Abstract
Objectives
We have previously shown that dexamethasone increases serum leptin in fed but not in fasted human subjects. We hypothesized that insulin and/or glucose mediated the effect of food intake. The primary aim of this study was to determine whether the administration of a pulse of insulin with dexamethasone was sufficient to increase serum leptin in vivo in fasted human subjects. Whether the presence of transient hyperglycemia and the dose of insulin were important was tested as a secondary aim.
Methods
Twenty-nine normal subjects were studied. In experiment 1 (meal-like), a pulse of insulin (0.03 U/kg s.c.) and of dexamethasone (2 mg i.v.) was given, and the blood glucose transiently elevated to 50 mg/dl above baseline for the first 2 h. In experiments 2 and 3 (dose-response), the effect of two doses of insulin (0.03 U/kg in experiment 2 and 0.06 U/kg in experiment 3) was tested in combination with dexamethasone, this time without transient hyperglycemia. Nine subjects were studied under fasting conditions, with or without dexamethasone, as a control experiment.
Results
A meal-like transient hyperinsulinemia and hyperglycemia, with a pulse of dexamethasone, increased serum leptin levels from baseline by 54±21% at 9 h (P = 0.038). In the absence of transient hyperglycemia, leptin increased significantly after doses of both insulin and dexamethasone. The effect of insulin was dose-dependent, with a larger increment of serum leptin at 9 h after the highest dose of insulin (75.2±15.7% vs 21.3±8.5%, P = 0.013). Fasting, with or without dexamethasone, resulted in a significant 20% decrease in leptin from morning basal levels. Conversely, the administration of a pulse of insulin and glucose, in the absence of dexamethasone, prevented the drop in serum leptin observed during fasting, regardless of the insulin dose or the serum glucose elevation.
Conclusions
With the permissive effect of dexamethasone, a single pulse of insulin triggered a rise in serum leptin in humans, even in the absence of transient hyperglycemia. A single pulse of insulin with glucose can prevent the drop in serum leptin normally observed during fasting.
Introduction
Leptin, the peptide hormone secreted by the fat cells, is secreted in proportion to fat mass (1) as well as regulated by nutritional (2, 3) and hormonal factors, such as glucocorticoids and insulin. Glucocorticoids and insulin have both been shown to be part of leptin regulation. Oral administration of dexamethasone at doses varying from 1.5 to 6 mg per day increases serum leptin in humans (4–8). We have shown that this effect is dependent on food intake (9). A pulse of dexamethasone followed by three meals stimulated leptin by 100% at 10 h. In contrast, the administration of dexamethasone did not increase leptin levels in fasted subjects. The factors related to food intake (gastrointestinal hormones, insulin and/or glucose or other nutrients) that synergize with dexamethasone to increase leptin are unknown.
Continuous infusion of insulin at supraphysiological (823 and 2843 pmol/l) (10) or more physiological levels (480 pmol/l) (11) increases serum leptin after 6 h in human subjects. A continuous infusion of insulin for 9 h, resulting in plasma insulin levels of between 380 and 790 pmol/l, increases plasma leptin by 26 and 43% from baseline at 9 h (12). These insulin levels are comparable with the ones achieved with meals in our previous study (360–1050 pmol/l) (9). There is some suggestion that glucose also plays a role in leptin regulation (2). Glucose infusion stimulates leptin in fasting human subjects (13).
The goal of this present study was to test the hypothesis that a transient rise in insulin and glucose, similar to that provoked by a meal in our previous study, would increase serum leptin after administration of a pulse of dexamethasone. Whether the presence of transient hyperglycemia and the dose of insulin were important was tested as a secondary aim.
Subjects and methods
Subjects
Twenty-nine subjects (25 males, 4 females), mean age 26±5 years, were studied. They were all healthy, non-smokers, non-obese (body mass index = 25±2 kg/m2), with a normal physical examination, routine blood work and thyroid function tests, taking no medication, not depressed as assessed by the Beck questionnaire (14), with an oral glucose tolerance test within the normal range, and a stable body weight for at least 3 months prior to the study. Total body fat (mean 21±6%) was measured by DEXA scan (15). Their baseline glucose, insulin and leptin levels were respectively 5.1±0.4 mmol/l, 71±29 pmol/l and 5±4 ng/ml. The study protocols were approved by the Institutional Review Board of St Luke's/Roosevelt Hospital, and informed written consent was obtained prior to inclusion in the study.
Experimental design
Subjects came to the Clinical Research Center at 0745 h after an overnight fast. An i.v. catheter was inserted into an antecubital vein, and the i.v. line was kept open with a slow infusion of 0.45% sodium chloride during the entire experiment. This line was used for blood sampling. Another i.v. catheter was inserted in an antecubital vein of the contralateral arm for 20% glucose infusion via a peristaltic pump (Gemini PC-1; Imed, San Diego, CA, USA).
