Abstract
Background: Adolescence is a critical period for rising adiposity and falling insulin sensitivity (IS), but the independent relation between adiposity and IS remains understudied.
Objective: The objective was to examine which adiposity measures are most strongly associated with IS in nondiabetic adolescents, whether sex-difference exists, and to what degree genetic or environmental factors affect the adiposity-IS relation.
Design: The study included 1613 rural Chinese adolescents (888 males) aged 13–20 y from a population-based twin cohort. We used graphic plots and linear mixed models to examine the relation of anthropometric and dual-energy X-ray absorptiometry–based measures of adiposity with IS [QUantitative Insulin-sensitivity ChecK Index (QUICKI), fasting serum insulin (FSI), homeostasis model assessment of insulin resistance (HOMA-IR)] and structural equation models to estimate genetic/environmental influences on these associations.
Results: In graphic analyses, participants in the highest quintile (quintile 5) of waist circumference (WC) and percentage body fat (%BF) had the lowest QUICKI and the highest FSI and HOMA-IR values for all age-sex groups. In regression models adjusted for age, Tanner stage, zygosity, and physical activity, all adiposity measures showed inverse associations with IS in both sexes, but WC explained the largest fraction of variance in IS measures (10–14%). Of the phenotypic correlations between adiposity measures and IS (−0.28 to −0.38), 74–85% were attributed to shared genetic factors and 15–26% to common unique environmental factors in both sexes.
Conclusions: In these relatively lean Chinese adolescents, WC and %BF (quintile 5) are the adiposity measures most consistently and strongly associated with decreased IS in both sexes. To a large degree, shared genetic factors contribute to this association.
INTRODUCTION
Over the past 2 decades, the prevalences of obesity and type 2 diabetes in adolescents have increased dramatically worldwide (1). In the United States, the percentage of overweight adolescents has more than tripled since 1980 (2), along with type 2 diabetes (3). In China, while experiencing economic and nutritional transition, obesity in adolescents has also markedly risen since the early 1990s (4).
Adolescence is a critical period for rising adiposity and falling insulin sensitivity (IS) (5, 6). During puberty, a period of tumultuous metabolic changes, dramatic changes occur in body composition and fat distribution in both sexes (7). There are also pubertal changes in IS: IS falls starting at the onset of puberty, is at a minimum at midpuberty, and returns to near prepubertal levels by the end of puberty (8).
The rise in obesity during adolescence has implications throughout life. More than 70% of overweight adolescents remain overweight as adults (2). Even among nonobese adolescents, those with above average body mass index (BMI at 50th–84th percentiles) are also at heightened risk of overweight as adults (9). Obesity has serious health consequences, related in part to its being a state of chronic systemic inflammation, which is linked with insulin resistance (IR) and type 2 diabetes (10).
In the context of the efforts to control the contemporary epidemic of obesity and associated diabetes—in which both start to rise during adolescence—a full understanding of the relation between adiposity and IS among adolescents is greatly needed. This need is further underscored by the fact that most published studies on the association between adiposity measures and IS have involved adults (and whites) (11); only a few studies have been conducted in children (12) and adolescents (13).
This study filled this knowledge gap by examining adiposity-IS associations in a large cohort of Chinese adolescents. This report aimed to address the following questions: Which adiposity measures are most closely associated with IS in adolescents, and does this differ by sex? Do genetic and/or environmental factors contribute to the relation between adiposity and IS? This study is unique in the following aspects. It was conducted in primarily a healthy lean population but with considerable variation in adiposity and IR measures. The measures of adiposity were comprehensive: BMI, waist circumference (WC), percentage body fat (%BF), and percentage trunk fat (%TF). This contrasts with previous studies, in which only BMI was used to measure adiposity (13, 14). IS was assessed by both the QUantitative Insulin-sensitivity ChecK Index (QUICKI) and IR [homeostasis model assessment of IR (HOMA-IR) and fasting serum insulin (FSI)]. Last, the twin design (as detailed in the methods) offers us the opportunity to estimate genetic and environmental contributions to associations between adiposity and IS, which have not previously been examined in adolescents and that is not possible in a general population design.
SUBJECTS AND METHODS
Study population and procedures
This study is part of an ongoing study of the metabolic syndrome in a large population-based twin cohort. The study protocol was approved by the Institutional Review Boards of Children's Memorial Hospital and the Institute of Biomedicine, Anhui Medical University, Hefei, China.
The baseline study was carried out in 8 counties of the Anqing region, Anhui Province, China in 1998–2000, and the follow-up study has been conducted since 2005. This report used the data from twins aged ≤20 y obtained at the follow-up survey. For the baseline study, as detailed elsewhere (15), the twins recruited were ≥6 y of age and both twins were available and consented to the study. For the follow-up study, twins were eligible if both twins participated in the baseline survey and both twins agreed and consented to participate in the follow-up. Participants were invited to a central office and stayed overnight to complete a questionnaire interview, an oral-glucose-tolerance test, a physical examination, and dual-energy X-ray absorptiometry (DXA) scan. Physical examinations were conducted by study physicians with specific training for the study. Tanner Stages (I-V) were assessed by the study physician on the basis of visual inspection of pubic hair, genitals (boys), and breasts (girls) (15).
Insulin sensitivity assessments and laboratory measurements
A standard oral-glucose-tolerance test (1.75 g/kg or a maximum of 75 g glucose) was performed after a fast of ≥10 h. Blood samples were obtained 0 and 120 min after glucose administration to measure plasma glucose and serum insulin. Glucose was measured by using a modified hexokinase enzymatic method (Hitachi 7020 Automatic Analyzer; Hitachi, Tokyo, Japan). Standard quality control procedures were performed each day with standard samples that came with the reagents (CV < 8%). Serum insulin was measured by electrochemiluminescence (ECL) on an Elecsys 2010 system (Roche, Basel, Switzerland). Duplicate analyses were also conducted daily by using samples collected from study participants (CV < 10%; mean = 3%). QUICKI was used to measure IS (a lower QUICK1 suggests lower IS):
HOMA-IR (higher values suggest lower IS) and FSI were used as markers of IR:
QUICKI ranged from 0.1209 to 0.2399, and HOMA-IR ranged from 0.16 to 9.68 in this sample.
Zygosity ascertainment
Zygosity was determined by microsatellite probes, or DNA fingerprinting techniques, which has an accuracy rate >99% (16).
Anthropometric and DXA measures of adiposity
Body weight and height were measured by using standard protocols while the subjects were without shoes or outerwear, as detailed elsewhere (16). WC was measured at the level of the umbilicus. BMI was calculated as weight (kg)/height2 (m). A standard whole-body scan was performed by DXA (GE-Lunar Prodigy, Madison, WI) to measure total BF and TF (chest, abdomen, and pelvis) (16). %BF was calculated as (total BF/body weight) × 100, and %TF was calculated as (TF/total BF) × 100.
Physical activity
Physical activity was assessed by using the short version of the international physical activity questionnaire (IPAQ-Short; http://www.ipaq.ki.se), as detailed elsewhere (17). The IPAQ generates a categorical indicator (low, moderate, and high) of regular physical activity.
Statistical analyses
General adiposity was assessed on the basis of BMI and %BF, and central adiposity was assessed on the basis of WC and %TF. IS was estimated on the basis of QUICKI; IR measures included HOMA-IR and FSI. Because the distribution of HOMA-IR and FSI was positively skewed, natural log-transformed values were used for subsequent analyses.
