Abstract
The objectives of this study were to determine the time course of the stimulatory effect of dexamethasone on serum leptin and whether it depends on food intake. Dexamethasone (4mg) was administered I. V. over 1 minute to healthy human volunteers (n=8) under fasting and feeding conditions (2000 kcal given at three meals over 7 hours). At 10 hours, serum leptin levels were increased only in the fed subjects (delta leptin 10.6±1.6 vs −2.4±1.9 ng/ml, p=0.01, n=8). To assess the interactive effect of food and dexamethasone on serum leptin, a subgroup (n=4) was studied under 4 conditions: 1) dexamethasone/fast; 2) dexamethasone/food; 3) saline/fast; 4) saline/food. Serum leptin declined from baseline under the fasting conditions, with or without dexamethasone. Feeding prevented the drop in serum leptin. In the dexamethasone/food condition, leptin levels rose from baseline after 7 hours and doubled after 10 hours (p<0.05). The rise in serum leptin was significantly greater in the food/dexamethasone condition compared to all other conditions (p<0.05). In summary, dexamethasone has no independent effect on serum leptin in the absence of food intake. Rather, dexamethasone appears to potentiate the food-induced increase in serum leptin. This synergism may be mediated by insulin and/or other factors associated with food ingestion.
Introduction
Serum leptin levels are elevated in obesity in proportion to body fat mass (1). It is clear, however, that leptin levels can change independently of fat mass, for example after fasting (2), overfeeding (3), administration of insulin (4–6) or dexamethasone (7–11). In humans, oral administration of glucocorticoids elevates serum leptin levels (7–11) and leptin mRNA levels in adipose tissue (10). Dexamethasone doubled serum leptin after 24–48 h (7–11), and a significant effect was apparent at 9 h (7). It is unclear from these studies whether the dexamethasone effect depends on concomitant food intake and the observed rise in serum insulin. Thus, the objectives of the present study were to determine whether the dexamethasone-induced increase in serum leptin occurs in the absence of food intake and to assess the time course of the effect.
Methods
Subjects
Subjects’ characteristics are listed in Table 1. All were non-smokers and showed normal physical exam, routine blood work, thyroid function tests and 24 h urinary cortisol. None were taking medications or depressed as assessed by the Beck questionnaire (12). The female subject was screened and tested during the follicular part of the menstrual cycle (13). The OGTT was within normal range. The body weight of the subjects was stable for at least 3 months prior to the study. Total body fat was measured by DEXA scan (14). Study protocols were approved by the Institutional Review Board of St. Luke’s/Roosevelt Hospital, and informed written consent was obtained.
Table 1.
Subject Characteristics
| Subject | Conditiona | Age | BMI | Fat | 0′ Glucose | 0′ Insulin | Basal Leptin |
|---|---|---|---|---|---|---|---|
| yr | kg/m2 | % | mmol/1 | pmol/1 | ng/ml | ||
| 1 | 1,2,3,4 | 43 | 26.7 | 38 | 4.8 | 115 | 11.8 |
| 2 | 1,2,3,4 | 24 | 24.6 | 25 | 4.9 | 58 | 5.1 |
| 3 | 1,2,3,4 | 28 | 25.2 | 19 | 4.7 | 67 | 3.6 |
| 4 | 1,2,3,4 | 26 | 22.5 | 21 | 4.9 | 77 | 3.8 |
| 5b | 1,2 | 24 | 23.0 | 28 | 4.3 | 81 | 8.8 |