Experiment 1: Effect of a transient ‘meal-like’ rise of glucose and insulin with a pulse of dexamethasone on serum leptin
This experiment was designed to mimic the meal-induced rises in insulin and glucose, with or without co-administration of dexamethasone. After baseline measurements, all subjects (n = 8) received a subcutaneous pulse of fast and short-acting insulin (Humalog; Lilly, Indianapolis, IL, USA) at a dose of 0.03 U/kg. Subjects simultaneously received either 2 mg dexamethasone (insulin plus dexamethasone condition) or saline (insulin-only condition) administered as an i.v. pulse at 0830 h. Blood glucose was determined with a glucose analyzer (Beckman, Brea, CA, USA) at short intervals and the glucose level was adjusted by varying the rate of the 20% glucose infusion. Blood glucose levels were maintained at 50 mg/dl above baseline for 1 h after treatment was given (hyperglycemic clamp), then at baseline levels (euglycemic clamp) for the rest of the experiment. Blood samples were taken every 30 min for the next 9 h for serum leptin, C-peptide, insulin and free fatty acids (FFA).
Experiments 2 and 3: Effect of two doses of insulin given as a pulse (0.03 U/kg in experiment 2 and 0.06 U/kg in experiment 3) and dexamethasone, in the absence of transient hyperglycemia
These experiments were designed to test the role of a transient rise in glucose. Each experiment was carried out exactly as described in experiment 1, except that blood glucose was maintained at baseline levels during the entire experiment. Additionally, to test the dose-response, the insulin dose was varied: 0.03 U/kg in experiment 2 (n = 7) and 0.06 U/kg in experiment 3 (n = 8).
Therefore, in all three experiments, subjects were studied under two conditions: the insulin and dexamethasone condition (insulin and dexamethasone, 2 mg i.v.) and the insulin-only condition (insulin and saline). The two conditions were randomly assigned for experiment 1. In experiments 2 and 3, the insulin and dexamethasone condition was always run first in order to match the slight hyperglycemic effect of dexamethasone during the insulin-only condition. For each experiment, the two conditions were separated by 1 week to 1 month. The dose of dexamethasone used in this study (2 mg) is half that which was used in our meal study (9). A preliminary dose-response study showed that 2 mg was as effective at increasing leptin (16), and likely to induce fewer side-effects.
Experiment 4: Control fasting
A control group of nine subjects was studied after an overnight fast for another 9 h of fast, with saline administration, with or without dexamethasone given as an i.v. pulse.
Statistical analysis
The response variables were the serum leptin and insulin levels at every time-point, and the area under the curve (AUC) for glucose, leptin, C-peptide, insulin and FFA. The relative change in leptin at 9 h was defined as: ((leptin at 540 min-leptin at 0 min)/leptin at 0 min) × 100. Delta increment was defined as the difference in the change of one variable between the two conditions of the same experiments. An independent t-test was used to compare a variable under two different conditions within each experiment. General linear model analysis with repeated measures was performed to evaluate the change in leptin from baseline separately for each condition of the three experiments. Correlation analysis was used between leptin increment and glucose infusion rate. Regression analysis between the delta of the change in leptin and FFA between the two conditions of each experiment was done. All data are presented as means±s.e.m. unless specified otherwise. The data were analyzed using SPSS for PC (17) with a level of significance of 0.05.
Assays
Glucose was measured with a Beckman glucose analyzer. Plasma insulin and leptin (kits from Linco, St Charles, MO, USA) and C-peptide (DPC, Los Angeles, CA, USA) were determined by radioimmunoassay. FFA were measured by an enzymatic method (Wako Chemicals USA, Inc., Richmond, VA, USA). All plasma samples were run in duplicate and data for each subject (two conditions) were analyzed simultaneously. The interassay coefficient of variation (CV) for leptin was 8.4% and 5.2% and the intra-assay CV 7.2% and 5.3% for CV levels of 2.8 ng/ml and 20.3 ng/ml respectively. The interassay CV for insulin was 8.5% and 6.9% and the intra-assay CV 6.2% and 5.2% for levels of 10.4 μU/ml and 45 μU/ml respectively.
Results
Insulin and dexamethasone conditions
Experiment 1
The rises in glucose and insulin, shown in Fig. 1, followed the same pattern as those observed after food intake in our previous experiments (9). The peak increases in serum glucose were 8.5± 0.6 mmol/l and 8.6±0.5 mmol/l, with and without dexamethasone respectively (P = 0.905), levels similar to the one seen in our previous meal studies. Peak serum insulin levels were 279±49 pmol/l and 382±52 pmol/l respectively. In previously published experiments, insulin levels peaked at 450 pmol/l after a breakfast and 4 mg dexamethasone (9) and at 600 pmol/l after a 1700 kcal meal and 2 mg dexamethasone (18). Plasma insulin and C-peptide (expressed as peak values and AUC) and plasma glucose (expressed as peak values) were not significantly different between the two conditions (with and without dexamethasone).