Locally weighted nonparametric smoothing plots (SAS LOESS; SAS Institute, Cary, NC) were used to graphically examine the relation of each adiposity measure (BMI, %BF, WC, and %TF) and each IS-related measure (QUICKI, HOMA-IR, and FSI). Adiposity measures were grouped into sex- and age-specific quintiles (Q1–Q5) for each year of age. Stratified by adiposity quintiles, QUICKI, HOMA-IR, and FSI were plotted against age, separately for males and females.
To investigate the associations of each adiposity measure with each IS measure, we applied sex-specific linear mixed models (residual maximum likelihood estimation; REML), adjusted for year of age (13 y is the reference group and 14, 15, 16, 17, 18, 19, and 20 y are the 7 indicator variables), Tanner Stage (1 is the reference and 2, 3, 4, and 5 are the 4 indicator variables in males; 1–2 is the reference and 3, 4, and 5 are the 3 indicator variables in females; Tanner stages 1 and 2 were combined because of the small sample size), zygosity [monozygotic (MZ) and dizygotic (DZ)], and physical activity (low, moderate, and high). The first quintile (Q1, the lowest quintile) of each adiposity measure was used as the reference group.
Similar linear mixed models were conducted with all adiposity measures treated as continuous variables to estimate the association of a 1-SD increase in each adiposity measurement (or 1-unit increase in z score) with IS. The adiposity measures were expressed as age- and sex-specific z scores, calculated as an observed value minus the mean value, divided by SD (within each year of age and sex stratum).
Because previous study in adult twins reported the effect of zygosity status on adiposity and metabolic disorders (18), we also stratified our analysis by zygosity to examine whether the relation between measures of adiposity and IS differed between MZ and DZ twins. Also, we tested the interactions between zygosity and each of the adiposity measures [zygosity adiposity-quintiles (or z score)] on the IS measures. Because the adiposity-IS associations in MZ twins were similar to those in DZ twins and the interaction terms were not statistically significant, we included zygosity in the final model as a covariate (as seen in the above linear mixed models).
To examine which adiposity measures provide the best prediction of IS, partial R2 was calculated for the various adiposity measures, based on maximum likelihood (ML) estimation to examine model fitting and assess the proportion of the variance in each IS outcome explained by each or combinations of adiposity measures (19):
where log LM is the log-likelihood of each model (IS measure = adiposity measures + covariates), log LN is the log-likelihood of the same model but without adiposity variables (IS measure = all covariates other than adiposity measures), and n is the number of observations. The SAS procedure MIXED was used, with the family effect treated as a random variable. All analyses were performed with SAS 9.1 software (SAS Institute Inc).
Finally, taking advantage of our twin cohort, we examined the relative contributions of genetic and environmental influences on the observed associations between adiposity and IS using structural equation modeling (20). Twin data, in which MZ twins are genetically identical and DZ twins are genetically related as siblings, provide information to help distinguish genetic from environmental factors. We first fitted a saturated model (ACE model) that allowed for additive genetic (A), common/familial environmental (C), and unique environmental (E) components for each adiposity and IS measure. We also fitted alternative models for which A, C, or E was equated to zero, ie, CE, AE, and AC models, respectively. Chi-square and Akaike Information Criterion (AIC) were used for comparison of goodness of fit of the models. We presented the estimates from the best-fitted models, which had the lowest AIC and did not have a significantly worse fit compared with the saturated model (ie, chi-square test P value > 0.05). Then, we fitted the bivariate Cholesky decomposition models to calculate genetic (rG), common (rC), and unique environmental correlations (rE) between IR and adiposity measures and 95% CIs. All variance components were estimated with inclusion of age, Tanner Stage, and physical activity as covariates in the models. Mx software (http:/www.psy.vu.nl/mxbib/) was used for the twin analysis.
RESULTS
Sample selection
Of a total of 1656 eligible participants, 43 were excluded: 15 because of missing DXA adiposity data, 2 because of missing BMI data, 21 because of missing FPG or serum insulin data, and 5 because of diabetes (FPG ≥ 7.0 mmol/L and/or a 2-h postload glucose concentration ≥11.1 mmol/L). This report includes the remaining 1613 nondiabetic subjects aged 13–20 y. The mean (±SD) age of the subjects was 16.6 ± 2.0 y for males (n = 888) and 16.7 ± 2.0 y for females (n = 725).
Anthropometric measures and maturity
The subjects were relatively short and lean. Mean height was near the 5th percentile of height-for-age on World Health Organization (WHO) charts for both sexes (see online Figure 1 under “Supplemental data” in the online issue) (21). The subjects' age and mean BMI were at the 25th−50th percentile BMI-for-age of WHO child growth standards (21) in females and near the 25th percentile in males (see online Figure 1 under “Supplemental data” in the online issue); 2.1% of the males and 2.6% of the females were overweight (14 males and 15 females) or obese (2 males and 1 female) in those aged ≤19 y (228 mo old; 763 males and 610 females), based on 2007 WHO sex-specific BMI-for-age criterion for children aged ≤19 y old (overweight: > +1 SD, equivalent to a BMI of 25 at 19 y; obesity: > +2 SD, equivalent to a BMI of 30 at 19 y) (21). The median Tanner Stage increased with age in both sexes (Table 1).
TABLE 1.
