Abstract
Arteries in vivo are subjected to large longitudinal stretch, which changes significantly due to vascular disease and surgery. However, little is known about the effect of longitudinal stretch on arterial endothelium. The aim of this study was to determine the morphologic adaptation of arterial endothelial cells (ECs) to elevated axial stretch. Porcine carotid arteries were stretched 20% more than their in vivo length while being maintained at physiological pressure and flow rate in an organ culture system. The ECs were elongated with the application of the axial stretch (aspect ratio 2.81 ± 0.25 vs. 3.65 ± 0.38, n=8, p<0.001). The elongation was slightly decreased after three days and the ECs recovered their normal shape after seven days, as measured by the shape index and aspect ratio (0.55 ±0.03 vs. 0.56 ± 0.04, and 2.93 ± 0.28 vs. 2.88 ± 0.20, respectively, n=5). Cell proliferation was increased in the intima of stretched arteries in three days as compared to control arteries but showed no difference after seven days in organ culture. These results demonstrate that the endothelial cells adapt to axial stretch and maintain their normal shape.
Keywords: Endothelium, Axial stretch, Cell shape, Cell proliferation, Ex vivo, Artery, Porcine
INTRODUCTION
Arterial endothelium plays a very important role in vascular biology, physiology, and pathology (Nerem et al. 1998; Lehoux and Tedgui 2003; Chien 2007). It is well documented that endothelial cell morphology and function are significantly affected by mechanical stresses including the circumferential stretch generated by the blood pressure and the wall shear stress generated by the flow (Ku 1997; Kakisis et al. 2004; Chien 2007). On the other hand, arteries in vivo are also subjected to significant axial stretch that may change dramatically due to vascular disease, surgery, or aging (Han and Fung 1995; Han et al. 1998; Nichols and O'Rourke 1998).The effects of axial stretch on arterial wall function and remodeling has been studied recently by us and others (Jackson et al. 2002; Han et al. 2003; Davis et al. 2005; Gleason and Humphrey 2005; Nichol et al. 2005). However, little is known about the effects of axial stretch on EC morphology, although the effect of blood flow on the EC morphology has been well-documented.
The objective of this study was to examine the effect of elevated axial stretch on the morphology of endothelium in organ culture.
MATERIALS AND METHODS
Artery Organ Culture
Porcine common carotid arteries were harvested from 6-7 month old farm pigs (100-150 kg) and mounted in a perfusion organ culture system, as previously described (Han et al. 2003). Briefly, arterial segments of 4-6 cm in length were maintained in flow loops inside incubators (37°C, 5% CO2) with medium perfusion at physiological pressures oscillating between 80 mmHg and 120 mmHg and a mean flow rate of ~160 ml/min.
Axial Stretch
Arteries were stretched 20% over their in vivo length following the protocol described previously (Han et al. 2003). Briefly, experimental arteries were stretched axially to an axial stretch ratio of λ=1.8 while control arteries were maintained at their in vivo lengths (λ=1.5). Left and right carotid arteries from each animal were harvested and randomly set as control or stretched arteries. Arteries were cultured for a period of three or seven days to examine the temporal changes.
Silver Staining
At the conclusion of the organ culture, arteries were removed from the flow loops and trimmed off both ends. Each artery was divided into two to three short ring segments (~0.5 cm) for light and fluorescent microscopy and a long segment (2-3 cm) for silver staining. The long segments were treated with 10 μM SNP, cleaned off the connective tissue and much of the adventitia, cut open with a longitudinal incision, and pinned down flat with the endothelium side up. The width and length of segments were adjusted to their perimeter and length in organ culture. The silver staining was then carried out to impregnate the endothelium using a protocol modified from Lautsch et al., (Lautsch et al. 1953) as follows:
The arterial segments were incubated in warm 5% dextrose for 5-10 minutes and an aqueous solution of 0.4% silver nitrate was dropped on the endothelial surface continuously for thirty seconds to impregnate the ECs. The arterial segments were then exposed to UV light for 30 seconds, washed in 5% dextrose, followed by fixation with 10% formalin for several hours and then washed in gently running tap water for 30 minutes. The specimens were then treated with anhydrous glycerol (98-100%) for 48-72 hours, carefully peeled off the outer layer, and mounted onto slides for en face microscopy.