| 6 | 1 | 28 | 24.1 | 22 | 5.3 | 110 | 5.4 |
| 7 | 2 | 32 | 24.9 | 17 | 4.9 | 85 | 3.7 |
| 8 | 2 | 33 | 29.4 | 38 | 4.9 | 123 | 18.9 |
| Mean ± SEM | 30±2 | 25±1 | 26±3 | 4.8±0.1 | 89±8 | 8±2 |
Conditions tested include 1) Dexamethasone/fast, 2) Dexamethasone/food, 3) Saline/fast, 4) Saline/food;
Female Subject
Experimental Design
Subjects came to the Clinical Research Center at 7:00 am after an overnight fast and rested during the entire study. An I. V. catheter was inserted in an ante-cubital vein, and the I.V. line was kept open with a slow infusion of 0.45% sodium chloride during the entire experiment. After baseline measurements were taken, the subjects were given 4 mg of dexamethasone or saline administered I.V. over 1 minute at 7:30. In the fed condition, subjects consumed 2000 kcal (55% CHO, 15% PROT, and 30%FAT): breakfast (700 kcal) at 8:00, lunch (960 kcal) at 12:00, and afternoon snack (340 kcal) at 14:00. Blood samples were taken every 30 minutes for the next 10 hours for measurements of leptin, glucose and insulin. In the first experiment, two conditions were compared: 1) dexamethasone/fast; 2) dexamethasone/food (diet as described above). In the second experiment, a subgroup of 4 subjects was retested under saline with fast and saline with food conditions. Thus, 4 subjects completed all four conditions: 1) dexamethasone/fast; 2) dexamethasone/food; 3) saline/fast and 4) saline/food. The experiments were separated by at least 1 week for each subject and no more than a month. The order of the four experimental conditions was randomly assigned. No side effects were reported during any of the experiments.
Analytical methods
Glucose was measured with a glucose analyzer (Beckman Instruments, Inc., Fullerton, CA). Serum insulin and leptin were measured by radio-immunoassay (Linco), with intra- and inter-assay coefficients of variation between 6 and 8%. The 24 h urinary Cortisol was measured by radio-immunoassay (DPC).
Calculations and Statistical Methods
The areas under the curves for leptin (LAUC), glucose (GAUC), and insulin (IAUC) versus time (10 hours) were calculated using the trapezoidal rule (15). The area used for analysis was obtained by subtracting the baseline area from the AUC. Delta values were defined as the difference between the final (10 h) value and the basal value for leptin. ANOVA was used to test for differences among the mean responses. Multiple comparisons were performed using Fisher’s protected least significant difference procedure. All statistical calculations were performed using the SAS statistical software package for personal computers (Ver. 6.12). The interaction between food intake and dexamethasone was tested using a contrast that compared the effect of dexamethasone with food to the effect of dexamethasone without food. The level of significance for all statistical tests of hypotheses was 0.05. Separate analyses were performed after removing the female subject and also after removing the 2 subjects with the highest BMI. The results gave a similar level of statistical significance. Therefore, all data were pooled.
Results
In fed subjects, an I.V. bolus of dexamethasone led to a significant rise in serum leptin by 7 h and a two-fold rise by 10 h (Table 2). In contrast, in fasted subjects, I.V. dexamethasone did not increase leptin over baseline.
Table 2.