Adiposity measures and insulin sensitivity (IS) estimates in Chinese twin adolescents aged 13–20 y1
| Tanner stage |
Adiposity measures2 |
IS-related measures |
OGTT-plasma glucose2 |
|||||||||
| Age (y) | n | Median | Mean ± SD | BMI | WC | %BF | %TF | QUICKI2 | HOMA-IR3 | FSI3 | FPG | 2-h PG |
| kg/m2 | cm | % | % | μU/mL | mmol/L | mmol/L | ||||||
| Male | ||||||||||||
| MZ | ||||||||||||
| 13 | 16 | 2 | 1.9 ± 0.8 | 17.8 ± 1.4 | 61.2 ± 3.4 | 12.1 ± 3.4 | 37.1 ± 4.9 | 0.15 ± 0.01 | 2.23 ± 1.49 | 9.0 ± 1.5 | 5.5 ± 0.2 | 6.0 ± 1.0 |
| 14 | 86 | 2 | 2.1 ± 1.0 | 17.5 ± 2.2 | 61.1 ± 5.5 | 11.5 ± 5.5 | 41.0 ± 4.6 | 0.15 ± 0.01 | 1.82 ± 1.49 | 7.4 ± 1.5 | 5.5 ± 0.4 | 6.3 ± 1.2 |
| 15 | 95 | 3 | 2.9 ± 1.1 | 18.0 ± 1.8 | 63.3 ± 4.6 | 10.7 ± 4.3 | 43.9 ± 6.5 | 0.15 ± 0.01 | 1.82 ± 1.82 | 8.2 ± 1.6 | 5.5 ± 0.4 | 6.2 ± 1.2 |
| 16 | 72 | 3 | 3.1 ± 0.8 | 18.9 ± 2.1 | 65.3 ± 5.8 | 12.6 ± 5.84 | 44.3 ± 5.2 | 0.15 ± 0.01 | 2.01 ± 1.49 | 8.2 ± 1.5 | 5.4 ± 0.4 | 6.2 ± 1.3 |
| 17 | 59 | 4 | 3.9 ± 0.8 | 20.1 ± 1.84 | 68.0 ± 4.84 | 11.8 ± 4.6 | 48.3 ± 5.1 | 0.15 ± 0.01 | 1.65 ± 1.49 | 7.4 ± 1.5 | 5.3 ± 0.4 | 5.6 ± 1.2 |
| 18 | 38 | 5 | 4.6 ± 0.6 | 19.9 ± 1.8 | 67.4 ± 4.5 | 11.7 ± 3.7 | 50.9 ± 4.4 | 0.15 ± 0.01 | 1.82 ± 1.65 | 7.4 ± 1.6 | 5.4 ± 0.3 | 6.1 ± 2.0 |
| 19 | 46 | 5 | 4.7 ± 0.5 | 20.1 ± 2.7 | 68.9 ± 7.4 | 11.5 ± 6.2 | 50.8 ± 5.3 | 0.16 ± 0.01 | 1.49 ± 1.82 | 6.0 ± 1.64 | 5.4 ± 0.4 | 5.2 ± 1.1 |
| 20 | 16 | 5 | 4.9 ± 0.3 | 20.7 ± 2.7 | 72.7 ± 7.1 | 14.3 ± 7.6 | 52.4 ± 5.4 | 0.15 ± 0.01 | 1.65 ± 1.82 | 6.7 ± 1.8 | 5.6 ± 0.45 | 5.9 ± 1.2 |
| DZ | ||||||||||||
| 13 | 29 | 2 | 1.8 ± 0.9 | 16.9 ± 1.9 | 59.7 ± 4.8 | 11.8 ± 4.5 | 40.3 ± 6.2 | 0.15 ± 0.01 | 2.23 ± 1.65 | 9.0 ± 1.6 | 5.5 ± 0.3 | 6.5 ± 1.4 |
| 14 | 72 | 2 | 2.3 ± 1.0 | 17.6 ± 1.9 | 62.5 ± 5.4 | 11.8 ± 5.9 | 40.7 ± 5.2 | 0.15 ± 0.01 | 1.82 ± 1.65 | 7.4 ± 1.5 | 5.4 ± 0.4 | 6.3 ± 1.0 |
| 15 | 67 | 3 | 2.2 ± 1.5 | 18.4 ± 1.9 | 63.9 ± 4.9 | 10.8 ± 4.5 | 42.2 ± 5.3 | 0.15 ± 0.01 | 1.82 ± 1.49 | 7.4 ± 1.5 | 5.4 ± 0.4 | 6.1 ± 1.5 |
| 16 | 51 | 3 | 3.3 ± 0.7 | 18.9 ± 1.6 | 65.1 ± 3.8 | 10.5 ± 3.0 | 44.2 ± 4.7 | 0.15 ± 0.01 | 1.82 ± 1.49 | 8.2 ± 1.5 | 5.4 ± 0.4 | 6.1 ± 1.4 |
| 17 | 37 | 4 | 3.8 ± 0.9 | 19.1 ± 1.5 | 65.4 ± 3.8 | 10.0 ± 3.0 | 46.8 ± 4.7 | 0.16 ± 0.01 | 1.49 ± 1.35 | 6.7 ± 1.4 | 5.3 ± 0.4 | 5.9 ± 1.3 |
| 18 | 42 | 4 | 4.3 ± 0.8 | 19.5 ± 1.7 | 68.1 ± 4.9 | 11.3 ± 5.0 | 48.8 ± 5.6 | 0.16 ± 0.01 | 1.65 ± 1.82 | 6.7 ± 1.6 | 5.3 ± 0.4 | 5.6 ± 1.3 |
| 19 | 28 | 5 | 4.6 ± 0.6 | 19.9 ± 2.2 | 67.7 ± 5.5 | 11.8 ± 5.8 | 50.8 ± 5.4 | 0.15 ± 0.01 | 1.82 ± 1.65 | 8.2 ± 1.6 | 5.3 ± 0.4 | 5.5 ± 1.1 |
| 20 | 31 | 5 | 4.9 ± 0.3 | 20.8 ± 2.3 | 70.0 ± 5.9 | 12.8 ± 6.6 | 53.1 ± 6.3 | 0.16 ± 0.02 | 1.49 ± 1.82 | 6.7 ± 1.8 | 5.3 ± 0.3 | 5.3 ± 1.3 |
| Female | ||||||||||||
| MZ | ||||||||||||
| 13 | 22 | 2 | 2.7 ± 0.8 | 17.6 ± 2.2 | 61.0 ± 4.6 | 22.0 ± 5.4 | 45.3 ± 6.2 | 0.15 ± 0.01 | 2.23 ± 1.65 | 9.0 ± 1.6 | 5.4 ± 0.3 | 6.7 ± 1.6 |
| 14 | 85 | 3 | 2.7 ± 0.7 | 18.5 ± 2.0 | 63.0 ± 5.6 | 24.0 ± 5.9 | 46.4 ± 4.0 | 0.15 ± 0.01 | 2.23 ± 1.65 | 10.0 ± 1.5 | 5.4 ± 0.4 | 5.8 ± 1.2 |
| 15 | 71 | 3 | 3.0 ± 0.6 | 19.8 ± 2.5 | 66.2 ± 7.1 | 27.3 ± 6.2 | 47.6 ± 4.2 | 0.15 ± 0.01 | 2.46 ± 1.35 | 10.0 ± 1.3 | 5.3 ± 0.4 | 5.9 ± 1.4 |
| 16 | 45 | 3 | 3.3 ± 0.9 | 19.8 ± 2.4 | 66.5 ± 6.8 | 27.6 ± 5.4 | 49.6 ± 2.7 | 0.14 ± 0.01 | 2.46 ± 1.65 | 11.0 ± 1.6 | 5.3 ± 0.5 | 6.6 ± 1.5 |
| 17 | 52 | 4 | 3.7 ± 0.8 | 20.6 ± 1.9 | 66.5 ± 6.4 | 28.8 ± 4.5 | 49.6 ± 3.5 | 0.14 ± 0.01 | 2.46 ± 1.49 | 11.0 ± 1.5 | 5.2 ± 0.5 | 5.9 ± 1.2 |
| 18 | 43 | 4 | 3.9 ± 0.8 | 21.1 ± 1.9 | 69.2 ± 5.0 | 30.3 ± 3.9 | 51.1 ± 3.3 | 0.14 ± 0.014 | 2.72 ± 1.494 | 11.0 ± 1.5 | 5.3 ± 0.5 | 5.9 ± 1.0 |
| 19 | 42 | 4 | 4.3 ± 0.7 | 21.2 ± 2.2 | 69.6 ± 5.9 | 30.1 ± 5.0 | 51.5 ± 4.0 | 0.15 ± 0.01 | 2.01 ± 1.49 | 8.2 ± 1.5 | 5.2 ± 0.3 | 5.3 ± 1.1 |
| 20 | 34 | 5 | 4.6 ± 0.7 | 21.6 ± 2.64 | 68.5 ± 5.7 | 30.0 ± 5.0 | 51.3 ± 3.4 | 0.15 ± 0.01 | 2.23 ± 1.49 | 9.0 ± 1.5 | 5.1 ± 0.3 | 5.2 ± 1.0 |
| DZ | ||||||||||||
| 13 | 19 | 2 | 2.2 ± 1.0 | 17.3 ± 2.0 | 59.6 ± 5.3 | 20.4 ± 5.5 | 45.1 ± 5.6 | 0.15 ± 0.01 | 2.23 ± 1.49 | 9.0 ± 1.5 | 5.3 ± 0.3 | 6.0 ± 0.8 |
| 14 | 28 | 3 | 2.7 ± 0.8 | 18.1 ± 2.1 | 62.1 ± 5.2 | 22.7 ± 6.6 | 45.7 ± 4.4 | 0.15 ± 0.01 | 2.46 ± 1.65 | 11.0 ± 1.5 | 5.3 ± 0.5 | 6.0 ± 1.1 |