In addition, a group of eight fresh arteries were cut into two segments of equal lengths each, stretched to 1.5x and 1.8x of their free lengths, respectively, and silver stained using the same protocol to determine the initial EC shape changes due to axial stretch.
Characterization of EC shape
EC morphology was examined en face under a light microscope and photographed at several (6-8) fields per specimen. The area, perimeter, major axis and minor axis lengths and orientation angle of several hundred cells were measured for each specimen using Image-Pro® Plus 4.5. Cell shape were quantified by the shape index and aspect ratio (Levesque and Nerem 1989):
| (1) |
| (2) |
The shape index is one for a circle and zero for a line. The aspect ratio is one for a circle and become larger in an elongated cell. The average values for all cells from each specimen were averaged to represent the value for the specimen.
Observation of Endothelial Nuclei
In a subset of arteries, the actin filaments and cell nuclei were labeled by rhodamine-phalloidin and Hoechst 33258 and examined en face using fluorescent microscopy following the methods described previously (Liu 1998; Han et al. 2003). Briefly, arteries were fixed with 4% formaldehyde under a pressure of 100 mmHg for 30 minutes, cut longitudinally into strips, and pinned down flat in a petri dish. The specimens were incubated with rhodamine-phalloidin at a concentration of 165 nmol/L (Molecular Probes) mixed with 1% BSA (bovine serum albumin) in PBS at 37 °C for 60 minutes. The specimens were then counterstained with Hoechst 33258 and examined under a Zeiss confocal microscope (Han et al. 2006).
Cell Proliferation Labeling, Immunostaining and Proliferated Cell Counting
Cell proliferation in the arteries were detected using bromodeoxyuridine labeling (BrdU at 5 μg/L, added 24 hours before harvesting) and anti-BrdU staining, then measured as described previously (Han et al. 2006). BrdU index, the percentage of BrdU-positive cells, was calculated to quantify cell proliferation.
Statistical Analysis
All values are presented as the mean ± SD. Statistical significance between means was determined using one way ANOVA with Tukey's pairwise comparisons and the significance level was set as a p value less than 0.05.
RESULTS