Figure 1.
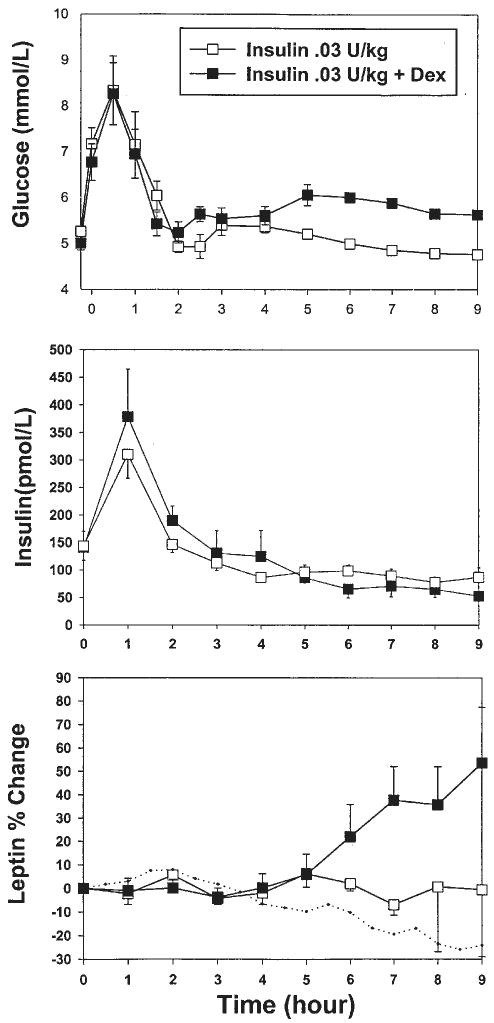
Experiment 1; ‘meal-like’. Changes over time in serum glucose, insulin and leptin levels after administration of a pulse of insulin with or without dexamethasone (Dex), in the presence of a transient rise in glucose (n = 8). The dotted line represents the change in serum leptin under fasting conditions (n = 9).
When given together with dexamethasone, a pulse of insulin and transient rise in glucose resulted in an increase in serum leptin at 9 h (54±21% above baseline, P = 0.038), with a significant effect starting 6.5 h after treatment (Fig. 1).
The AUC for FFA was significantly higher in the insulin plus dexamethasone condition than in the insulin-only condition (73±24 vs 44±18 mmol/l per 9 h, P = 0.015). The administration of insulin resulted in a decrease in FFA levels from baseline for 5 h, followed by a subsequent increase in FFA levels to baseline (Fig. 2). In the insulin plus dexamethasone condition, the suppressing effect of insulin on FFA was shorter, about 2 h, after which FFA levels increased to reach levels 44% higher than the insulin-only condition, indicating a lipolytic effect of dexamethasone. The effect of dexamethasone on leptin persisted after adjustment for FFA changes.
Figure 2.
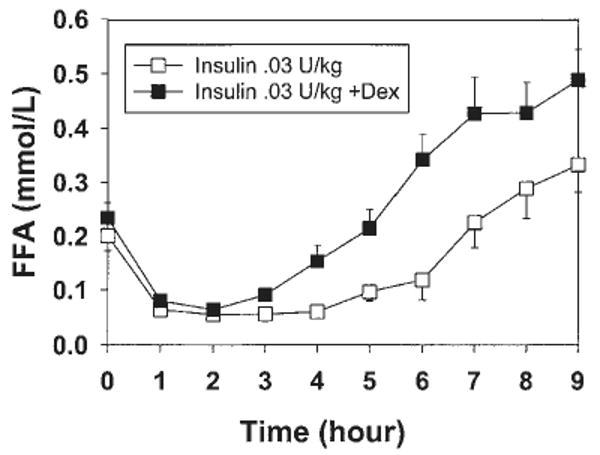
Experiment 1; ‘meal-like’. Changes over time in serum FFA levels after administration of a pulse of insulin with or without dexamethasone (Dex), in the presence of a transient rise in glucose (n = 8).
Experiments 2 and 3
In experiments 2 and 3, glucose levels were not raised above baseline for the entire experiment, and the glucose level was not different between the two conditions with and without dexamethasone (Fig. 3). The peak increase in serum insulin was, respectively, for the insulin-only and the insulin plus dexamethasone conditions, 25.0±2.2 μU/ml and 25.7±2.9 μU/ml after 0.03 U/kg, and 39.5± 1.9 μU/ml and 44.0±4.1 μU/ml after 0.06 U/kg s.c. insulin. Insulin levels returned to baseline levels 2 h after insulin administration in all the experiments (Fig. 3). In experiments 2 and 3, plasma glucose, insulin and C-peptide (expressed as AUC) were not significantly different between the conditions (with and without dexamethasone).
