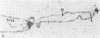Abstract
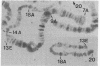
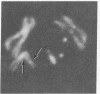
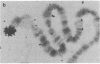
Ring chromosomes that have been opened to give linear chromosomes offer an opportunity to study the DNA sequences associated with new chromosome ends. The Drosophila melanogaster chromosome C(1)A was originally a ring chromosome, consisting of two linked X chromosomes, and thus had no telomeres. This chromosome has spontaneously opened in polytene region 13, a region near the middle of the euchromatic arm of the X chromosome. The opening of the ring has produced two new telomeres on the C(1)A chromosome. Each of the new telomeres has acquired He-T DNA sequences. He-T DNA is a complex family of repeated sequences found in the telomeric and pericentric heterochromatin of D. melanogaster chromosomes. He-T DNA sequences are detected, at various levels, in the most distal band on the end of each polytene chromosome in all D. melanogaster stocks. To our knowledge, these sequences have never been detected within the euchromatic chromosomal regions in any stock. The strong correlation between He-T DNA sequences and telomeric regions suggests that He-T sequences may have a role in organizing or maintaining the ends of chromosomes. The association of He-T DNA with newly acquired telomeres in a formerly euchromatic region, polytene region 13, strengthens this correlation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackburn E. H. Telomeres: do the ends justify the means? Cell. 1984 May;37(1):7–8. doi: 10.1016/0092-8674(84)90295-2. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. The molecular structure of centromeres and telomeres. Annu Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- Chan C. S., Tye B. K. Organization of DNA sequences and replication origins at yeast telomeres. Cell. 1983 Jun;33(2):563–573. doi: 10.1016/0092-8674(83)90437-3. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985 Dec;43(2 Pt 1):405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Jones J. D., Flavell R. B. Chromosomal structure and arrangement of repeated DNA sequences in the telomeric heterochromatin of Secale cereale and its relatives. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1209–1213. doi: 10.1101/sqb.1983.047.01.136. [DOI] [PubMed] [Google Scholar]
- Morin G. B., Cech T. R. Mitochondrial telomeres: surprising diversity of repeated telomeric DNA sequences among six species of Tetrahymena. Cell. 1988 Feb 12;52(3):367–374. doi: 10.1016/s0092-8674(88)80029-1. [DOI] [PubMed] [Google Scholar]
- Moyzis R. K., Buckingham J. M., Cram L. S., Dani M., Deaven L. L., Jones M. D., Meyne J., Ratliff R. L., Wu J. R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue M. L., Brown D. D., Birnstiel M. L. Location of the genes for 5S ribosomal RNA in Xenopus laevis. Chromosoma. 1973;42(2):191–203. doi: 10.1007/BF00320940. [DOI] [PubMed] [Google Scholar]
- Pardue M. L., Dawid I. B. Chromosomal locations of two DNA segments that flank ribosomal insertion-like sequences in Drosophila: flanking sequences are mobile elements. Chromosoma. 1981;83(1):29–43. doi: 10.1007/BF00286014. [DOI] [PubMed] [Google Scholar]
- Renkawitz-Pohl R., Bialojan S. A DNA sequence of Drosophila melanogaster with a differential telomeric distribution. Chromosoma. 1984;89(3):206–211. doi: 10.1007/BF00295001. [DOI] [PubMed] [Google Scholar]
- Richards E. J., Ausubel F. M. Isolation of a higher eukaryotic telomere from Arabidopsis thaliana. Cell. 1988 Apr 8;53(1):127–136. doi: 10.1016/0092-8674(88)90494-1. [DOI] [PubMed] [Google Scholar]
- Rubin G. M. Isolation of a telomeric DNA sequence from Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1041–1046. doi: 10.1101/sqb.1978.042.01.104. [DOI] [PubMed] [Google Scholar]
- Shampay J., Szostak J. W., Blackburn E. H. DNA sequences of telomeres maintained in yeast. Nature. 1984 Jul 12;310(5973):154–157. doi: 10.1038/310154a0. [DOI] [PubMed] [Google Scholar]
- Syvanen M. The evolutionary implications of mobile genetic elements. Annu Rev Genet. 1984;18:271–293. doi: 10.1146/annurev.ge.18.120184.001415. [DOI] [PubMed] [Google Scholar]
- Young B. S., Pession A., Traverse K. L., French C., Pardue M. L. Telomere regions in Drosophila share complex DNA sequences with pericentric heterochromatin. Cell. 1983 Aug;34(1):85–94. doi: 10.1016/0092-8674(83)90138-1. [DOI] [PubMed] [Google Scholar]