Arterial vasomotor responses were checked after three and seven days in organ culture as described previously (Han et al. 2003). The diameter responses showed no statistically significant difference between the stretched arteries and the corresponding control arteries. Visual inspection and measurements by an observer blinded to conditions failed to identify difference in microstructure and wall dimensions.
Adaptation of EC Shape
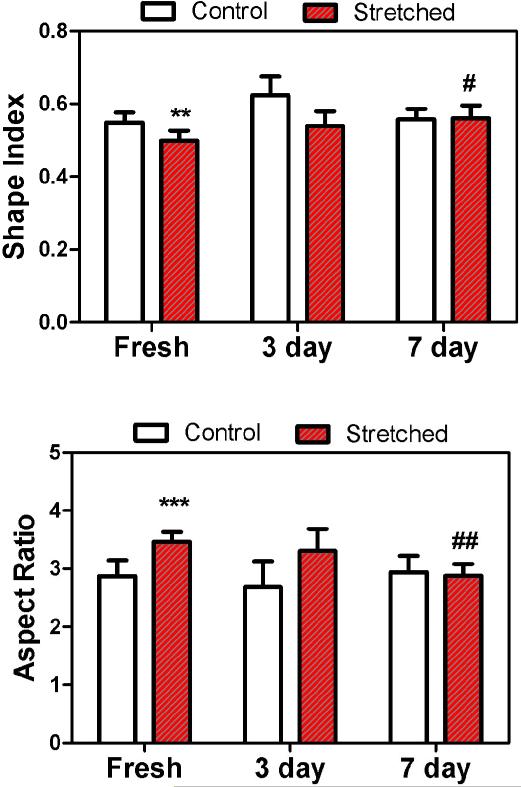
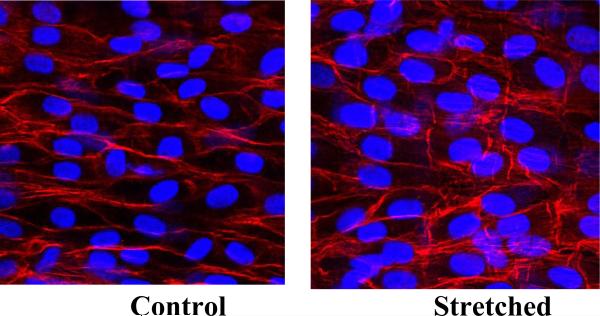
The ECs in the arteries were significantly elongated after the initial application of a 20% increase in axial stretch (p<0.01, Figs. 1 and 2). The ECs in the stretched arteries remained elongated after three days but regained their normal shape after seven days. While ECs in control arteries demonstrated no statistical differences among the fresh, three-day, and seven-day groups in terms of either shape index or aspect ratio, ECs in stretched arteries changed significantly among the fresh, three-day, and seven-day groups (p<0.005). There were no statistical differences between the 7-day stretched and control groups in terms of either shape index or aspect ratio. In addition, ECs in all arteries aligned in the axial direction and there was no statistical difference in the cell alignment angle between the stretched and control arteries (88.2° ± 6.7° versus 87.0° ± 8.1° for fresh (n = 8) and 84.5° ± 3.3° versus 89.2° ± 7.4° for 7-day (n = 5), respectively). No significant difference in cell density was observed between the stretched and control arteries after 7 days either (31.5 ± 2.1 versus 33.5 ± 4.6 nuclei per view field). The shape index and aspect ratios of EC nuclei (Fig. 3) of the stretched arteries were not statistically different from those of control arteries (0.76 ± 0.02 versus 0.78 ± 0.03 and 1.60 ± 0.05 versus 1.58 ± 0.05, respectively).
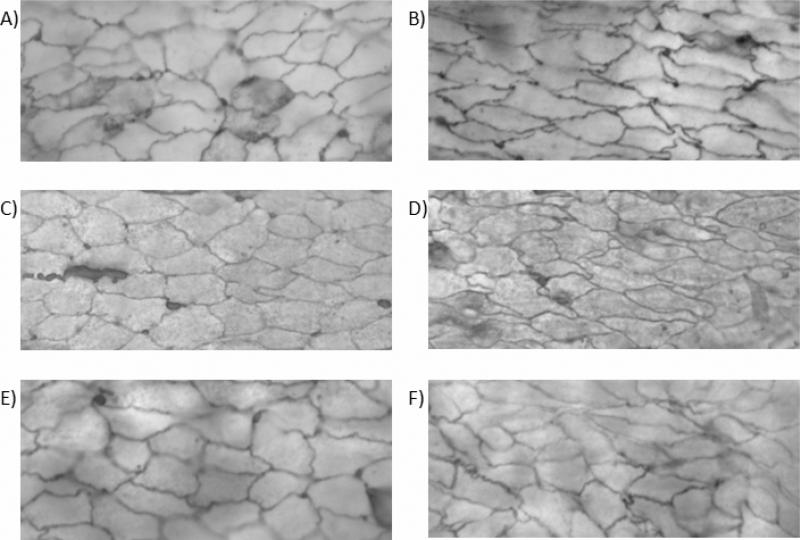
Figure 1.
Micrographs from en face light microscopy illustrating the contour of endothelial cell visualized by silver nitrate. A) control fresh artery, B) stretched fresh artery, C) control artery cultured for 3 days, D) stretched artery cultured for 3 days, E) control artery cultured for 7 days, and F) stretched artery cultured for 7 days. Magnification 100X.
Figure 2.
Comparison of the shape index (Top) and aspect ratio (Bottom) of the endothelial cells in the stretched and control arteries in fresh (n =8), 3 and 7 days after organ cultured (n = 5). ** P<0.01, ***P<0.001 vs. Fresh control; # P<0.05, ## P<0.01 vs. Fresh stretched.