| 15 | 72 | 3 | 2.9 ± 0.7 | 19.3 ± 2.2 | 64.6 ± 5.8 | 26.6 ± 5.0 | 46.5 ± 4.1 | 0.15 ± 0.01 | 2.46 ± 1.49 | 10.0 ± 1.5 | 5.4 ± 0.4 | 6.0 ± 1.1 |
| 16 | 45 | 3 | 3.3 ± 0.9 | 20.0 ± 2.4 | 66.6 ± 6.2 | 28.4 ± 5.4 | 49.0 ± 4.1 | 0.15 ± 0.01 | 2.23 ± 1.65 | 10.0 ± 1.6 | 5.3 ± 0.5 | 6.0 ± 1.6 |
| 17 | 32 | 4 | 3.8 ± 0.7 | 21.0 ± 2.0 | 68.3 ± 5.5 | 29.4 ± 5.1 | 48.1 ± 4.3 | 0.15 ± 0.01 | 2.46 ± 1.49 | 10.0 ± 1.5 | 5.3 ± 0.4 | 5.8 ± 0.8 |
| 18 | 29 | 4 | 4.3 ± 0.7 | 20.5 ± 1.8 | 67.7 ± 4.9 | 28.5 ± 4.7 | 49.2 ± 3.6 | 0.15 ± 0.01 | 2.01 ± 1.49 | 9.0 ± 1.5 | 5.1 ± 0.4 | 5.6 ± 1.3 |
| 19 | 14 | 5 | 4.6 ± 0.5 | 21.5 ± 1.6 | 69.8 ± 5.0 | 29.2 ± 3.4 | 50.2 ± 3.4 | 0.15 ± 0.01 | 2.01 ± 1.65 | 9.0 ± 1.6 | 5.3 ± 0.4 | 5.4 ± 1.0 |
| 20 | 19 | 5 | 4.8 ± 0.4 | 19.8 ± 2.0 | 66.3 ± 6.0 | 27.4 ± 4.2 | 50.3 ± 4.0 | 0.15 ± 0.01 | 2.01 ± 1.82 | 9.0 ± 1.6 | 5.1 ± 0.5 | 5.4 ± 1.4 |
WC, waist circumference; %BF, percentage body fat; %TF, percentage trunk fat; QUICKI, QUantitative Insulin-sensitivity ChecK Index; HOMA-IR, homeostasis model assessment of insulin resistance; FSI, fasting serum insulin; FPG, fasting plasma glucose; 2-h PG, 2-h postload glucose; OGTT, oral-glucose-tolerance test; MZ, monozygotic; DZ, dizygotic.
Values are means ± SDs.
Values are geometric means ± SDs.
Sex-specific generalized estimating equation linear regressions were used to test the difference of each continuous value between MZ and DZ twins within each 1-y age (2-sided P values): 4P < 0.05, 5P < 0.01.
Distribution of adiposity and IS measures
Of 1437 participants in whom zygosity was determined, 407 pairs (male/male 212, female/female 195) were MZ and 300 pairs (male/male 132, female/female 84, and male/female 84) were DZ. The mean (± SD) adiposity measures (BMI, WC, %BF, and %TF), IS-related measures (QUICKI, HOMA-IR, and FSI), and measures of plasma glucose (FPG and 2-h postload glucose concentration) by zygosity, age, and sex are shown in Table 1. Overall, there were no differences between MZ and DZ twins in these measurements for most age-sex subgroups (P > 0.05), with only a few exception. For example, in males, the mean %BF in MZ twins was 2.1% higher than that in DZ twins at age 16 y (P = 0.045).
Relations between adiposity measures (%BF, BMI, WC, and %TF) and IS
Graphic analysis
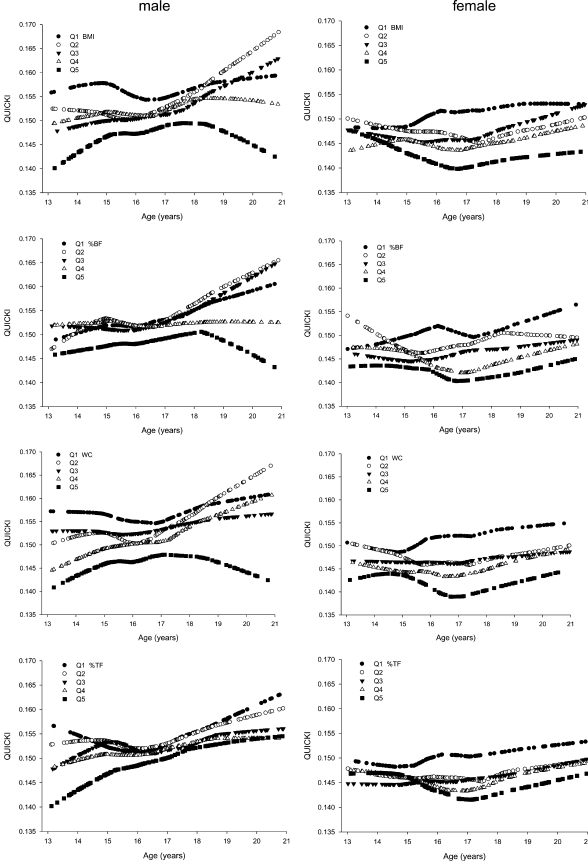
Levels of QUICKI by age, stratified by age- and sex-specific quintiles for each adiposity measure, are shown in Figure 1; those for FSI and HOMA-IR are available elsewhere (see online Figures 2 and 3, respectively, under “Supplemental data” in the online issue). A general pattern emerged from these plots: subjects in the highest quintile (Q5) of adiposity measures had the lowest level of QUICKI (Figure 1), the highest FSI levels (see online Figure 2 under “Supplemental data” in the online issue), and the highest HOMA-IR levels (see online Figure 3 under “Supplemental data” in the online issue) in most age-sex subgroups. These patterns were most evident for WC, %BF, and BMI.
FIGURE 1.
Smoothing plots of QUantitative Insulin-sensitivity ChecK Index (QUICKI) by age, stratified by each 1-y age group and sex-specific quintiles (Q1–Q5) of adiposity measures in 888 males and 725 females aged 13–20 y. %BF, percentage body fat; WC, waist circumference; %TF, percentage trunk fat.
Linear mixed modeling
After adjustment for age, Tanner stage, zygosity, and physical activity, linear mixed models confirmed that all measures of adiposity were associated with reduced IS in both sexes, and this was true when adiposity was analyzed categorically and when it was analyzed continuously. The analyses for QUICKI and log (FSI) are shown in Table 2 (categorical) and Table 3 (continuous). For example, in males, relative to Q1 WC, Q5 WC was associated with a 0.0123 lower QUICKI (P for trend <0.0001) (Table 2), and a 1-SD increase in WC was associated with a 0.0044 lower QUICKI (Table 3) (P < 0.0001). For other measures of adiposity, IS was lower for Q2–Q5 than for Q1 in females; but an association with reduced IS was seen only for Q4–Q5 %BF and Q4–Q5 %TF in males (Table 2). The associations between each adiposity measure and HOMA-IR were similar to those for log (FSI) levels in both sexes (see online Table 1 under “Supplemental data” in the online issue).