Effects of dexamethasone, with or without food, on serum leptin, glucose and insulin
| Condition | 1 Dexa/Fast | 2 Dex/Food | P |
|---|---|---|---|
| n | 6 | 7 | |
| LAUCb ng/ml/10h | −17±7 | 19±6 | 0.02 |
| Delta L ng/ml | −2.4±1.9 | 10.6±1.6 | 0.01 |
| GAUC mmol/l/10h | 6.2±2.6 | 16.4±2.2 | 0.05 |
| IAUC pmol/l/10h | 100±251 | 2440±215 | 0.01 |
Dexamethasone;
Areas under the curve (AUC) were calculated as a change from baseline; a negative number indicates a decrease
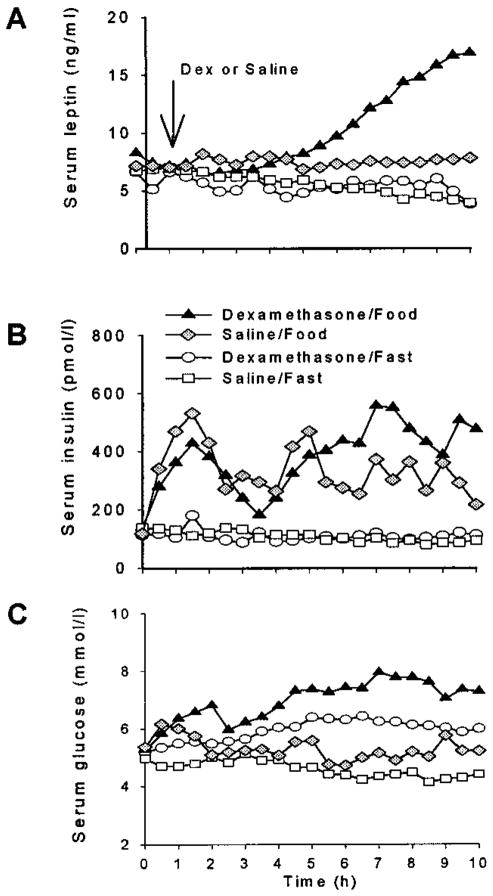
To dissect the interactions between food and dexamethasone, the effects of feeding on leptin with and without dexamethasone were studied in a subgroup of 4 subjects. In the two fasted groups, leptin levels decreased to a similar extent over the 10 hours, by 41% without dexamethasone and by 35% with dexamethasone (Figure 1A, Table 3). In fed subjects (food/saline), no change of serum leptin was observed.
Figure 1.
Effects of dexamethasone and/or food on serum leptin (A), insulin (B), and glucose (C). Subjects (n=4) were studied under 4 conditions: 1) dexamethasone (4mg) administered I.V. over one minute with fast, 2) dexamethasone with food (2000 kcal given at 3 meals), 3) saline with fast and 4) saline with food. SEM are not shown for clarity. In the food condition, breakfast (700 kcal), lunch (960 kcal) and snack (340 kcal) were given 15 min., 4 h and 6 h, respectively, after dexamethasone or saline administration.
Table 3.
Effects of dexamethasone and food on serum leptin, glucose and insulin
| 1 Dexa/Fast | 2 Dex/Food | 3 Saline/Fast | 4 Saline/Food | P | |
|---|---|---|---|---|---|
| n | 4 | 4 | 4 | 4 | |
| GAUC mmol/l/10h | 133±27 | 307±57 | −68±24 | −12±47 | 0.001 |
| IAUC pmol/l/time | −6±13 | 336±54 | −42±15 | 304±78 | 0.001 |
| LAUC ng/ml/time | −12.4±6 | 17.4±11 | −11±5 | 3±2.5 | 0.02 |
| Delta L ng/ml | −2.3±1.5 | 8.6±3.0 | −2.8±1.0 | 0.7±0.2 | 0.002 |
Dexamethasone; data are mean ± SEM Discussion
When compared to the dexamethasone/fast condition, the combination of dexamethasone and food increased serum leptin starting at 4 hours (Figure 1A). The increase in serum leptin over basal levels was significant starting at 7 hours after dexamethasone administration (p<0.05). As in experiment 1 (Table 2), when dexamethasone was administered, LAUC and delta leptin were higher under feeding conditions than under fasting conditions (Table 3). There was a significant interaction between the effects of food and dexamethasone on delta L (p<0.05), indicating that the magnitude of the effect of feeding was higher in the dexamethasone versus the saline condition. When dexamethasone was given together with food serum leptin levels doubled from a baseline of 8.3 ng/ml to 16.9 ng/ml at 10 hours (n=4, Figure 1A and Table 3).
Serum insulin (Figure 1B) levels declined from baseline in the saline/fast group (p=0.0001) but did not change in the dexamethasone/fast group. Delta I and IAUC did not differ between dexamethasone/food and dexamethasone/fast conditions (Table 3).
Serum glucose levels decreased over time in the fast/saline condition but increased over time in all other conditions (Figure 1C). Feeding produced a greater rise in serum glucose in the dexamethasone group compared to the saline group (p<0.05) (Table 3).