Figure 3.
Microscopic photographs from en face fluorescent microscopy demonstrating normal endothelial cell nuclei morphology and density. Magnification 400X.
Cell Proliferation
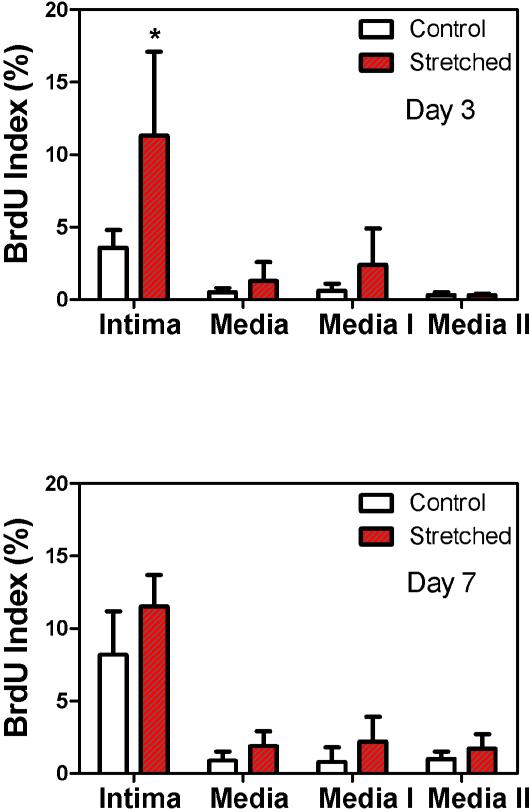
Across the arterial wall, the BrdU index was significantly higher in the intima than in the media in both stretched and control arteries (p<0.05, Fig.4). Within the media, there were no statistical differences between the inner half layer (Media I) and the outer half layer (Media II) suggesting a uniform distribution of proliferating cells across the media. The BrdU index in the intima was significantly higher in the stretched arteries cultured for three days than in the corresponding controls (Fig 4, Top, p<0.05). Overall, stretched arteries showed a slightly higher BrdU index in the intima and media than the control arteries, but the difference was statistically insignificant.
Figure 4.
Comparison of the percentage of BrdU-positive cell nuclei in the control and stretched arteries after being cultured for three days (n = 4) and seven days (n = 6) in organ culture. Media I and Media II represent the inner and outer media layers; each covers half of the media thickness. * p< 0.05.
DISCUSSION AND CONCLUSION
We studied the adaptation of the EC shape in arteries under axial stretch using an organ culture model. The results showed that ECs were initially elongated by the axial stretch but eventually adapted to the axial stretch, regaining their normal shape.
It has been well documented that ECs change their shape, cytoskeleton, and function under shear flow and cyclic stretch (Nerem et al. 1998; Kakisis et al. 2004). It was suggested that the “wisdom of the cell” is to adjust its structure and function to maintain its homeostasis in face of the external perturbations (Chien 2007). Our results demonstrate the capability of ECs to maintain their normal shape, and probably their cellular function, under axial stretch. This adaptation is an important aspect of the wall remodeling and is closely related to the adaptation of their subcellular structure, cellular functions, and extracellular matrix (Jackson et al. 2002; Sipkema et al. 2003).
Our results showed that the adaptation of EC shape is slower in the arteries than in cell culture (Nerem et al. 1998; Kakisis et al. 2004). The slower adaptation may be due to the either the presence of flow or the strong binding between ECs and their natural extracellular matrix environment. It has been documented that extracellular matrix has significant effect on the shape, alignment, and migration of ECs (Levesque and Nerem 1989; Sprague and Palmaz 2005). This speculation is also supported by the observation that EC migration is slower in natural arteries than in cell culture in vitro (Sprague et al. 2007).