TABLE 2.
Association of adiposity measures with QUantitative Insulin-sensitivity ChecK Index (QUICKI) and fasting serum insulin (FSI) in male and female twins aged 13–20 y1
| Males |
Females |
|||||||||||||
| QUICKI |
log (FSI) (μU/mL) |
QUICKI |
log (FSI) (μU/mL) |
|||||||||||
| n | β | SE | P value | β | SE | P value | n | β | SE | P value | β | SE | P value | |
| WC quintile | ||||||||||||||
| Q1 (low) | 153 | Ref | Ref | 124 | Ref | Ref | ||||||||
| Q2 | 158 | −0.0032 | 0.0014 | 0.0236 | 0.13 | 0.05 | 0.0122 | 135 | −0.0035 | 0.0011 | 0.0024 | 0.15 | 0.05 | 0.0017 |
| Q3 | 162 | −0.0034 | 0.0014 | 0.0162 | 0.14 | 0.05 | 0.0107 | 132 | −0.0044 | 0.0012 | 0.0002 | 0.19 | 0.05 | 0.0002 |
| Q4 | 155 | −0.0053 | 0.0015 | 0.0004 | 0.21 | 0.05 | 0.0001 | 132 | −0.0067 | 0.0012 | <0.0001 | 0.29 | 0.05 | <0.0001 |
| Q5 (high) | 157 | −0.0123 | 0.0015 | <0.0001 | 0.51 | 0.06 | <0.0001 | 129 | −0.0094 | 0.0013 | <0.0001 | 0.42 | 0.05 | <0.0001 |
| Trend | 785 | <0.0001 | <0.0001 | 652 | <0.0001 | <0.0001 | ||||||||
| Partial R2 | 0.100 | 0.119 | 0.086 | 0.097 | ||||||||||
| %BF quintile | ||||||||||||||
| Q1 (low) | 154 | Ref | Ref | 127 | Ref | Ref | ||||||||
| Q2 | 159 | 0.0003 | 0.0014 | 0.8318 | 0.003 | 0.05 | 0.958 | 132 | −0.0026 | 0.0012 | 0.0313 | 0.11 | 0.05 | 0.032 |
| Q3 | 158 | −0.0021 | 0.0014 | 0.1548 | 0.08 | 0.05 | 0.123 | 133 | −0.0036 | 0.0012 | 0.0035 | 0.17 | 0.05 | 0.001 |
| Q4 | 159 | −0.0031 | 0.0015 | 0.0358 | 0.14 | 0.06 | 0.015 | 132 | −0.0066 | 0.0012 | <0.0001 | 0.29 | 0.05 | <0.0001 |
| Q5 (high) | 155 | −0.0073 | 0.0015 | <0.0001 | 0.32 | 0.06 | <0.0001 | 128 | −0.0074 | 0.0013 | <0.0001 | 0.35 | 0.06 | <0.0001 |
| Trend | 785 | <0.0001 | <0.0001 | 652 | <0.0001 | <0.001 | ||||||||
| Partial R2 | 0.047 | 0.058 | 0.065 | 0.076 | ||||||||||
| BMI quintile | ||||||||||||||
| Q1 (low) | 154 | Ref | Ref | 127 | Ref | Ref | ||||||||
| Q2 | 159 | −0.0051 | 0.0014 | 0.0003 | 0.18 | 0.05 | 0.001 | 132 | −0.0037 | 0.0012 | 0.0017 | 0.15 | 0.05 | 0.002 |
| Q3 | 158 | −0.0053 | 0.0015 | 0.0004 | 0.20 | 0.06 | 0.001 | 133 | −0.0047 | 0.0012 | 0.0001 | 0.21 | 0.05 | <0.0001 |
| Q4 | 159 | −0.0053 | 0.0015 | 0.0005 | 0.19 | 0.06 | 0.001 | 132 | −0.0057 | 0.0012 | <0.0001 | 0.25 | 0.05 | <0.0001 |
| Q5 (high) | 155 | −0.0121 | 0.0016 | <0.0001 | 0.48 | 0.06 | <0.0001 | 128 | −0.0086 | 0.0013 | <0.0001 | 0.39 | 0.06 | <0.0001 |
| Trend | 785 | <0.0001 | <0.0001 | 652 | <0.0001 | <0.0001 | ||||||||
| Partial R2 | 0.086 | 0.096 | 0.067 | 0.077 | ||||||||||
| %TF quintile | ||||||||||||||
| Q1 (low) | 154 | Ref | Ref | 127 | Ref | Ref | ||||||||
| Q2 | 159 | −0.0006 | 0.0014 | 0.6803 | 0.03 | 0.05 | 0.610 | 132 | −0.0039 | 0.0012 | 0.0009 | 0.17 | 0.05 | 0.0008 |
| Q3 | 158 | −0.0010 | 0.0014 | 0.5102 | 0.05 | 0.05 | 0.381 | 133 | −0.0046 | 0.0012 | 0.0002 | 0.19 | 0.05 | 0.0002 |
| Q4 | 159 | −0.0030 | 0.0015 | 0.0490 | 0.11 | 0.06 | 0.045 | 132 | −0.0042 | 0.0012 | 0.0007 | 0.19 | 0.05 | 0.0003 |
| Q5 (high) | 155 | −0.0039 | 0.0016 | 0.0149 | 0.18 | 0.06 | 0.003 | 128 | −0.0060 | 0.0013 | <0.0001 | 0.27 | 0.06 | <0.0001 |
| Trend | 785 | 0.0049 | 0.0014 | 652 | <0.0001 | <0.001 | ||||||||
| Partial R2 | 0.014 | 0.018 | 0.036 | 0.040 | ||||||||||
Sample size varies from 785 to 649 for males and from 652 to 640 for females because of missing data for Tanner stage. Adiposity measures were categorized based on each 1-y age- and sex-specific quintiles (Q) of each adiposity measure. WC, waist circumference; %BF, percentage body fat; %TF, percentage trunk fat; Ref, referent. All regression models were adjusted for Tanner stage (1, 2, 3, 4, and 5 for males; 1–2, 3, 4, and 5 for females), age (13, 14, 15, 16, 17, 18, 19, and 20 y), zygosity (monozygosity or dizygosity), and physical activity (low, moderate, and high). Partial R2 = 1 − exp[−2/n (log LM − log LN)], where log LM is the log-likelihood of the model of interest (which includes fixed and random effects and a correlated error structure): insulin sensitivity measure = each adiposity quintiles + covariates; log LN is the log-likelihood of the model: insulin sensitivity measure = all covariates other than adiposity measures; and n is the number of observations.
TABLE 3.