Figure 3.
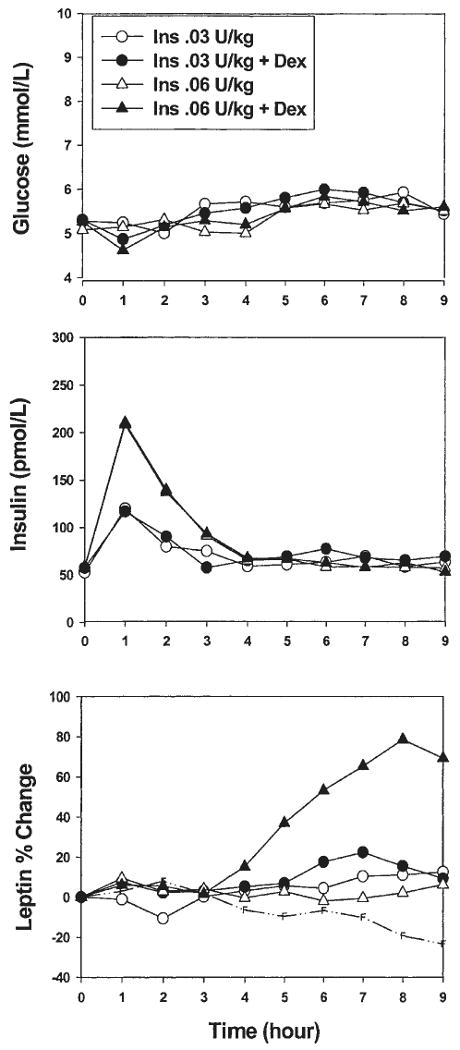
Experiments 2 and 3. Changes over time in serum glucose, insulin and leptin levels after administration of a pulse of insulin (Ins) at two doses (0.03 U/kg, n = 7 or 0.06 U/kg, n = 8) with or without dexamethasone (Dex), in the absence of a transient rise in glucose. The broken line represents the change in serum leptin during the fasting condition (n = 9).
Even without a transient rise in serum glucose, a pulse of insulin plus dexamethasone increased serum leptin compared with the insulin-only condition. With the highest dose of insulin (0.06 U/kg), serum leptin rose above baseline (69±14%) at 9 h (P = 0.002) (Fig. 3). The effect was significant starting at 4.5 h, when compared with baseline. With the smallest low dose of insulin (0.03 U/kg), leptin rose significantly by 14-22% between 5.5 h and 8.5 h after treatment (P < 0.05). However, the duration of the effect was shorter than with the highest dose of insulin and had already disappeared at 9 h (9±4% increment, P = 0.093).
To test the possibility of a dose-effect of insulin, the delta leptin increment after insulin and dexamethasone was compared between experiment 2 and experiment 3. There was a larger increment in serum leptin at 9 h after the highest dose of insulin (75.2±15.7% vs 21.3±8.5%, P = 0.013). The increase in serum leptin after 0.03 U/kg insulin was greater in the presence of transient hyperglycemia (experiment 1 vs experiment 2). However, the insulin output was also greater in the later condition. In the two experiments with comparable serum levels of insulin (experiment 1, hyperglycemia and 0.03 U/kg, and experiment 3, euglycemia and 0.06 U/kg), the increase in leptin after dexamethasone was not significantly different (P = 0.505), in spite of different glycemic levels. In experiments 2 and 3, the FFA changes were comparable with those seen in experiment 1 (Figs 2 and 4).
Figure 4.
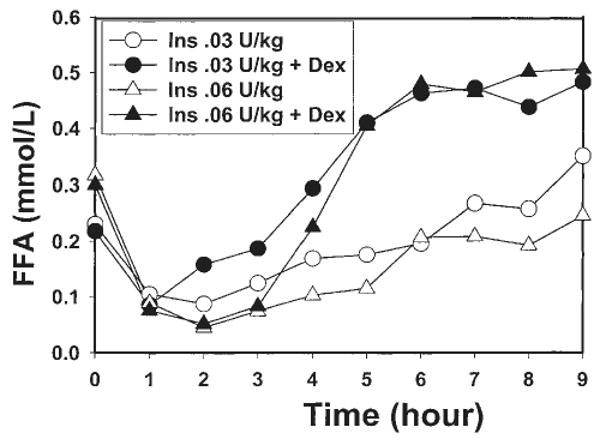
Experiments 2 and 3. Changes over time in serum FFA after administration of a pulse of insulin (Ins) at two doses (0.03 U/kg, n = 7 or 0.06 U/kg, n = 8) with or without dexamethasone (Dex), in the absence of a transient rise in glucose.
