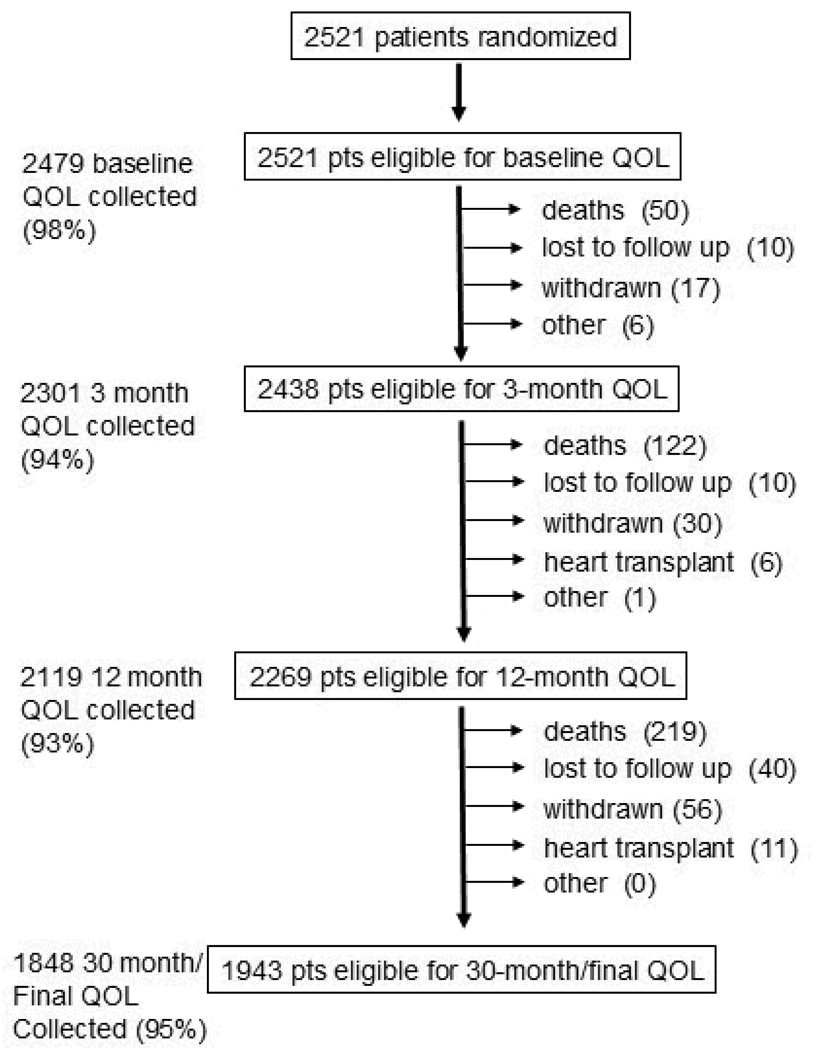
Of 2521 patients randomized, 2479 (98%) completed baseline quality-of-life questionnaires (Figure 1). The initial demographic and clinical characteristics of these patients were well balanced by treatment group (Table 1)1. At each follow-up interval, questionnaires were collected from 93% to 95% of eligible subjects. Overall, from a total of 9170 expected patient contacts, 8747 quality of life questionnaires were collected (95%). Patient refusal was rare (1.2%). Only 1.4% of forms were judged incomplete. In 69 interviews (0.11%), proxy forms were substituted for the full questionnaire.
Figure 1.
Graphic display of completeness of quality-of-life data collection according to follow-up anniversary. Major reasons for missing data are provided. Follow-up for vital status was 100% complete. QOL = quality of life.
Table 1.
Baseline Characteristics of Patients with a Baseline Quality-of-Life Assessment
| Amiodarone (n= 830) |
Placebo (n= 833) |
ICD (n= 816) |
|
|---|---|---|---|
| Age, years | 59.6 (±11.9) | 59.1 (±11.9) | 59.9 (±11.9) |
| female | 204 (24.6) | 190 (22.8) | 187 (22.9) |
| non-white | 188 (22.7) | 197 (23.6) | 184 (22.5) |
| NYHA class II | 592 (71.3) | 583 (70.0) | 556 (68.1) |
| Ischemic etiology |
420 (50.6) | 449 (53.9) | 425 (52.1) |
| Ejection fraction, % |
23.9 (±7.0) | 24.0 (±6.8) | 23.6 (±7.0) |
| Diabetes | 238 (28.7) | 263 (31.6) | 250 (30.6) |
| Hypertension | 457 (55.1) | 474 (56.9) | 448 (54.9) |
| Current smoker | 123 (14.8) | 139 (16.7) | 141 (17.3) |
| Pulmonary disease |
145 (17.5) | 158 (19.0) | 174 (21.3) |
| Atrial fibrillation/flutter |
129 (15.5) | 114 (13.7) | 139 (17.0) |
| Prior MI | 353 (84.0) | 388 (86.4) | 364 (85.6) |
| Prior Stroke | 53 (6.4) | 64 (7.7) | 43 (5.3) |
NYHA=New York Heart Association; MI=myocardial infarction; ICD = implantable cardioverter-defibrillator.
Age and ejection fraction values are means (standard deviation). All other values are N (percent). Race was self reported on the clinical case report form.