Discussion
We have demonstrated that an I.V. bolus of dexamethasone increases serum leptin only when subjects are fed. Dexamethasone did not affect serum leptin in fasted subjects. These results are in agreement with previous reports that oral dexamethasone increases serum leptin in subjects allowed to eat a fixed amount of food (7) or to consume food freely (8–11).
In agreement with previous studies (2,6,16), feeding alone prevented the fall in leptin associated with fasting. Although insulin was increased to the same extent with the food/saline treatment as with the food/dexamethasone treatment, serum leptin was increased only in the latter condition. It is unlikely that the magnitude of the effect on serum leptin (+ 100%) after dexamethasone and food can be explained solely as an insulin effect. Increasing serum insulin by I.V. administration to levels comparable to those achieved with meals in our study (384–792 pmol/L), increased plasma leptin by only 26–43% at 9 hours (6). We therefore conclude that dexamethasone must interact with factors associated with meal ingestion (insulin, gastrointestinal hormones and/or glucose) to increase serum leptin.
Some studies have suggested a role for glucose or glucose utilization (17,18) in the regulation of leptin secretion. The administration of glucose after a prolonged fast induces an acute increase in serum leptin, apparently independent of insulin (18). The fact that glucose was increased by dexamethasone in both fasting and feeding conditions, but leptin increased only in the feeding condition, makes it unlikely that glucose per se is the factor responsible for the increase in leptin when dexamethasone is given with food. Although the combination of dexamethasone and food leads to a greater rise in serum glucose, it seems unlikely that this may have contributed to the rise in serum leptin because glucose utilization by the adipocyte was most likely decreased by dexamethasone (9, 19).
There was a time lag of 7 hours after administration of dexamethasone and food before leptin increased. This is similar to that observed with overfeeding (5 to 10 hours) (3), insulin administration (4 to 6 hours) (4–6), or a shift in meal timing (20). This time lag may be necessary to elicit leptin transcription and/or post-transcriptional steps leading to leptin secretion. In vitro, an effect of insulin plus dexamethasone on leptin secretion in subcutaneous adipose tissue was detected at 5 h (21).
Results of some in vitro experiments (21,22) suggest that additive or synergistic effects of insulin plus dexamethasone are likely mechanisms of the increase in serum leptin shown in the present study. Two studies indicate that the combination of insulin and dexamethasone increased the release of leptin from human subcutaneous adipocytes to a greater extent than insulin or dexamethasone alone (21,22). However, others reported an inhibitory effect of insulin on dexamethasone-stimulated leptin secretion from isolated adipocytes (23,24). Differences in incubation conditions are likely to explain these in vitro discrepancies. In vivo, however, clamped hyperinsulinemia after oral dexamethasone did not decrease serum leptin (25).
Further studies are needed to define the role of insulin and/or other meal-associated factors in mediating the effect of dex on leptin in the fed state. In addition, it would be important to extend these studies to a greater numbers of both lean and obese subjects and to compare different modes of glucocorticoid administration.
It may appear paradoxical that glucocorticoids increase serum leptin, yet serum levels of leptin and Cortisol exhibit reciprocal diurnal rhythms (26). This apparent contradiction can be resolved by also considering the level of insulinemia and/or the feeding state. In the fasted state, Cortisol is high, and insulin and leptin are low. In the fed state, a rise in insulin, with at least a permissive level of glucocorticoids, leads to a slow rise in leptin. Moreover, hypercortisolism caused by Cushing's syndrome (27,28) or pharmacological glucocorticoid administration (7–11) combined with hyperinsulinemia, leads to a marked rise in leptin.
In summary, the present study demonstrates that I.V. dexamethasone administered to fed subjects increases serum leptin after 5–7 hours. In contrast, neither dexamethasone alone (without feeding) nor feeding alone (without dexamethasone) are able to increase leptin levels over basal levels. We conclude that dexamethasone must interact with factors associated with feeding to increase leptin in humans.