Insulin and glucose conditions - fasting conditions
The effect of insulin and glucose on leptin was studied in the absence of dexamethasone. The administration of a subcutaneous pulse of insulin (0.03 U/kg) and a transient rise in serum glucose, in the insulin-only condition of experiment 1 did not increase serum leptin levels over baseline at any time-point studied. Similarly, in experiments 2 and 3, despite the lack of a rise in serum glucose, serum leptin levels did not significantly change from baseline after the low- or high-dose insulin pulse.
In the control group of nine fasted subjects, leptin levels decreased by 24±6% from baseline at 9 h (P = 0.02). The effect was significant starting 7.5 h after the beginning of the study period (P = 0.002). The addition of dexamethasone had no effect on the decrement of serum leptin during the fast, as we have already shown (9). The percent change of leptin from baseline at 9 h was significantly different between the fasting conditions (with and without dexamethasone) and the insulin and glucose pulse condition for each of the three experiments (−24±6% vs + 0.61±7.1%, P = 0.015 for experiment 1, −24±6% vs +12.3± 7.9%, P = 0.002 for experiment 2, and −24±6% vs +6±6%; P = 0.003 for experiment 3). Thus, the administration of a pulse of insulin and/or glucose in experiments 1, 2 and 3, although unable to raise serum leptin levels from baseline, prevented the decline in serum leptin that occurred under fasting conditions.
Amount of glucose administered and leptin increase
The total amount of glucose administered during the 9-h experiment was always higher for the non-dexamethasone condition when compared with the dexamethasone condition, although the degree of statistical significance varied according to the experiment: meal-like experiment: 93.3±10.8 g/9 h vs 87.1±18.1 g/9 h, P = 0.065: dose-response experiments: 68.1±10.8 g/9 h vs 14.5±3.6 g/9 h, P = 0.012 after 0.03 U/kg insulin, and 87.4±10.8 g/9 h vs 53.5±11.8 g/9 h, P = 0.851 after 0.06 U/kg. In the meal-like experiment (experiment 1, transient glucose elevation), there was a positive correlation between the increment of leptin at 9 h and the amount of glucose infused during the experiment among the subjects given insulin plus dexamethasone (r = 0.729, P = 0.04).
Discussion
The combination of a transient, 2-h elevation in insulin and glucocorticoid, with or without a transient increase in serum glucose, was sufficient to trigger an increase in leptin 6–9 h later. Neither hormone alone was effective. We know from previous experiments (9) that dexamethasone increases leptin in fed but not fasting subjects. Thus, the glucocorticoid stimulation of serum leptin seems to require either a positive feeding state (9) or a hyperinsulinemic state, albeit a very transient one (1–2 h). Dexamethasone must interact with insulin to increase serum leptin, and transient hyperglycemia does not appear to be necessary.
The magnitude of the increase in leptin (+53%) after dexamethasone and insulin is approximately half of the increase we observed in our previous meal experiment (+100%) (9). This is likely due to the lesser dose of dexamethasone used in the present study (2 mg instead of 4 mg). Also, it is possible that the gastric and intestinal peptides released after a meal could also have an additive effect, an effect lacking when glucose and insulin are administered i.v.
The effect of a pulse of insulin on leptin is obtained with physiological levels of serum insulin. The magnitude of the effect of insulin and dexamethasone on serum leptin varies with the dose of insulin administered. The highest dose of insulin led to the greatest increase in serum leptin. Our data are consistent with in vitro experiments, indicating that the combination of insulin and dexamethasone increased the release of leptin from human subcutaneous adipocytes to a greater extent than insulin or dexamethasone alone (19, 20).
Others have shown a clear effect of 6- to 9-h insulin infusion on leptin stimulation (10–12). In our study, although insulin levels reached a peak of 380 pmol/l after insulin administration and hyperglycemia, levels comparable with ones from other studies (12), serum leptin levels failed to increase from baseline. It is likely that a longer time exposure to insulin and/or the highest dose of insulin, as used by others (10–12), are key determinants of the stimulating effect of insulin on leptin.
Although a transient rise in insulin and/or glucose was not able to stimulate serum leptin levels, it seems sufficient to maintain them at baseline levels. Conversely, fasting decreased serum leptin by 24% at 9 h. Therefore, a transient rise in insulin and glucose, similar to the metabolic response observed after a single meal, appears to prevent the decline in leptin observed with fasting. This confirmed, as shown by many (10, 12, 21), the role of insulin in the short-term regulation of leptin. Moreover, our data showed that a physiological rise in insulin levels, and not a continuous infusion, was enough to prevent the fast-induced decline in serum leptin, demonstrating a physiological role of metabolic status, i.e. food intake and/or insulin and glucose, in the short-term regulation of serum leptin.