Quality of Life Outcomes
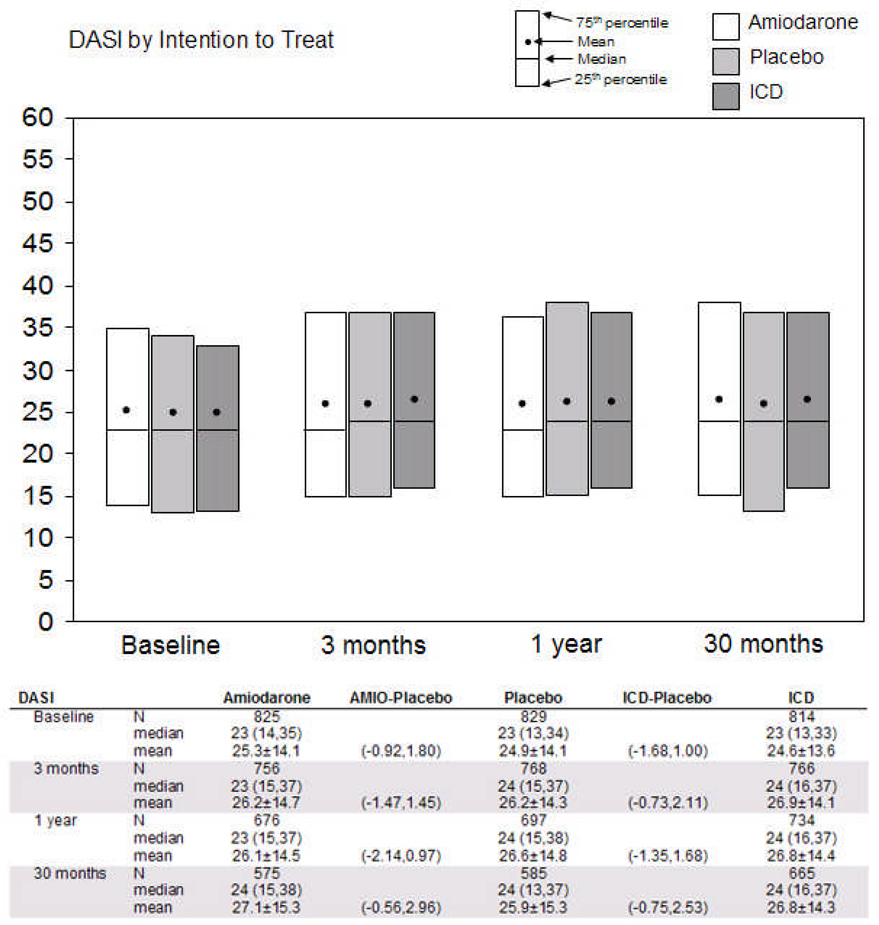
In unadjusted comparisons by treatment group, cardiac-specific physical functioning was not significantly different in the ICD and placebo arms at baseline (median DASI scores 23 vs. 23, p=0.76), 3 months, 12 months, and 30 months (median scores 24 vs. 24 for all 3 intervals, p>0.10) (Figure 2A). There were also no significant differences at any point between the amiodarone and placebo arms.
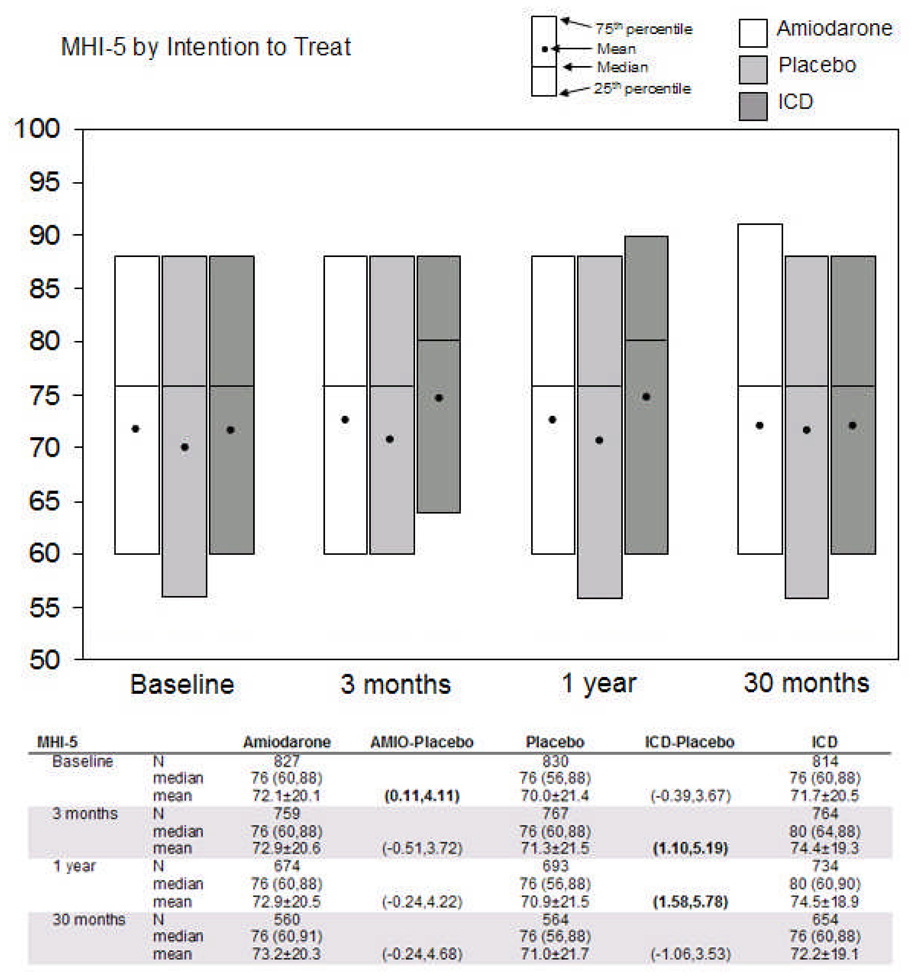
Figure 2.
Comparisons of two principal quality-of-life measures by intention-to-treat. Cardiac physical functioning is represented in 2A by the Duke Activity Status Index (DASI), which ranges from 0 (worst) to 58 (best). Psychological well-being is represented in 2B by the Short Form-36 Mental Health Inventory (SF-36 MHI-5), which ranges from 0 (worst) to 100 (best). Tables below the figures show medians with 25th and 75th percentiles, means with standard deviations, and 95% confidence intervals around the difference in means (active treatment minus placebo) at each time point. Comparisons with bolded values were statistically significant at a p≤ 0.05 level. ICD = implantable cardioverter-defibrillator; AMIO = amiodarone.