In contrast to a previous report that cyclic axial stretch of arteries could realign the EC to the circumferential direction in the absence of blood flow (Sipkema et al. 2003), we did not see EC realignment under the steady axial stretch. This may be due to the differences in the stretch load (static vs cyclic), the extracellular matrix (Asanuma et al. 2003; Gupta and Grande-Allen 2006), or the presence of lumen flow and cyclic circumferential stretch. The shear stress and circumferential cyclic stretch, which are known to influence the EC alignment, may have played a more dominant role in regulating the EC alignment than the step increase in axial stretch ratio. The recovery of normal cell shape could be a response to the normal flow in the arteries.
Associated with the EC shape changes, we found that the cell proliferation was significantly increased in the intima of stretched arteries after three days, which is consistent with previous in vivo results (Jackson et al. 2002). The increase in cell proliferation may have led to the normalization of the cell density in the stretched arteries. Furthermore, we observed a homogenous distribution of the proliferating cells across the media thickness which is different from the proliferating cell distribution in hypertensive arteries (Han et al. 2006). This difference may be due to the difference in wall stress: while an increase in pressure results in a higher increase in the circumferential stress at the inner wall than at the outer wall (Matsumoto and Hayashi 1996), an elevation in axial strain leads to an nearly homogenous increase in the axial stress and the circumferential stress remains nearly unchanged (Gleason and Humphrey 2005).
One limitation of this study was that only one level of step increase in axial stretch was studied based on the fact that changes in axial stretch ratio due to vascular surgery are often a step change. Different level of axial stretch and cyclic axial stretch may lead to different cell proliferation and EC shape adaptation (Sipkema et al. 2003; Nichol et al. 2005) and their long-term effects need to be examined in future studies. Nevertheless, our data clearly demonstrated the effects of axial stretch on EC adaptation. Furthermore, previous reports have showed that stretch lead to cellular protein changes and extracellular matrix remodeling (Asanuma et al. 2003; Lehoux and Tedgui 2003; Gupta and Grande-Allen 2006). These changes need further investigation.
In conclusion, our results demonstrated that endothelial cells can regain their normal shape after the arteries are moderately stretched axially. These results enhance our understanding of vascular remodeling and endothelial response to injury, which is very important to atherosclerotic pathology and the prognosis of artery after grafting or repairing operations.
ACKNOWLEDGMENTS
This work was partially supported by the National Science Foundation under award numbers EEC-9731643 and CBET-0602834, a Beginning Grant-in-Aid #0160215B from the American Heart Association Southeast Affiliate, and an MBRS-SCORE program from NIH under award number S06GM08194. We thank Drs. N. Peter Davis, Merry Lindsey, Ms. Tracey Couse, Mr. Vinay Challa, Mr. Kurtis Johnson, and Mr. Marcello Pilia for their help throughout this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Asanuma K, Magid R, Johnson C, Nerem RM, Galis ZS. Uniaxial strain upregulates matrix-degrading enzymes produced by human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;284(5):H1778–84. doi: 10.1152/ajpheart.00494.2002. [DOI] [PubMed] [Google Scholar]
- Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292(3):H1209–24. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- Davis NP, Han HC, Wayman B, Vito RP. Sustained axial loading lengthens arteries in organ culture. Ann Biomed Eng. 2005;33(7):869–879. doi: 10.1007/s10439-005-3488-x. [DOI] [PubMed] [Google Scholar]