The leptin increase after insulin and dexamethasone was not statistically different between experiments 1 and 3, with similar levels of hyperinsulinemia. It seems that the transient elevation of glucose in experiment 1 is not additive to the effect of insulin and dexamethasone on leptin. Many have shown an effect of glucose in the regulation of leptin (22, 23). In our study, however, we could not separate the effect of insulin from that of glucose. Although we did not measure the level of glucose utilization under our experimental conditions, the amount of glucose infused to maintain blood glucose at the levels required for each experimental condition was always higher in the absence of dexamethasone. This indicated a lesser degree of insulin sensitivity in the presence of dexamethasone. It seems unlikely, therefore, that the combination of dexamethasone and insulin contributed to the rise in serum leptin via an increase in adipocyte glucose utilization. This is in agreement with in vitro data showing that glucocorticoids tend to decrease human adipocyte glucose utilization (24, 25).
The role of lipolysis in regulating leptin has been proposed (26) but remains unclear (27). Dexamethasone increases lipolysis (28) and it is conceivable that this increased lipolysis could play a role in the leptin increase after dexamethasone and insulin. However, the increase in FFA does not occur until 5 h after the administration of insulin and dexamethasone, and is therefore unlikely to be a key factor in the leptin increment. Furthermore, our data suggest that the change in leptin after insulin and dexamethasone is unlikely to be FFA-dependent.
The time lag of 6.5 h after administration of dexamethasone and insulin before leptin increases is very similar to the one we observed after dexamethasone and a meal (9). This time-lag was also observed with overfeeding (5–10 h) (3), insulin administration (4–6 h) (10–12), or a shift in meal timing (21). This time-lag may be necessary to elicit leptin transcription and/or post-transcriptional steps leading to leptin secretion. In vitro, an effect of insulin plus dexamethasone on leptin secretion in subcutaneous adipose tissue was detected at 5 h (19).
The role of glucocorticoids in the physiological regulation of leptin has been questioned. Because of the discrepancy between the effects of an endogenous hypercortisolism (Cushing's syndrome) and of administered glucocorticoids on circulating leptin, some have argued that the elevation of serum leptin after exogenous steroid administration has no physiological relevance (29). Others have shown that the alteration of physiological levels of cortisol did not affect the pattern of leptin secretion (30), nor did the administration of methylprednisolone (31). However, the situation is not clear. Prior studies of the inverse diurnal changes of leptin and cortisol have not taken into account the co-variations of insulin with meals and the expected time-lag in the leptin response. The present data argue that the combination of dexamethasone and insulin increases serum leptin many hours later. Such a situation may occur with the cortisol and insulin increase after the lunch meal in humans (32) or after overfeeding (33). Thus, it is conceivable that the meal-associated nocturnal leptin secretion could be, in fact, a late response to daytime cortisol and insulin secretion under normal feeding conditions.
In summary, a single pulse of insulin administered in place of a meal can act synergistically with dexamethasone to increase serum leptin. Also, a transient increase of insulin can maintain baseline leptin levels and prevent the decline in leptin observed during fasting, demonstrating a physiological role of insulin on leptin regulation. Both effects of insulin are independent of glucose levels, as long as glucose levels are maintained at or above, even transiently, fasting levels. The present study demonstrates that there is a clear synergism of action between the effects of glucocorticoids and insulin on serum leptin in humans.
Acknowledgments
This work was supported by a grant from the American Diabetes Association, and by NIH grants K08 DK 02572-01, M01RR00645 and DK-26687. A C was supported by the Fulbright Program–Generalitat de Catalunya 2000–2001. We thank Yim Dam and Ping Zhou for their excellent help with hormonal assays. We are grateful to Wendy Nash and Ansley Roche for their assistance in writing this manuscript.
Contributor Information
B Laferrère, Obesity Research Center, St Luke's/Roosevelt Hospital Center, Columbia University, 1111 Amsterdam Avenue, New York, New York 10025, USA.
A Caixas, Unitat de Diabetis, Endocrinologia I Nutricio, Hospital de Sabadell, Corporacio Parc Tauli, 08208 Sabadell, Barcelona, Spain.
S K Fried, Rutgers University, New Brunswick, New Jersey 08901, USA.
C Bashore, Obesity Research Center, St Luke's/Roosevelt Hospital Center, Columbia University, 1111 Amsterdam Avenue, New York, New York 10025, USA.
J Kim, Obesity Research Center, St Luke's/Roosevelt Hospital Center, Columbia University, 1111 Amsterdam Avenue, New York, New York 10025, USA.
F X Pi-Sunyer, Obesity Research Center, St Luke's/Roosevelt Hospital Center, Columbia University, 1111 Amsterdam Avenue, New York, New York 10025, USA.