Association of adiposity measures with QUantitative Insulin-sensitivity ChecK Index (QUICKI) and fasting serum insulin (FSI) among adolescents aged 13–20 y1
| Males (n = 785) |
Females (n = 652) |
|||||||||
| Adiposity measures (z scores) | QUICKI |
log (FSI) (μU/mL) |
QUICKI | log (FSI) (μU/mL) |
||||||
| β (SE) | Partial R2 | β (SE) | Partial R2 | β (SE) | Partial R2 | β (SE) | Partial R2 | |||
| WC | −0.0044 (0.0005)2 | 0.113 | 0.19 (0.02)2 | 0.142 | −0.0034 (0.0004)2 | 0.100 | 0.15 (0.02)2 | 0.112 | ||
| %BF | −0.0036 (0.0005)2 | 0.084 | 0.16 (0.02)2 | 0.108 | −0.0029 (0.0004)2 | 0.070 | 0.14 (0.02)2 | 0.085 | ||
| BMI | −0.0043 (0.0005)2 | 0.106 | 0.18 (0.02)2 | 0.126 | −0.0032 (0.0004)2 | 0.084 | 0.14 (0.02)2 | 0.097 | ||
| WC+%BF | ||||||||||
| WC | −0.0034 (0.0007)2 | 0.14 (0.02)2 | −0.0028 (0.0006)2 | 0.12 (0.02)2 | ||||||
| %BF | −0.0014 (0.0006)3 | 0.120 | 0.06 (0.02)4 | 0.151 | −0.0009 (0.0006) | 0.104 | 0.05 (0.02)3 | 0.119 | ||
| WC+BMI | ||||||||||
| WC | −0.0028 (0.0009)4 | 0.13 (0.03)2 | −0.0025 (0.0007)5 | 0.11 (0.03)5 | ||||||
| BMI | −0.0020 (0.0009)3 | 0.120 | 0.07 (0.03)3 | 0.147 | −0.0012 (0.0007) | 0.104 | 0.06 (0.03) | 0.118 | ||
Sample size varies from 785 to 649 for males and from 652 to 640 for females because of missing data for Tanner stage. WC, waist circumference; %BF, percentage body fat. All regression models were adjusted for Tanner stage (1, 2, 3, 4, and 5 for males; 1–2, 3, 4, and 5 for females), age (13, 14, 15, 16, 17, 18, 19, and 20 y), zygosity (monozygosity or dizygosity), and physical activity (low, moderate, and high). Partial R2 = 1 − exp[−2/n (log LM − log LN)], where Log LM is the log-likelihood of the model of interest (which includes fixed and random effects and a correlated error structure): insulin sensitivity measure = z score of each adiposity + covariates; log LN is the log-likelihood of the model: insulin sensitivity measure = all covariates other than adiposity measures; n is the number of observations. z Scores were age- and sex-specific for each adiposity measure.
P < 0.0001.
P < 0.05.
P < 0.01.
P < 0.001.
Further analyses focused on WC, %BF, and BMI as measures of adiposity. As shown in Table 3, the proportion of the variance in IS (QUICKI or FSI) explained by adiposity varied slightly by each adiposity measure (7–14%). For WC, partial R2 ranged from a low of 0.100 for QUICKI in females to a high of 0.142 for FSI in males. For %BF, the range was 0.070–0.108; for BMI, the range was 0.084–0.126. When 2 factor measures (general and central adiposity) were examined (WC and %BF; WC and BMI), the R2 estimates did not change appreciably from those for single factor measures.
Genetic and environmental contributions to the adiposity-IR associations
We examined the relative contribution of genetic and environmental influences on the associations between adiposity measures (BMI, %BF, and WC) and IS in adolescents. The following analysis included only paired same-sex twins.
IS, BMI, %BF, and WC were all traits influenced by genetic factors in both sexes. This was reflected by a higher intrapair correlation (which measures the within-pair similarity of the traits) in MZ twins than in DZ twins (Table 4). After adjustment for age, Tanner stage, and physical activity, the estimates of heritability (the ratio of genetic variance to the total phenotypic variance) for IS measures (QUICKI, HOMA-IR, and FSI) ranged from 50% to 58% in both sexes. For adiposity measures, heritability estimates ranged from 48% for BMI in females to 87% for %BF in males (Table 4; see online Table 2 under “Supplemental data” in the online issue).
TABLE 4.
Estimates of genetic (A), common environmental (C), and individual specific environmental (E) effects on insulin sensitivity (IS)–related measures and adiposity measures in adolescents aged 13–20 y by using univariate model fitting1
| Intrapair correlations |
Parameter estimates |
|||||||
| MZ | DZ | A (95% CI) | C (95% CI) | E (95%CI) | χ2 | P value | AIC2 | |
| Male | ||||||||
| IS-related measures | ||||||||
| QUICKI | 0.58 | 0.10 | 0.50 (0.37, 0.61) | — | 0.50 (0.39, 0.63) | 0.00 | — | −2.00 |
| HOMA-IR3 | 0.62 | 0.13 | 0.57 (0.45, 0.66) | — | 0.43 (0.34, 0.55) | 0.00 | — | −2.00 |
| FSI3 | 0.63 | 0.11 | 0.58 (0.46, 0.68) | — | 0.42 (0.32, 0.54) | 0.00 | — | −2.00 |
| Adiposity measures | ||||||||
| BMI | 0.91 | 0.58 | 0.86 (0.82, 0.89) | — | 0.14 (0.11, 0.18) | 0.00 | — | −2.00 |
| WC | 0.87 | 0.56 | 0.77 (0.70, 0.82) | — | 0.23 (0.18, 0.30) | 0.28 | 0.599 | −1.73 |
| %BF | 0.87 | 0.48 | 0.87 (0.82, 0.90) | — | 0.13 (0.10, 0.18) | 0.29 | 0.589 | −1.71 |
| Female | ||||||||
| IS-related measures | ||||||||
| QUICKI | 0.53 | 0.30 | 0.55 (0.44–0.64) | — | 0.45 (0.36, 0.56) | 0.29 | 0.590 | −1.71 |
| HOMA-IR3 | 0.54 | 0.30 | 0.56 (0.45–0.65) | — | 0.44 (0.35, 0.55) | 0.24 | 0.622 | −1.76 |
| FSI3 | 0.55 | 0.30 | 0.57 (0.47–0.66) | — | 0.43 (0.34, 0.53) | 0.21 | 0.646 | −1.79 |
| Adiposity measures | ||||||||
| BMI | 0.89 | 0.57 | 0.48 (0.24, 0.81) | 0.35 (0.02, 0.58) | 0.17 (0.13, 0.22) | — | — | — |
| WC | 0.83 | 0.52 | 0.78 (0.72, 0.83) | — | 0.22 (0.17, 0.28) | 3.04 | 0.081 | 1.04 |
| %BF | 0.86 | 0.49 | 0.81 (0.76, 0.86) | — | 0.19 (0.14, 0.24) | 0.70 | 0.402 | −1.30 |
QUICKI, QUantitative Insulin-sensitivity ChecK Index; HOMA-IR, homeostasis model assessment of insulin resistance; FSI, fasting serum insulin; WC, waist circumference; %BF, percentage body fat; AIC, Akaike's Information Criterion; MZ, monozygotic; DZ, dizygotic. Age, Tanner stage, and physical activity were adjusted as covariates in the structural equation modelings.
Chi-square and AIC were used to compare the goodness-of-fit of the models (AE and CE models) with the saturated fat model (ACE model). Only the estimates from the best-fit models are presented.
Natural log transformed.