- Gleason RL, Humphrey JD. Effects of a sustained extension on arterial growth and remodeling: a theoretical study. J Biomech. 2005;38(6):1255–61. doi: 10.1016/j.jbiomech.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Gupta V, Grande-Allen KJ. Effects of static and cyclic loading in regulating extracellular matrix synthesis by cardiovascular cells. Cardiovasc Res. 2006;72(3):375–83. doi: 10.1016/j.cardiores.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Han HC, Fung YC. Longitudinal strain of canine and porcine aortas. J Biomech. 1995;28(5):637–41. doi: 10.1016/0021-9290(94)00091-h. [DOI] [PubMed] [Google Scholar]
- Han HC, Ku DN, Vito RP. Arterial wall adaptation under elevated longitudinal stretch in organ culture. Ann Biomed Eng. 2003;31(4):403–11. doi: 10.1114/1.1561291. [DOI] [PubMed] [Google Scholar]
- Han HC, Marita S, Ku DN. Changes of opening angle in hypertensive and hypotensive arteries in 3-day organ culture. J Biomech. 2006;39(13):2410–8. doi: 10.1016/j.jbiomech.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Han HC, Zhao L, Huang M, Hou LS, Huang YT, Kuang ZB. Postsurgical changes of the opening angle of canine autogenous vein graft. J Biomech Eng. 1998;120(2):211–6. doi: 10.1115/1.2798304. [DOI] [PubMed] [Google Scholar]
- Jackson ZS, Gotlieb AI, Langille BL. Wall tissue remodeling regulates longitudinal tension in arteries. Circ Res. 2002;90(8):918–25. doi: 10.1161/01.res.0000016481.87703.cc. [DOI] [PubMed] [Google Scholar]
- Kakisis JD, Liapis CD, Sumpio BE. Effects of cyclic strain on vascular cells. Endothelium. 2004;11(1):17–28. doi: 10.1080/10623320490432452. [DOI] [PubMed] [Google Scholar]
- Ku DN. Blood flow in arteries. Ann Rev Fluid Mech. 1997;29:399–434. [Google Scholar]
- Lautsch EV, McMillan GC, Duff GL. Technics for the Study of the Normal and Atherosclerotic Arterial Intima from Its Endothelial Surface. Laboratory Investigations. 1953;2(6):397–407. [PubMed] [Google Scholar]
- Lehoux S, Tedgui A. Cellular mechanics and gene expression in blood vessels. J Biomech. 2003;36(5):631–43. doi: 10.1016/s0021-9290(02)00441-4. [DOI] [PubMed] [Google Scholar]
- Levesque MJ, Nerem RM. The study of rheological effects on vascular endothelial cells in culture. Biorheology. 1989;26(2):345–57. doi: 10.3233/bir-1989-26218. [DOI] [PubMed] [Google Scholar]
- Liu SQ. Influence of tensile strain on smooth muscle cell orientation in rat blood vessels. J Biomech Eng. 1998;120(3):313–20. doi: 10.1115/1.2797996. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Hayashi K. Stress and strain distribution in hypertensive and normotensive rat aorta considering residual strain. J Biomech Eng. 1996;118(1):62–73. doi: 10.1115/1.2795947. [DOI] [PubMed] [Google Scholar]
- Nerem RM, Alexander RW, Chappell DC, Medford RM, Varner SE, Taylor WR. The study of the influence of flow on vascular endothelial biology. Am J Med Sci. 1998;316(3):169–75. doi: 10.1097/00000441-199809000-00004. [DOI] [PubMed] [Google Scholar]
- Nichol JW, Petko M, Myung RJ, Gaynor JW, Gooch KJ. Hemodynamic conditions alter axial and circumferential remodeling of arteries engineered ex vivo. Ann Biomed Eng. 2005;33(6):721–32. doi: 10.1007/s10439-005-4494-8. [DOI] [PubMed] [Google Scholar]
- Nichols WW, O'Rourke MF. McDonald's Blood Flow in Arteries: Theoretical, Experimental, and Clinical Principles. 4th Edition. Arnold Publisher; London: 1998. Chapter 4. [Google Scholar]
- Sipkema P, van der Linden PJ, Westerhof N, Yin FC. Effect of cyclic axial stretch of rat arteries on endothelial cytoskeletal morphology and vascular reactivity. J Biomech. 2003;36(5):653–9. doi: 10.1016/s0021-9290(02)00443-8. [DOI] [PubMed] [Google Scholar]
- Sprague E, Luo J, Lee Y, Han H. Flow increases endothelial healing and inhibits SMC proliferation in parallel artery and co-culture models.. BMES Annual Fall Meeting; Los Angels, CA. 2007. [Google Scholar]
- Sprague EA, Palmaz JC. A model system to assess key vascular responses to biomaterials. J Endovasc Ther. 2005;12(5):594–604. doi: 10.1583/05-1555.1. [DOI] [PubMed] [Google Scholar]