Psychological well-being did not differ significantly in the ICD and the placebo arms at baseline (median MHI-5 scores 76 vs. 76, p=0.17) but was better in the ICD arm at 3 months (median scores 80 vs. 76, p=0.01) and at 12 months (median scores 80 vs. 76, p=0.003) compared with placebo. At 30 months, the two arms were again not significantly different (median scores 76 vs. 76, p=0.79) (Figure 2B). Comparing the amiodarone and placebo arms, we did not observe significant differences at any point during follow-up.
Each of the six other SF-36 scales showed at least one interval comparison with significantly better scores for ICD patients. However, values were clinically similar and not statistically significantly different at baseline or at 30 months for any of these scales (Table 2). Patients in the amiodarone arm gave significantly higher scores on the SF-36 pain index at all four intervals; there were no significant differences for any of the other SF-36 scales.
Table 2.
Additional Quality-of-Life Measures by Intention to Treat
| Amiodarone | AMIO- Placebo* |
Placebo | ICD-Placebo* | ICD | ||
|---|---|---|---|---|---|---|
|
SF role function – physical (0–100)† |
||||||
| Baseline | N | 820 | 821 | 797 | ||
| median | 25 (0,50) | 0 (0,50) | 25 (0,50) | |||
| mean | 32.7±37.2 | (−1.54,5.78) | 30.6±38.4 | (−2.97,4.51) | 31.4±38.3 | |
| 3 months | N | 740 | 757 | 743 | ||
| median | 25 (0,75) | 25 (0,75) | 25 (0,75) | |||
| mean | 41.2±39.2 | (−2.00,6.08) | 39.2±40.5 | (0.34,8.47) | 43.6±39.8 | |
| 1 year | N | 665 | 684 | 719 | ||
| median | 50 (0,75) | 25 (0,100) | 50 (0,100) | |||
| mean | 45.0±40.0 | (−2.57,6.07) | 43.2±40.9 | (−0.63,7.92) | 46.9±40.8 | |
| 30 months | N | 541 | 556 | 634 | ||
| median | 25 (0,100) | 25 (0,75) | 25 (0,100) | |||
| mean | 44.4±40.6 | (−1.01,8.66) | 40.6±41.0 | (−1.05,8.28) | 44.2±40.8 | |
|
SF role function – emotional (0–100) |
||||||
| Baseline | N | 821 | 820 | 798 | ||
| median | 100 (33,100) | 100 (33,100) | 100 (33,100) | |||
| mean | 65.9±41.03 | (−1.04,7.06) | 62.9±42.5 | (−0.71,7.48) | 66.3±41.4 | |
| 3 months | N | 738 | 758 | 742 | ||
| median | 100 (33,100) | 100 (33,100) | 100 (33,100) | |||
| mean | 70.3±39.9 | (−0.42,7.87) | 66.6±41.8 | (1.37,9.54) | 72.0±38.9 | |
| 1 year | N | 666 | 682 | 720 | ||
| median | 100 (33,100) | 100 (33,100) | 100 (33,100) | |||
| mean | 69.7±40.0 | (−2.34,6.35) | 67.7±41.3 | (0.04,8.37) | 71.9±38.3 | |
| 30 months | N | 541 | 554 | 631 | ||
| median | 100 (33,100) | 100 (33,100) | 100 (33,100) | |||
| mean | 71.3±40.4 | (−3.20,6.48) | 69.6±41.2 | (−5.48,3.96) | 68.9±41.4 | |
| SF general health (0–100) | ||||||
| Baseline | N | 826 | 829 | 814 | ||
| median | 42 (30,57) | 40 (25,60) | 45 (30,60) | |||
| mean | 44.6±21.4 | (−1.27,2.93) | 43.8±22.2 | (−0.66,3.51) | 45.2±20.9 | |
| 3 months | N | 754 | 764 | 759 | ||
| median | 45 (30,62) | 45 (29,62) | 47 (32,65) | |||
| mean | 46.2±22.0 | (−1.58,2.96) | 45.6±23.1 | (1.02,5.53) | 48.8±21.7 | |
| 1 year | N | 669 | 692 | 726 | ||
| median | 45 (30,62) | 45 (27,62) | 47 (30,67) | |||
| mean | 46.9±22.8 | (−1.39,3.52) | 45.8±23.37 | (0.76,5.56) | 49.0±22.7 | |
| 30 months | N | 558 | 561 | 646 | ||
| median | 45 (30,62) | 45 (30,62) | 42 (30,62) | |||
| mean | 45.9±22.4 | (−2.16,3.22) | 45.4±23.4 | (−1.85,3.29) | 46.1±22.1 | |
| SF social function (0–100) | ||||||
| Baseline | N | 828 | 831 | 816 | ||
| median | 75 (50,100) | 75 (50,100) | 75 (50,100) | |||
| mean | 69.3±27.8 | (0.03,5.47) | 66.6±28.7 | (−1.13,4.35) | 68.2±28.0 | |
| 3 months | N | 760 | 770 | 764 | ||
| median | 75 (50,100) | 75 (50,100) | 75 (50,100) | |||
| mean | 71.3±27.4 | (−1.69,3.86) | 70.2±28.0 | (0.81,6.18) | 73.7±25.6 | |
| 1 year | N | 678 | 695 | 735 | ||
| median | 75 (50,100) | 75 (50,100) | 75 (50,100) | |||
| mean | 71.4±26.6 | (−2.34,3.31) | 70.9±26.8 | (1.12,6.50) | 74.7±25.1 | |
| 30 months | N | 560 | 571 | 657 | ||
| median | 75 (50,100) | 75 (50,100) | 75 (50,100) | |||