The magnitude of cross-twin cross-trait correlations (r) between z score of IS and z scores of each adiposity measure in MZ was much higher than in DZ twins. Cross-twin cross-trait correlations were statistically significant for MZ (BMI, %BF, and WC: r = −0.31, −0.20, and −0.33 in males and −0.19, −0.21, and −0.23 in females; all P < 0.001) but not for DZ (r = −0.01, −0.001, 0.03 in males; −0.14 for BMI and −0.13 for WC in females; all P > 0.05), with one exception (for female DZ twins, r = −0.22 for IS and %BF, P < 0.01).
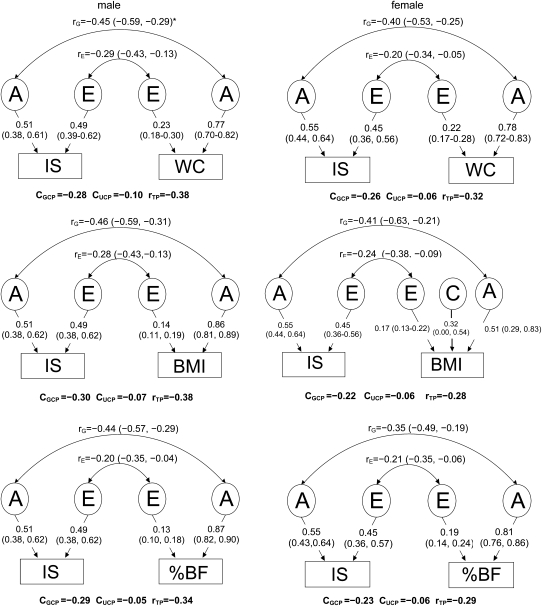
As shown in Figure 2, in this sample, a common set of genetic and unique environmental factors contributed to the observed inverse associations of IS compared with BMI, WC, and %BF. Bivariate Cholesky decomposition models showed that each adiposity measure and QUICKI were moderately correlated genetically (rG = slightly <0.5 in males and ≈0.4 in females), which indicated that these paired traits share some common genetic factors. The corresponding environmental correlations (rE) were ≈ −0.2 to −0.3 in both sexes. None of the 95% CIs for rG and rE was across zero. Seventy-four to 85% of the total phenotypic correlations between each adiposity measure and IS (rTP = −0.28 to −0.38) is determined by common genes [CGCP (genetic contribution to the correlation between two phenotypes) = −0.22 to −0.30], whereas the remaining 15–26% is due to common unique environmental factors [GUCP (unique environmental contribution to the correlation between two phenotypes) = −0.05 to −0.10] in both sexes. For example, in males, of the phenotypic correlation between WC and IS (−0.38), 74% (= 0.28/0.38) is due to genetic factors.
FIGURE 2.
Estimates of genetic (rG) and environmental (rE) correlations between adiposity measures [BMI, percentage body fat (%BF), and waist circumference (WC)] and insulin sensitivity [IS, estimated by QUantitative Insulin-sensitivity ChecK Index (QUICKI)] in males and females. Age, Tanner stage, and physical activity were adjusted as covariates in all bivariate structural equation modeling. rG, genetic correlation between 2 phenotypes; rC, common environmental correlation; rE, unique environmental correlation between 2 phenotypes; CGCP, CCCP, and CUCP, genetic, common environmental, and unique environmental contribution to the correlation between 2 phenotypes, respectively; rTP, phenotype correlation between IS and adiposity measures. 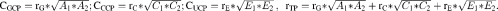 The genetic (A), common environment (C), and individual specific environment (E) components for each phenotype were similar to those from univariate genetic models, but they were not identical because bivariate analysis included covariance between the 2 variables examined.
The genetic (A), common environment (C), and individual specific environment (E) components for each phenotype were similar to those from univariate genetic models, but they were not identical because bivariate analysis included covariance between the 2 variables examined.
DISCUSSION
Main findings in this study and findings in the previous literature
Our study provides new information on how adiposity and IS are related in the relatively lean adolescent sample (98% with normal weight) examined. Notably, we found an inverse relation between adiposity measurements and IS, independent of age, Tanner stage, zygosity, and physical activity. The relation was most consistent for Q5 WC (a measure of central adiposity) and for Q5 %BF (a measure of total body adiposity). Our data suggest that these measures should be used in future research concerning adolescent adiposity-IS relations. A unique feature of this study was that it showed an inverse adiposity-IS relation in relatively lean Chinese adolescents, which complements and extends the findings from previous studies in mostly overweight, adult, white populations.
There have been few large-scale epidemiologic studies of the association between adiposity and IS in adolescents. Studies in the United States (using BMI and FSI) (14) and in Hong Kong (using BMI and HOMA-IR) (13) have shown falling IS with rising adiposity. Our findings confirm those findings and show that they remained after adjustment for age, Tanner stage, zygosity, and physical activity. Our study used comprehensive measures of adiposity and IS to expands an understanding of the manifestation of this relation. Our finding that Q5 WC and %BF are most consistently and strongly related to IS is consistent with the findings of some previous small studies (22–24). This reinforces the importance of measuring these variables in future studies in this field.
This study also provided new information on the influence of genetic and environmental factors on adiposity, IS, and the inverse adiposity-IS association. The heritability of adiposity measures and IS measures was near to or >50%; 74–85% of the total phenotypic correlation between each adiposity measure and IS was due to genetic factors in both sexes. Thus, there is a strong genetic influence on adiposity and IS separately and also on the adiposity-IS correlation. At the same time, environmental factors also contribute, particularly related to adiposity and IS separately.
Previous studies have shown that the relation between BF/fat distribution and IS involves both shared genetic and common environmental factors in adults (11, 25, 26). Our estimated heritabilities were within the range observed in those previous studies. Our findings expanded our knowledge on the genetic and environmental contribution to adiposity-IS associations during adolescence, a critical time period for the development of type 2 diabetes and cardiovascular disease risk factors.
Study strengths and limitations
This study had several strengths. First, the data came from a population-based twin cohort with accurate ascertainment of zygosity. Second, DXA-based adiposity measures were included. In addition, standard oral-glucose-tolerance tests were performed in this study, so that it was possible to ensure that only nondiabetic participants were included. Study participants were healthy rural Chinese adolescents and young adults, which prevented some confounders such as medication use, widespread Western dietary intake, and sedentary lifestyles.
Our study also had several limitations. First, the cross-sectional design limits any temporal inference or cause-effect conclusion. Second, birth weight was not included in the final modeling. Previous studies suggest that MZ twins are exposed to a more adverse intrauterine environment than are DZ twins, and birth weight has a potential influence on adult adiposity and IS levels (27). However, of 500 participants who reported birth weight in the present study, no birth weight differences (mean ± SD) were observed between MZ and DZ twins in males (2731 ± 689 g compared with 2784 ± 636 g) or in females (2658 ± 562 g compared with 2678 ± 663 g). In addition, no associations were found between birth weight and adiposity measures or IS measures after adjustment for age, Tanner stage, and physical activity in both sexes, except for %TF in males (β = −1.54 per kg birth weight, SE = 0.68, P = 0.023). Thus, birth weight was less likely to confound our findings on the adiposity-IR association in this study sample. Third, because our rural Chinese sample was relatively short and lean, caution is needed when generalizing our findings to other populations. Finally, although the twin analyses presented provide evidence of the contribution of genetics and environment to the adiposity-IS association, it did not identify specific genes or address associated mechanisms.