Acknowledgments
This work was supported by a grant from the American Diabetes Association and a grant from the National Institute of Health (DK26687). We are grateful to Colleen Russell for her comments on the manuscript and to Claudine Lombardi for her help in preparing this manuscript.
References
- 1.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. New Eng J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 2.Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab. 1996;81(9):3419–3423. doi: 10.1210/jcem.81.9.8784108. [DOI] [PubMed] [Google Scholar]
- 3.Kolaczynski JW, Ohannesian JP, Considine RV, Marco C, Caro JF. Response of leptin to short-term and prolonged overfeeding in humans. J Clin Endocrinol Metab. 1996;81(11):4162–4165. doi: 10.1210/jcem.81.11.8923877. [DOI] [PubMed] [Google Scholar]
- 4.Malmstrom R, Taskinen MR, Karonen SL, Yki-Jarvinen H. Insulin increases plasma leptin concentrations in normal subjects and patients with NIDDM. Diabetologia. 1996;39(8):993–996. doi: 10.1007/BF00403921. [DOI] [PubMed] [Google Scholar]
- 5.Utriainen T, Malmstrom R, Makimattila S, Yki-Jarvinen H. Supraphysiological hyperinsulinemia increases plasma leptin concentrations after 4 h in normal subjects. Diabetes. 1996;45:1364–1366. doi: 10.2337/diab.45.10.1364. [DOI] [PubMed] [Google Scholar]
- 6.Saad MF, Khan A, Sharma A, et al. Physiological insulinemia acutely modulates plasma leptin. Diabetes. 1998;47:544–549. doi: 10.2337/diabetes.47.4.544. [DOI] [PubMed] [Google Scholar]
- 7.Miell JP, Englaro P, Blum WF. Dexamethasone induces an acute and sustained rise in circulating leptin levels in normal human subjects. Horm Metab Res. 1996;28:704–707. doi: 10.1055/s-2007-979882. [DOI] [PubMed] [Google Scholar]
- 8.Kiess W, Englaro P, Hanistsch S, Rascher W, Attanasio A, Blum WF. High leptin concentrations in serum of very obese children are further stimulated by dexamethasone. Horm Metab Res. 1996;28:708–710. doi: 10.1055/s-2007-979883. [DOI] [PubMed] [Google Scholar]
- 9.Larsson H, Ahrén B. Short-term dexamethasone treatment increases plasma leptin independently of changes in insulin sensitivity in healthy women. J Clin Endocrinol Metab. 1996;81:4428–4431. doi: 10.1210/jcem.81.12.8954054. [DOI] [PubMed] [Google Scholar]
- 10.Papaspyrou-Rao S, Schneider SH, Petersen RN, Fried SK. Dexamethasone increases leptin expression in humans in vivo. J Clin Endocrinol Metab. 1997;82:1635–1637. doi: 10.1210/jcem.82.5.3928. [DOI] [PubMed] [Google Scholar]
- 11.Dagogo-Jack S, Selke G, Melson A, Newcomer JW. Robust leptin secretory responses to dexamethasone in obese subjects. J Clin Endocrinol Metab. 1997;82:3230–3233. doi: 10.1210/jcem.82.10.4154. [DOI] [PubMed] [Google Scholar]
- 12.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- 13.Hardie L, Trayhurn P, Abramovich D, Fowler P. Circulating leptin in women: a longitudinal study in the menstrual cycle and during pregnancy. Clin Endocrinol. 1997;47:101–106. doi: 10.1046/j.1365-2265.1997.2441017.x. [DOI] [PubMed] [Google Scholar]
- 14.Heymsfield SB, Wang J, Aulet M, et al. Dual photon absorptiometry: validation of mineral and fat measurements. Basic Life Sciences. 1990;55:327–37. doi: 10.1007/978-1-4613-1473-8_45. [DOI] [PubMed] [Google Scholar]