References
- 1.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. New England Journal of Medicine. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 2.Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in normal subjects. Journal of Clinical Endocrinology and Metabolism. 1996;81:3419–3423. doi: 10.1210/jcem.81.9.8784108. [DOI] [PubMed] [Google Scholar]
- 3.Kolaczynski JW, Ohannesian JP, Considine RV, Marco C, Caro JF. Response of leptin to short-term and prolonged overfeeding in humans. Journal of Clinical Endocrinology and Metabolism. 1996;81:4162–4165. doi: 10.1210/jcem.81.11.8923877. [DOI] [PubMed] [Google Scholar]
- 4.Miell JP, Englaro P, Blum WF. Dexamethasone induces an acute and sustained rise in circulating leptin levels in normal human subjects. Hormone and Metabolic Research. 1996;28:704–707. doi: 10.1055/s-2007-979882. [DOI] [PubMed] [Google Scholar]
- 5.Kiess W, Englaro P, Hanistsch S, Rascher W, Attanasio A, Blum WF. High leptin concentrations in serum of very obese children are further stimulated by dexamethasone. Hormone and Metabolic Research. 1996;28:708–710. doi: 10.1055/s-2007-979883. [DOI] [PubMed] [Google Scholar]
- 6.Larsson H, Ahrén B. Short-term dexamethasone treatment increases plasma leptin independently of changes in insulin sensitivity in healthy women. Journal of Clinical Endocrinology and Metabolism. 1996;81:4428–4431. doi: 10.1210/jcem.81.12.8954054. [DOI] [PubMed] [Google Scholar]
- 7.Papaspyrou-Rao S, Schneider SH, Petersen RN, Fried SK. Dexamethasone increases leptin expression in humans in vivo. Journal of Clinical Endocrinology and Metabolism. 1997;82:1635–1637. doi: 10.1210/jcem.82.5.3928. [DOI] [PubMed] [Google Scholar]
- 8.Dagogo-Jack S, Selke G, Melson A, Newcomer JW. Robust leptin secretory responses to dexamethasone in obese subjects. Journal of Clinical Endocrinology and Metabolism. 1997;82:3230–3233. doi: 10.1210/jcem.82.10.4154. [DOI] [PubMed] [Google Scholar]
- 9.Laferrère B, Fried SK, Hough K, Campbell SA, Thornton J, Pi-Sunyer FX. Synergistic effects of feeding and dexamethasone on serum leptin levels. Journal of Clinical Endocrinology and Metabolism. 1998;83:3742–3745. doi: 10.1210/jcem.83.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Utriainen T, Malmstrom R, Makimattila S, Yki-Jarvinen H. Supraphysiological hyperinsulinemia increases plasma leptin concentrations after 4 h in normal subjects. Diabetes. 1996;45:1364–1366. doi: 10.2337/diab.45.10.1364. [DOI] [PubMed] [Google Scholar]
- 11.Malmstrom R, Taskinen MR, Karonen SL, Yki-Jarvinen H. Insulin increases plasma leptin concentrations in normal subjects and patients with NIDDM. Diabetologia. 1996;39:993–996. doi: 10.1007/BF00403921. [DOI] [PubMed] [Google Scholar]
- 12.Saad MF, Khan A, Sharma A, Michael R, Riad-Gabriel M, Boyadjian R, et al. Physiological insulinemia acutely modulates plasma leptin. Diabetes. 1998;47:544–549. doi: 10.2337/diabetes.47.4.544. [DOI] [PubMed] [Google Scholar]
- 13.Grinspoon SK, Askari H, Landt ML, Nathan DM, Schoenfeld DA, Hayden DL, et al. Effects of fasting and glucose infusion on basal and overnight leptin concentrations in normal-weight women. American Journal of Clinical Nutrition. 1997;66:1352–1356. doi: 10.1093/ajcn/66.6.1352. [DOI] [PubMed] [Google Scholar]
- 14.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1998;8:77–100. [Google Scholar]
- 15.Heymsfield SB, Wang J, Aulet M, Kehayias J, Lichtman S, Kamen Y, et al. Dual photon absorptiometry: validation of mineral and fat measurements. Basic Life Sciences. 1990;55:327–337. doi: 10.1007/978-1-4613-1473-8_45. [DOI] [PubMed] [Google Scholar]
- 16.Hough K, Thornton J, Fried SK, Pi-Sunyer FX, Laferrère B. Oral dexamethasone increases serum leptin levels in a dose dependent manner in humans. International Journal of Obesity. 1998;22:S167. [Google Scholar]
- 17.SPSS/windows. SPSS Manuals. Chicago: 1995. [Google Scholar]