| mean | 71.8±26.6 | (−1.63,4.62) | 70.3±27.0 | (−2.06,3.90) | 71.2±26.2 | |
| SF pain index (0–100) | ||||||
| Baseline | N | 828 | 828 | 813 | ||
| median | 64 (41,84) | 62 (41,84) | 64 (41,84) | |||
| mean | 65.1±25.8 | (0.04,5.22) | 62.5±27.1 | (−0.16,5.02) | 64.9±26.5 | |
| 3 months | N | 761 | 770 | 764 | ||
| median | 72 (51,100) | 62 (41,84) | 72 (42,100) | |||
| mean | 68.8±26.7 | (3.18,8.62) | 62.9±27.7 | (2.42,7.75) | 68.0±25.5 | |
| 1 year | N | 677 | 695 | 736 | ||
| median | 72 (42,100) | 62 (41,84) | 72 (42,84) | |||
| mean | 66.9±26.6 | (1.51,7.16) | 62.6±26.7 | (1.95,7.42) | 67.3±26.1 | |
| 30 months | N | 561 | 570 | 657 | ||
| median | 72 (41,100) | 62 (41,84) | 64 (41,84) | |||
| mean | 67.8±27.5 | (2.59,8.83) | 62.1±26.1 | (−0.98,4.86) | 64.0±26.0 | |
| SF vitality (0–100) | ||||||
| Baseline | N | 827 | 830 | 814 | ||
| median | 45 (30,60) | 45 (25,60) | 40 (25,55) | |||
| mean | 44.3±22.4 | (−0.94,3.47) | 43.0±23.3 | (−2.84,1.57) | 42.4±22.2 | |
| 3 months | N | 760 | 768 | 764 | ||
| median | 50 (30,65) | 45 (30,65) | 50 (30,65) | |||
| mean | 46.1±23.6 | (−1.93,2.86) | 45.7±24.1 | (0.22,4.91) | 48.2±22.7 | |
| 1 year | N | 674 | 693 | 734 | ||
| median | 50 (30,65) | 50 (30,65) | 50 (35,65) | |||
| mean | 47.8±23.5 | (−1.17,3.83) | 46.4±23.6 | (−0.61,4.19) | 48.2±22.6 | |
| 30 months | N | 561 | 565 | 654 | ||
| median | 50 (30,60) | 50 (30,65) | 50 (30,60) | |||
| mean | 46.5±23.2 | (−2.24,3.21) | 46.0±23.4 | (−2.31,2.84) | 46.3±22.4 | |
| Bed days | ||||||
| Baseline | N | 814 | 823 | 811 | ||
| median | 0 (0,2) | 0 (0,2) | 0 (0,2) | |||
| mean | 2.1±5.0 | (−0.71,0.31) | 2.3±5.5 | (−0.69,0.31) | 2.1±4.8 | |
| 3 months | N | 750 | 761 | 759 | ||
| median | 0 (0,1) | 0 (0,1) | 0 (0,1) | |||
| mean | 1.7±4.5 | (−0.74,0.21) | 2.0±4.9 | (−0.81,0.13) | 1.7±4.5 | |
| 1 year | N | 660 | 690 | 723 | ||
| median | 0 (0,1) | 0 (0,1) | 0 (0,0) | |||
| mean | 1.8±4.8 | (−0.55,0.48) | 1.9±4.8 | (−0.91,−0.03) | 1.4±3.5 | |
| 30 months | N | 546 | 567 | 646 | ||
| median | 0 (0,1) | 0 (0,1) | 0 (0,1) | |||
| mean | 1.9±4.7 | (−0.99,0.19) | 2.3±5.4 | (−1.00,0.13) | 1.8±4.6 | |
| Disability days | ||||||
| Baseline | N | 811 | 821 | 807 | ||
| median | 2 (0,15) | 3 (0,15) | 3 (0,15) | |||
| mean | 8.3±10.7 | (−1.15,0.92) | 8.4±10.6 | (−0.72,1.33) | 8.7±10.6 | |
| 3 months | N | 749 | 758 | 754 | ||
| median | 0 (0,10) | 1 (0,10) | 0 (0,7) | |||
| mean | 6.9±10.2 | (−0.75,1.26) | 6.7±9.7 | (−1.86,0.04) | 5.7±9.1 | |
| 1 year | N | 653 | 687 | 725 | ||
| median | 1 (0,10) | 0 (0,10) | 0 (0,7) | |||
| mean | 6.4±9.5 | (−0.91,1.14) | 6.2±9.6 | (−1.53,0.42) | 5.7±9.1 | |
| 30 months | N | 545 | 563 | 643 | ||
| median | 0 (0,10) | 0 (0,10) | 0 (0,10) | |||
| mean | 6.2±9.5 | (−0.82,1.37) | 5.9±9.0 | (−1.03,1.00) | 5.9±8.9 | |
| Driving a car | ||||||
| Baseline | N | 827 | 833 | 814 | ||
| yes | 710 (85.9%) | (−0.03,0.10) | 700 (84.0%) | (−0.02,0.12) | 705 (86.6%) | |
| 3 months | N | 756 | 770 | 767 | ||
| yes | 637 (84.3%) | (−0.08,0.06) | 653 (84.8%) | (−0.04,0.10) | 662 (86.3%) | |
| 1 year | N | 677 | 697 | 738 | ||
| yes | 575 (84.9%) | (−0.07,0.08) | 589 (84.5%) | (−0.03,0.12) | 639 (86.6%) | |
| 30 months | N | 576 | 587 | 666 | ||
| yes | 490 (85.0%) | (−0.03,0.13) | 484 (82.5%) | (0.01,0.16) | 579 (86.9%) | |
| Manages money | ||||||
| Baseline | N | 825 | 829 | 813 | ||
| yes | 751 (91.0%) | (−0.12,0.05) | 763 (92.0%) | (−0.08,0.10) | 750 (92.3%) | |
| 3 months | N | 756 | 769 | 767 | ||
| yes | 690 (91.3%) | (−0.11,0.07) | 707 (91.9%) | (−0.10,0.09) | 704 (91.8%) | |
| 1 year | N | 676 | 697 | 735 | ||
| yes | 638 (94.4%) | (−0.07,0.15) | 651 (93.4%) | (−0.13,0.08) | 682 (92.8%) | |
| 30 months | N | 574 | 586 | 664 | ||
| yes | 526 (91.6%) | (−0.12,0.09) | 540 (92.2%) | (−0.07,0.14) | 618 (93.1%) | |
| Working full or part time | ||||||
| Baseline | N | 830 | 833 | 816 | ||
| yes | 244 (29.4%) | (−0.00,0.10) | 211 (25.3%) | (−0.04,0.07) | 215 (26.4%) | |