Summary and future research needs
In this sample of relatively lean, healthy, rural Chinese adolescents, we observed an inverse association between adiposity and IS. WC and %BF are the adiposity measures most consistently and strongly associated with decreased IS in both sex. Although the inverse associations between adiposity measures and IS are clear, the R2 values of the models were relatively low, <21% after accounting for the major determinants of IR, including adiposity, Tanner Stage, age, sex, zygosity, and physical activity. Thus, other factors influencing IS are yet to be identified. Of the phenotypic correlations between adiposity measures and IS, 74–85% were attributed to shared genetic factors, whereas 15–26% were attributed to common unique environmental factors in both sexes. Although our data underscore the importance of genetic influences, further studies are needed to determine specific genes or gene-environmental interactions that are important for adiposity, IR, and their phenotypic correlation. This study was cross-sectional in nature. Continued follow-up of this cohort would provide more insight into the temporal relation between adiposity and IR and the complex interplay of environmental and genetic determinants. This is especially important because China is undergoing a rapid economic and nutritional transition.
Supplementary Material
Acknowledgments
We acknowledge the assistance and cooperation of the faculty and staff of the Anhui Institute of Biomedicine, Anhui Medical University, and thank all of the study participants for their support.
The authors’ responsibilities were as follows—FO: completed the data analyses, contributed to data interpretation, and drafted the manuscript; KKC: contributed to data interpretation and intensive editing; WJB and DZ: participated in the data interpretation and critically reviewed the manuscript; BW, HX, SZ, GW, RL, and XX: participated in data collection; LMA: assisted with the R2 calculation; and XW: contributed to the study design, quality control, data analysis, intensive editing of the manuscript, and ascertainment of grant support for this study. None of the authors had any conflicts of interest.
REFERENCES
- 1.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet 2002;360:473–82 [DOI] [PubMed] [Google Scholar]
- 2.MacKay AP, Duran C. Adolescent health in the United States, 2007. Atlanta, GA: National Center for Health Statistics, 2007 [Google Scholar]
- 3.Fagot-Campagna A, Pettitt DJ, Engelgau MM, et al. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr 2000;136:664–72 [DOI] [PubMed] [Google Scholar]
- 4.Ji CY. The prevalence of childhood overweight/obesity and the epidemic changes in 1985-2000 for Chinese school-age children and adolescents. Obes Rev 2008;9(suppl 1):78–81 [DOI] [PubMed] [Google Scholar]
- 5.Morrison JA, Barton BA, Biro FM, Daniels SR, Sprecher DL. Overweight, fat patterning, and cardiovascular disease risk factors in black and white boys. J Pediatr 1999;135:451–7 [DOI] [PubMed] [Google Scholar]
- 6.Morrison JA, Sprecher DL, Barton BA, Waclawiw MA, Daniels SR. Overweight, fat patterning, and cardiovascular disease risk factors in black and white girls: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr 1999;135:458–64 [DOI] [PubMed] [Google Scholar]
- 7.Cruz ML, Shaibi GQ, Weigensberg MJ, Spruijt-Metz D, Ball GD, Goran MI. Pediatric obesity and insulin resistance: chronic disease risk and implications for treatment and prevention beyond body weight modification. Annu Rev Nutr 2005;25:435–68 [DOI] [PubMed] [Google Scholar]
- 8.Moran A, Jacobs DR, Jr, Steinberger J, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes 1999;48:2039–44 [DOI] [PubMed] [Google Scholar]
- 9.Field AE, Cook NR, Gillman MW. Weight status in childhood as a predictor of becoming overweight or hypertensive in early adulthood. Obes Res 2005;13:163–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006;116:1793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samaras K, Nguyen TV, Jenkins AB, et al. Clustering of insulin resistance, total and central abdominal fat: same genes or same environment? Twin Res 1999;2:218–25 [DOI] [PubMed] [Google Scholar]
- 12.Zhang CX, Tse LA, Deng XQ, Jiang ZQ. Cardiovascular risk factors in overweight and obese Chinese children: a comparison of weight-for-height index and BMI as the screening criterion. Eur J Nutr 2008;47:244–50 [DOI] [PubMed] [Google Scholar]
- 13.Kong AP, Choi KC, Ko GT, et al. Associations of overweight with insulin resistance, beta-cell function and inflammatory markers in Chinese adolescents. Pediatr Diabetes 2008;9:488–95 [DOI] [PubMed] [Google Scholar]
- 14.Ford ES, Li C, Imperatore G, Cook S. Age, sex, and ethnic variations in serum insulin concentrations among U.S. youth: findings from the National Health and Nutrition Examination Survey 1999-2002. Diabetes Care 2006;29:2605–11 [DOI] [PubMed] [Google Scholar]
- 15.Ouyang F, Wang B, Arguelles LM, et al. Bone growth patterns in Chinese children and adolescents: a 6 year follow-up study provides evidence for sexual dimorphism and tracking. Arch Osteoporos 2007;2:29–43 [Google Scholar]
- 16.Wang B, Necheles J, Ouyang F, et al. Monozygotic co-twin analyses of body composition measurements and serum lipids. Prev Med 2007;45:358–65 [DOI] [PubMed] [Google Scholar]
- 17.Yu Y, Lu BS, Wang B, et al. Short sleep duration and adiposity in Chinese adolescents. Sleep 2007;30:1688–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poulsen P, Levin K, Beck-Nielsen H, Vaag A. Age-dependent impact of zygosity and birth weight on insulin secretion and insulin action in twins. Diabetologia 2002;45:1649–57 [DOI] [PubMed] [Google Scholar]
- 19.Magee L. R2 measures based on Wald and likelihood ratio joint significance tests. Am Stat 1990;44:250–3 [Google Scholar]
- 20.Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Brief Bioinform 2002;3:119–33 [DOI] [PubMed] [Google Scholar]
- 21.WHO. Growth reference 5-19 years. http://www.who.int/growthref/who2007_bmi_for_age/en/ (cited 25 May 2009)
- 22.Roemmich JN, Clark PA, Lusk M, et al. Pubertal alterations in growth and body composition. VI. Pubertal insulin resistance: relation to adiposity, body fat distribution and hormone release. Int J Obes Relat Metab Disord 2002;26:701–9 [DOI] [PubMed] [Google Scholar]
- 23.Grant AM, Taungapeau FK, McAuley KA, et al. Body mass index status is effective in identifying metabolic syndrome components and insulin resistance in Pacific Island teenagers living in New Zealand. Metabolism 2008;57:511–6 [DOI] [PubMed] [Google Scholar]
- 24.Lee S, Bacha F, Gungor N, Arslanian SA. Waist circumference is an independent predictor of insulin resistance in black and white youths. J Pediatr 2006;148:188–94 [DOI] [PubMed] [Google Scholar]
- 25.Nelson TL, Vogler GP, Pedersen NL, Hong Y, Miles TP. Genetic and environmental influences on body fat distribution, fasting insulin levels and CVD: are the influences shared? Twin Res 2000;3:43–50 [DOI] [PubMed] [Google Scholar]
- 26.Comuzzie AG, Blangero J, Mahaney MC, et al. Genetic and environmental correlations among hormone levels and measures of body fat accumulation and topography. J Clin Endocrinol Metab 1996;81:597–600 [DOI] [PubMed] [Google Scholar]
- 27.Monrad RN, Grunnet LG, Rasmussen EL, Malis C, Vaag A, Poulsen P. Age-dependent nongenetic influences of birth weight and adult body fat on insulin sensitivity in twins. J Clin Endocrinol Metab 2009;94:2394–9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.