- 15.Kaiser KS. Numerical Analysis. New York: McGraw-Hill Book Company; 1957. p. 146. [Google Scholar]
- 16.Kolaczynski JW, Considine RV, Ohannesian J, et al. Response of leptin to short-term fasting and refeeding in humans. Diabetes. 1996;45:1511–1515. doi: 10.2337/diab.45.11.1511. [DOI] [PubMed] [Google Scholar]
- 17.Mueller WM, Gregoire FM, Stanhope KL, et al. Evidence that glucose metabolism regulates leptin secretion from cultured rat adipocytes. Endocrinology. 1998;139(2):551–558. doi: 10.1210/endo.139.2.5716. [DOI] [PubMed] [Google Scholar]
- 18.Grinspoon SK, Askari H, Landt ML, et al. Effects of fasting and glucose infusion on basal and overnight leptin concentrations in normal-weight women. Am J Clin Nutr. 1997;66:1352–1356. doi: 10.1093/ajcn/66.6.1352. [DOI] [PubMed] [Google Scholar]
- 19.Tappy C, Randin D, Vollenweider P, et al. Mechanisms of dexamethasone-induced insulin resistance in healthy humans. J Clin Endocrinol Metab. 1994;79(4):1063–1069. doi: 10.1210/jcem.79.4.7962275. [DOI] [PubMed] [Google Scholar]
- 20.Schoeller DA, Cella LK, Sinha MK, Caro JF. Entrainment of the diurnal rhythm of serum leptin to meal timing. J Clin Invest. 1997;100:1882–1887. doi: 10.1172/JCI119717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell CD, Petersen RN, Rao SP, et al. Leptin expression in adipose tissue from obese humans: depot-specific regulation by insulin and dexamethasone. Am J Physiol. 1998 doi: 10.1152/ajpendo.1998.275.3.E507. (in press) [DOI] [PubMed] [Google Scholar]
- 22.Wabitsch M, Jensen P, Blum W, et al. Insulin and Cortisol promote leptin production in cultured human fat cells. Diabetes. 1996;45:1435–1438. doi: 10.2337/diab.45.10.1435. [DOI] [PubMed] [Google Scholar]
- 23.Considine RV, Nyce MR, Kolaczynski JW, et al. Dexamethasone stimulates leptin release from human adipocytes: unexpected inhibition by insulin. J Cell Biochem. 1997;65:254–258. doi: 10.1002/(sici)1097-4644(199705)65:2<254::aid-jcb10>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 24.Halleux CM, Servais I, Reul BA, Detry R, Brichard SM. Multihormonal control of ob gene expression and leptin secretion from cultured human visceral adipose tissue: increased responsiveness to glucocorticoids in obesity. J Clin Endocrinol Metab. 1998;83:902–910. doi: 10.1210/jcem.83.3.4644. [DOI] [PubMed] [Google Scholar]
- 25.Kolaczynski JW, Goldstein B, Considine RV. Dexamethasone, Ob gene, and leptin in humans: effect of exogenous hyperinsulinemia. J Clin Endocrinol Metab. 1997;82:3895–3897. doi: 10.1210/jcem.82.11.4341. [DOI] [PubMed] [Google Scholar]
- 26.Licinio J, Mantzoros C, Negrao AB, et al. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat Med. 1997;3(5):575–579. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- 27.Leal-Cerro A, Considine RV, Peino R, et al. Serum immunoreactive-leptin levels are increased in patients with Cushing’s syndrome. Horm Metab Res. 1996;28:711–713. doi: 10.1055/s-2007-979884. [DOI] [PubMed] [Google Scholar]
- 28.Masuzaki H, Ogawa Y, Hosoda K, et al. Glucocorticoid regulation of leptin synthesis and secretion in humans: elevated plasma leptin levels in Cushing’s syndrome. J Clin Endocrinol Metab. 1997;82:2542–2547. doi: 10.1210/jcem.82.8.4128. [DOI] [PubMed] [Google Scholar]