| 3 months | N | 761 | 772 | 768 | ||
| yes | 214 (28.1%) | (−0.02,0.09) | 197 (25.5%) | (−0.06,0.06) | 196 (25.5%) | |
| 1 year | N | 681 | 698 | 740 | ||
| yes | 187 (27.5%) | (0.00,0.12) | 159 (22.8%) | (−0.05,0.08) | 176 (23.8%) | |
| 30 months | N | 585 | 590 | 673 | ||
| yes | 154 (26.3%) | (−0.01,0.12) | 131 (22.2%) | (−0.09,0.05) | 140 (20.8%) | |
|
Living with Heart Failure (105-0) |
||||||
| Baseline | N | 824 | 819 | 805 | ||
| median | 42 (22,61) | 43 (21,63) | 41 (24,62) | |||
| mean | 42.0±24.6 | (−3.51,1.35) | 43.1±25.6 | (−2.92,1.93) | 42.6±24.1 | |
| 3 months | N | 745 | 750 | 736 | ||
| median | 33 (17,54) | 36 (14,59) | 30 (13,52) | |||
| mean | 36.7±24.2 | (−3.42,1.63) | 37.6±25.6 | ( −6.43,−1.42) | 33.7±23.6 | |
| 1 year | N | 638 | 666 | 704 | ||
| median | 33 (14,54) | 36 (14,59) | 32 (14,52) | |||
| mean | 35.6±24.6 | (−4.93,0.61) | 37.8±26.3 | ( −5.73,−0.45) | 34.7±23.6 | |
| 30 months | N | 458 | 492 | 549 | ||
| median | 33 (14,55) | 36 (17,59) | 32 (15,52) | |||
| mean | 36.5±24.8 | (−5.00,1.38) | 38.3±25.3 | ( −6.04,−0.05) | 35.3±24.0 | |
| Time Trade-Off (0–1) | ||||||
| Baseline | N | 818 | 824 | 808 | ||
| median | 0.98 (0.8,1.0) | 0.98 (0.8, 1.0 ) | 0.98 (0.8, 1.0) | |||
| mean | 0.8±0.3 | (−0.02,0.04) | 0.8±0.3 | (−0.04,0.03) | 0.8±0.3 | |
| 3 months | N | 752 | 761 | 758 | ||
| median | 0.98 (0.8, 1.0) | 0.98 (0.8, 1.0) | 0.98 (0.9, 1.0 ) | |||
| mean | 0.8±0.3 | (−0.02,0.05) | 0.8±0.3 | (0.01,0.07) | 0.9±0.3 | |
| 1 year | N | 667 | 694 | 728 | ||
| median | 0.98 (0.8, 1.0) | 0.98 (0.9, 1.0) | 0.98 (0.8, 1.0) | |||
| mean | 0.8±0.3 | (−0.03,0.03) | 0.9±0.3 | (−0.03,0.03) | 0.9±0.3 | |
| 30 months | N | 551 | 562 | 643 | ||
| median | 0.98 (0.8, 1.0) | 0.98 (0.9, 1.0 ) | 0.98 (0.9, 1.0) | |||
| mean | 0.9±0.3 | (−0.03,0.03) | 0.9±0.3 | (−0.03,0.03) | 0.9±0.3 | |
| Self-rating scale (0–100) | ||||||
| Baseline | N | 786 | 791 | 781 | ||
| median | 65 (50,80) | 60 (50,80) | 65 (50,80) | |||
| mean | 62.9±20.7 | (−1.82,2.39) | 62.6±21.9 | (−2.03,2.22) | 62.7±21.1 | |
| 3 months | N | 719 | 702 | 712 | ||
| median | 70 (50,80) | 70 (50,80) | 75 (50,85) | |||
| mean | 65.9±20.6 | (−2.32,2.00) | 66.1±21.0 | (1.39,5.61) | 69.6±19.5 | |
| 1 year | N | 629 | 645 | 681 | ||
| median | 70 (50,85) | 70 (50,85) | 75 (51,85) | |||
| mean | 67.6±21.2 | (−1.28,3.44) | 66.5±21.7 | (0.68,5.11) | 69.4±19.4 | |
| 30 months | N | 506 | 529 | 599 | ||
| median | 70 (50,80) | 70 (50,80) | 70 (50,85) | |||
| mean | 67.1±20.9 | (−1.60,3.63) | 66.1±21.9 | (−0.56,4.47) | 68.0±21.1 | |
Continuous variables shown as median (25th to 75th percentiles). Categorical variables are shown as percentages. Means are shown with standard deviations. AMIO = amiodarone; ICD = implantable cardioverter-defibrillator; SF = Short Form 36.
Columns show 95% confidence intervals for difference in mean values for continuous variables or in percentages for categorical variables (active treatment minus placebo). Intervals corresponding to a nominal p-value ≤0.05, uncorrected for multiple comparisons, are shown in bold.
Ranges for each scale are provided in parenthesis.
At baseline, patients reported a mean of approximately 2 bed days and 8 to 9 disability days over the preceding 42 days. In addition, 86% of patients were able to drive a car, 92% could manage their finances independently, and 27% were employed outside the home. Compared to medical therapy alone, we were not able to detect an effect of ICD therapy on the number of bed days or disability days, nor on the proportion of patients who were able to drive a car, manage their finances, or maintain employment over the follow-up period.
The Minnesota Living with Heart Failure scores were well balanced at baseline (median scores 41 for ICD vs. 43 for placebo, p=0.77). They were generally lower (i.e., better) in the ICD arm at 3 months (median scores 30 vs. 36, p=0.006), 12 months (median scores 32 vs. 36, p=0.07), and 30 months (median scores 32 vs. 36, p=0.05).