- 18.Laferrère B, Fried SK, Osborne T, Pi-Sunyer FX. Effect of one morning meal and a bolus of dexamethasone on 24-hour variation of serum leptin levels in humans. Obesity Research. 2000;8:481–486. doi: 10.1038/oby.2000.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell CD, Petersen RN, Rao SP, Ricci MR, Prasad A, Brolin RE, et al. Leptin expression in adipose tissue from obese humans: depot-specific regulation by insulin and dexamethasone. American Journal of Physiology. 1998;275:E507–E515. doi: 10.1152/ajpendo.1998.275.3.E507. [DOI] [PubMed] [Google Scholar]
- 20.Wabitsch M, Jensen PB, Blum WF, Christoffersen CT, Englaro P, Heinze E, et al. Insulin and cortisol promote leptin production in cultured human fat cells. Diabetes. 1996;45:1435–1438. doi: 10.2337/diab.45.10.1435. [DOI] [PubMed] [Google Scholar]
- 21.Schoeller DA, Cella LK, Sinha MK, Caro JF. Entrainment of the diurnal rhythm of serum leptin to meal timing. Journal of Clinical Investigation. 1997;100:1882–1887. doi: 10.1172/JCI119717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller WM, Gregoire FM, Stanhope KL, Mobbs CV, Mizuno TM, Warden CH, et al. Evidence that glucose metabolism regulates leptin secretion from cultured rat adipocytes. Journal of Clinical Endocrinology and Metabolism. 1998;139:551–558. doi: 10.1210/endo.139.2.5716. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Liu R, Hawkins M, Barzilai N, Rossetti L. A nutrient sensitive pathway regulates leptin gene expression in muscle and fat. Nature. 1998;393:684–688. doi: 10.1038/31474. [DOI] [PubMed] [Google Scholar]
- 24.Appel B, Fried SK. Effects of insulin and dexamethasone on lipoprotein lipase in human adipose tissue. American Journal of Physiology. 1992;262:E695–E699. doi: 10.1152/ajpendo.1992.262.5.E695. [DOI] [PubMed] [Google Scholar]
- 25.Sakoda H, Ogihara T, Anai M, Funaki M, Inukai K, Katagiri H, et al. Dexamethasone-induced insulin resistance in 3T3-L1 adipocytes is due to inhibition of glucose transport rather than insulin signal transduction. Diabetes. 2000;49:1700–1708. doi: 10.2337/diabetes.49.10.1700. [DOI] [PubMed] [Google Scholar]
- 26.Stefan N, Fritsche A, Häring H, Stumvoll M. Acute stimulation of leptin concentrations in humans during hyperglycemic hyperinsulinemia. Influence of free fatty acids and fasting. International Journal of Obesity. 2001;25:138–142. doi: 10.1038/sj.ijo.0801527. [DOI] [PubMed] [Google Scholar]
- 27.Peino R, Fernandez Alvarez J, Peñalva A, Considine RV, Rodriguez-Segade S, Rodriguez-Garcia J, et al. Acute change in free-fatty acids (FFA) do not alter serum leptin levels. Journal of Endocrinological Investigation. 1998;21:526–530. doi: 10.1007/BF03347339. [DOI] [PubMed] [Google Scholar]
- 28.Divertie GD, Jensen MD, Miles JM. Stimulation of lipolysis in humans by physiological hypercortisolemia. Diabetes. 1991;40:1228–1232. doi: 10.2337/diab.40.10.1228. [DOI] [PubMed] [Google Scholar]
- 29.Torpy DJ, Bornstein SR, Cizza G, Chrousos GP. The effect of glucocorticoids on leptin levels in humans may be restricted to acute pharmacological dosing. Journal of Clinical Endocrinology and Metabolism. 1998;83:1821–1822. doi: 10.1210/jcem.83.5.4821-1. [DOI] [PubMed] [Google Scholar]
- 30.Purnell JQ, Samuels MH. Levels of leptin during hydrocortisone infusions that mimic normal and reverse diurnal cortisone levels in subjects with adrenal insufficiency. Journal of Clinical Endocrinology and Metabolism. 1999;84:3125–3128. doi: 10.1210/jcem.84.9.5990. [DOI] [PubMed] [Google Scholar]
- 31.Tataranni PA, Pratley R, Maffei M, Ravussin E. Acute and prolonged administration of glucocorticoids (methylprednisolone) does not affect plasma leptin concentration in humans. International Journal of Obesity. 1997;21:327–330. doi: 10.1038/sj.ijo.0800397. [DOI] [PubMed] [Google Scholar]
- 32.Van Cauter E, Shapiro ET, Tillil H, Polonsky KS. Circadian modulation of glucose and insulin responses to meal: relationship to cortisol rhythm. American Journal of Physiology. 1992;262:E467–E475. doi: 10.1152/ajpendo.1992.262.4.E467. [DOI] [PubMed] [Google Scholar]
- 33.De Schepper J, Zhou X, De Bock S, Smitz J, Louis O, Hooghe-Peters E, et al. Study of serum leptin in cafeteria-diet-overfed rats. Influence of diet, insulin and corticosterone. Hormone Research. 1998;50:271–275. doi: 10.1159/000023289. [DOI] [PubMed] [Google Scholar]