Time trade-off utilities averaged 0.80 at baseline in all 3 treatment groups; there was a significant improvement in the ICD arm over the placebo arm at 3 months, but not at any of the other time points. On a scale of 0 (worst) to 100 (best), ICD patients rated their overall health more highly than did placebo patients at 3 months (median scores 75 vs. 70, p=0.002) and 12 months (median scores 75 vs. 70, p=0.05) but there was no statistically significant difference at 30 months (median scores 70 vs. 70, p=0.18).
Effect of ICD Shocks on Quality of Life
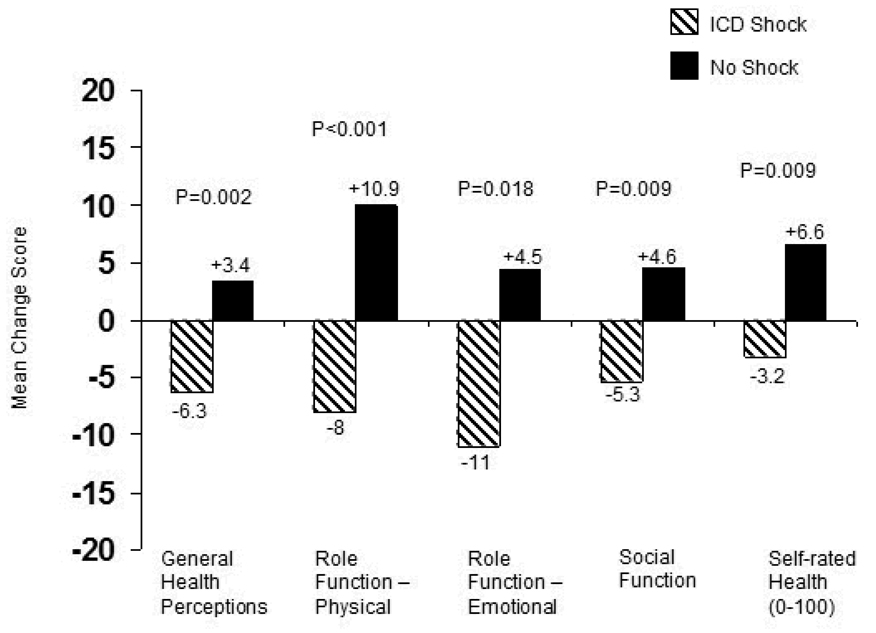
In the ICD arm, 49 patients had an ICD shock within 1 month of a subsequent scheduled quality-of-life assessment. Compared with ICD patients without a shock, quality of life in the month following a shock was characterized by significant decreases in general health perceptions, role function physical , role function emotional, social function, and self rated health 0–100 (Figure 3, all comparisons unadjusted).
Figure 3.
Effect of ICD shocks on Short Form-36 quality-of-life domains. Patients in the ICD arm who had an ICD shock within 1 month following a scheduled quality-of-life follow-up assessment were compared with ICD patients without an ICD shock. Change scores for the ICD shock group were calculated as the post-shock value minus the most recent pre-shock value. Change scores for the comparison group were the 3 month quality of life values minus baseline. When we used other follow-up contacts (12 months, 24 months) to construct the “no shock” change scores, results were unaltered.
Including patients who had a shock within 2 months of a scheduled quality-of-life assessment (n=66) showed the same patterns but diminished magnitude of differences. When we compared the 12-month quality of life of surviving patients who had an ICD shock at any time in the first study year (n=100) with those who did not (n=638), no statistically significant differences were evident. We also did not find a statistically significant effect on subsequent quality of life of ICD discharges above an arbitrary number, ranging from 2 to ≥5.
Survival-Adjusted Analyses
To account for the improved survival of the ICD-arm patients, we estimated the survival average causal effect for each quality-of-life variable. Overall, the results were not materially different from the unadjusted comparisons described above (Table 3).
Table 3.
Treatment Differences in Quality-of-Life Measures, Taking Mortality Differences into Account*
| Amiodarone vs. Placebo | |||
|---|---|---|---|
| 3 Months (95% CI) | 12 Months (95% CI) | 30 Months (95% CI) | |
| Principal Quality-of-Life End Points | |||
| DASI | −0.06 (−1.42,1.31) | −0.72 (−2.29, 0.84) | 0.99 (−0.81,2.80) |
| MHI-5 | 1.57 (−0.51, 3.65) | 1.92 (−0.32, 4.16) | 2.05 (−0.55, 4.64) |
| Additional Quality-of-Life End Points | |||
| SF-Body Pain | 5.82 (3.07, 8.58) | 4.12 (1.28, 6.96) | 5.54 (2.71, 8.37) |
| SF-General | 0.69 (−1.50, 2.88) | 0.83 (−1.56, 3.23) | 0.44 (−2.23, 3.12) |
| Health | |||
| SF-Role | 3.66 (−0.37, 7.69) | 1.96 (−2.23, 6.15) | 1.14 (−4.01, 6.29) |
| Emotional | |||
| SF-Role Physical | 1.99 (−1.71, 5.69) | 1.46 (−2.32, 5.24) | 3.44 (−1.21, 8.09) |
| SF-Social | 1.10 (−1.84, 4.05) | 0.36 (−2.32, 3.03) | 1.36 (−1.62, 4.34) |
| Functioning | |||
| SF-Vitality | 0.45 (−1.83, 2.73) | 1.17 (−1.35, 3.69) | 0.23 (−2.30, 2.77) |
| 0–100 rating scale | −0.15 (−2.20, 1.89) | 1.01 (−1.30, 3.32) | 1.02 (−1.63, 3.68) |
| Time Tradeoff | 0.08 (−0.09, 0.24) | −0.02 (−0.19, 0.14) | −0.00 (−0.18, 0.17) |
| ICD vs. Placebo | |||
| 3 Months(95% CI) | 12 Months(95% CI) | 30 Months(95% CI) | |
| Principal Quality-of-Life End Points | |||
| DASI | 0.64 (−0.84, 2.12) | 0.01 (−1.55, 1.58) | 0.61 (−1.03, 2.26) |
| MHI-5 | 3.13 (1.09, 5.18) | 3.56 (1.43, 5.69) | 1.02 (−1.38, 3.41) |
| Additional Quality-of-life End Points | |||
| SF-Body Pain | 4.99 (2.18, 7.81) | 4.48 (1.64, 7.32) | 1.58 (−1.20, 4.36) |
| SF-General | 3.24 (0.84, 5.64) | 2.97 (0.54, 5.40) | 0.53 (−2.02, 3.09) |
| Health | |||
| SF-Role | 5.38 (1.45, 9.32) | 4.06 (−0.02, 8.14) | −0.98 (−6.10, 4.14) |
| Emotional | |||
| SF-Role Physical | 4.36 (0.50, 8.22) | 3.48 (−0.61, 7.57) | 3.35 (−1.54, 8.23) |
| SF-Social | 3.43 (0.65, 6.20) | 3.68 (1.21, 6.14) | 0.79 (−2.07, 3.64) |
| Functioning | |||
| SF-Vitality | 2.50 (0.09, 4.91) | 1.62 (−0.74, 3.99) | 0.03 (−2.62, 2.67) |
| 0–100 rating scale | 3.49 (1.49, 5.50) | 2.83 (0.63, 5.02) | 1.94 (−0.57, 4.45) |
| Time Tradeoff | 0.19 (0.03, 0.34) | −0.01 (−0.16, 0.14) | −0.00 (−0.16, 0.16) |
Values in table are mean differences in quality-of-life scales between treatment groups at each follow-up point, adjusted for differences in survival. Positive values indicate active treatment better than placebo. Comparisons with bolded values were statistically significant at a p≤ 0.05 level.
DASI = Duke Activity Status Index; MHI-5 = Short Form 36 Mental Health Inventory; SF = Short Form 36; ICD = implantable cardioverter-defibrillator.
DISCUSSION
In the SCD-HeFT trial, single-lead ICD therapy enhanced survival and did not detectably diminish health-related quality of life during follow-up of up to 30 months in stable patients with moderately symptomatic heart failure. Although ICD therapy for primary prevention of sudden death was not expected to improve quality of life, the possibility of harm from either psychological or physical complications of the therapy was of significant concern. In our overall comparisons by randomized treatment assignment, we found no statistically or clinically significant evidence of either. In a double-blind comparison of amiodarone with placebo, we failed to detect an effect on either of the two principal quality-of-life measures.
In ICD patients who fortuitously had an ICD shock within the month preceding their scheduled follow-up, quality of life was diminished in multiple domains. Including patients who received shocks out to two months before a quality-of-life assessment showed the same trends but with reduced magnitude and statistical significance. Extending the window between the ICD shock and subsequent assessment to 1 year eliminated these trends altogether. While it may be plausible to assume that this association is causal, our analyses do not have sufficient statistical power to examine the relative contributions of the ICD shocks and concomitant deteriorations in clinical status to these observations.
Among other primary prevention trials of ICD therapy, only the Coronary Artery Bypass Graft (CABG)-Patch trial has reported on quality-of-life outcomes.18 At six months, the ICD arm had significantly lower levels of psychological well-being than the control arm. Further, patients who had received at least one ICD shock had reduced quality of life in several different dimensions. Important differences between the CABG-Patch quality-of-life study and the present study include the ICD technology (large, bulky ICD versus small, low profile ICD), the method of ICD implantation (open chest with abdominal pocket versus outpatient transvenous with pectoral pocket), and the target population (patients referred for CABG with ejection fraction ≤35% versus stable heart failure with ejection fractions ≤35% and about 50% non-ischemic etiology).
The largest prior study of quality of life with ICD therapy was an analysis from the Antiarrhythmics Versus Implantable Defibrillators (AVID) trial, a secondary prevention trial that was stopped prematurely by the Data and Safety Monitoring Board for efficacy.4,19 The ICD arm and antiarrhythmic therapy arm showed similar changes in SF-36 physical and mental component scores. Among ICD patients with complete data on ICD shocks and follow-up quality-of-life data, the occurrence of 1 or more ICD shocks was significantly associated with subsequent reductions in both physical functioning and mental well-being. Major differences with the present study include the different study populations (patients with life-threatening arrhythmias versus stable heart failure) and a significantly lower quality-of-life data collection rate in AVID (83% at baseline and 61% at one year). Nonetheless, a reasonable conclusion from the AVID analysis is that, in the absence of administered shock therapy, ICDs were well tolerated and did not diminish the quality of life of recipients.
The Canadian Implantable Defibrillator Study (CIDS), another secondary prevention ICD trial, obtained quality-of-life data at 6 and 12 months of follow-up.5 Emotional and physical health scores improved in the ICD arm relative to the amiodarone arm. ICD patients who received ≥5 shocks did not show improvement in these quality-of-life scales. However, ICD patients who received 1 to 4 shocks during follow-up were not distinguishable in quality of life from ICD patients who received no shocks.
All studies of the effects of ICD therapy on quality of life, including the present one, are limited by inability to blind the therapy. Thus, the perceived effects of ICDs found in this study may reflect attitudes of the study doctors and nurses transmitted to the patients, as well as the beliefs and expectations of the patients themselves. Patients may view the ICD either as an electronic security blanket or as an unpredictable and uncontrollable source of physical and emotional discomfort. In this unblinded comparison, we observed small improvements in some domains of quality of life during the first year of follow-up in the ICD group. We have no direct means of testing whether these improvements reflect the effects of such biases.
Caution should be exercised in interpreting significant differences in quality-of-life measures by treatment given the numerous statistical tests performed in this study. P values shown are uncorrected for multiple comparisons. Since quality of life was a secondary end point, the study was not constructed to test formally for non-inferiority of ICD therapy on these outcomes.
Our evaluation of the effects of ICD shocks on subsequent quality of life was limited by the lack of quality-of-life data linked to the occurrence of the ICD therapies (which was judged logistically infeasible) and by the relatively small number of patients with a quality-of-life assessment shortly after a shock episode. We did not have enough patients with multiple shock episodes over 24 hours (“ICD storm”) to define the effects of that phenomenon on subsequent quality of life.
In conclusion, we evaluated the quality of life of moderately symptomatic stable heart failure patients enrolled in the SCD-HeFT trial. Randomization to the ICD arm was not associated with adverse effects on health-related quality of life over the first 30 months of follow-up.
Supplementary Material
Acknowledgments
We gratefully acknowledge the editorial support provided by Melanie R. Daniels and the assistance of Judy Stafford in preparing the data for analysis. We are particularly indebted to the SCD-HeFT site coordinators who worked so hard to collect the data for this study and the SCD-HeFT patients who volunteered to be a part of the study.
Supported by grants (UO1 HL55496, UO1 HL55766, UO1 HL55297) from the National Heart, Lung, and Blood Institute/ National Institutes of Health, Bethesda, MD. Additional support to the SCD-HeFT clinical trial from Medtronic, Wyeth-Ayerst Laboratories, and Knoll Pharmaceuticals.
Footnotes
Disclosures
Dr. Mark reports having received grant support and speaking fees from Medtronic. Dr. Anstrom reports having received grant support from Medtronic Vascular. Dr. Bardy reports having received research funding from Philips Medical, Medtronic, and Laerdal Medical, having served as a consultant to Philips Medical, and having intellectual property rights from Medtronic; he is a founder of, board member of, and equity holder in Cameron Health. Dr. Lee reports having received research funding from Medtronic and Wyeth–Ayerst pharmaceuticals and having received speaking fees from Guidant and Medtronic.
REFERENCES
- 1.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 2.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 3.Kolata G. Defibrillator May Prolong Patient's Life. The New York Times. 2002 April 1; [Google Scholar]
- 4.Schron EB, Exner DV, Yao Q, Jenkins LS, Steinberg JS, Cook JR, Kutalek SP, Friedman PL, Bubien RS, Page RL, Powell J. Quality of life in the antiarrhythmics versus implantable defibrillators trial: impact of therapy and influence of adverse symptoms and defibrillator shocks. Circulation. 2002;105:589–594. doi: 10.1161/hc0502.103330. [DOI] [PubMed] [Google Scholar]
- 5.Irvine J, Dorian P, Baker B, O'Brien BJ, Roberts R, Gent M, Newman D, Connolly SJ. Quality of life in the Canadian Implantable Defibrillator Study (CIDS) Am Heart J. 2002;144:282–289. doi: 10.1067/mhj.2002.124049. [DOI] [PubMed] [Google Scholar]
- 6.Bigger JT., Jr for the Coronary Artery Bypass Graft (CABG) Patch Trial Investigators. Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary-artery bypass graft surgery. N Engl J Med. 1997;337:1569–1575. doi: 10.1056/NEJM199711273372201. [DOI] [PubMed] [Google Scholar]
- 7.Doval HC, Nul DR, Grancelli HO, Perrone SV, Bortman GR, Curiel R Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA) Randomised trial of low-dose amiodarone in severe congestive heart failure. Lancet. 1994;344:493–498. doi: 10.1016/s0140-6736(94)91895-3. [DOI] [PubMed] [Google Scholar]
- 8.Singh SN, Fletcher RD, Fisher SG, Singh BN, Lewis HD, Deedwania PC, Massie BM, Colling C, Lazzeri D. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure. N Engl J Med. 1995;333:77–82. doi: 10.1056/NEJM199507133330201. [DOI] [PubMed] [Google Scholar]
- 9.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, Cobb FR, Pryor DB. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index) Am J Cardiol. 1989;64:651–654. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 10.Ware JE, Jr, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual & Interpretation Guide. Boston: Nimrod Press; 1993. [Google Scholar]
- 11.Hlatky MA, Rogers WJ, Johnstone I, Boothroyd D, Brooks MM, Pitt B, Reeder G, Ryan T, Smith H, Whitlow P, Wiens R, Mark DB. Medical care costs and quality of life after randomization to coronary angioplasty or coronary bypass surgery. Bypass Angioplasty Revascularization Investigation (BARI) Investigators. N Engl J Med. 1997;336:92–99. doi: 10.1056/NEJM199701093360203. [DOI] [PubMed] [Google Scholar]
- 12.Rector TS, Kubo SH, Cohn JN. Patients' self-assessment of their congestive heart failure. Part 2: Content, reliability and validity of a new measure, The Minnesota Living with Heart Failure Questionnaire. Heart Failure. 1987 October/November;:198–209. [Google Scholar]
- 13.Rector TS. Overview of the Minnesota Living with Heart Failure(TM) Questionnaire. 2005 http://www.mlhfq.org/_dnld/mlhfq_overview.pdf.
- 14.Torrance GW, Feeny D. Utilities and quality-adjusted life years. Tech Assess Health Care. 1989;5:559–575. doi: 10.1017/s0266462300008461. [DOI] [PubMed] [Google Scholar]
- 15.Hayden D, Pauler DK, Schoenfeld D. An estimator for treatment comparisons among survivors in randomized trials. Biometrics. 2005;61:305–310. doi: 10.1111/j.0006-341X.2005.030227.x. [DOI] [PubMed] [Google Scholar]
- 16.Dawid AP. Causal Inference without Counterfactuals. J Am Stat Assoc. 2000;95:407–448. [Google Scholar]
- 17.Efron E, Tibshirani RJ. An introduction to the bootstrap. New York: Chapman and Hall; 1993. [Google Scholar]
- 18.Namerow PB, Firth BR, Heywood GM, Windle JR, Parides MK. Quality-of-life six months after CABG surgery in patients randomized to ICD versus no ICD therapy: findings from the CABG Patch Trial. Pacing Clin Electrophysiol. 1999;22:1305–1313. doi: 10.1111/j.1540-8159.1999.tb00623.x. [DOI] [PubMed] [Google Scholar]
- 19.The Antiarrhythmics Versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576–1583. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.