Abstract
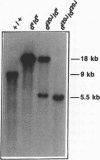
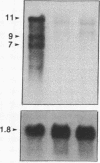
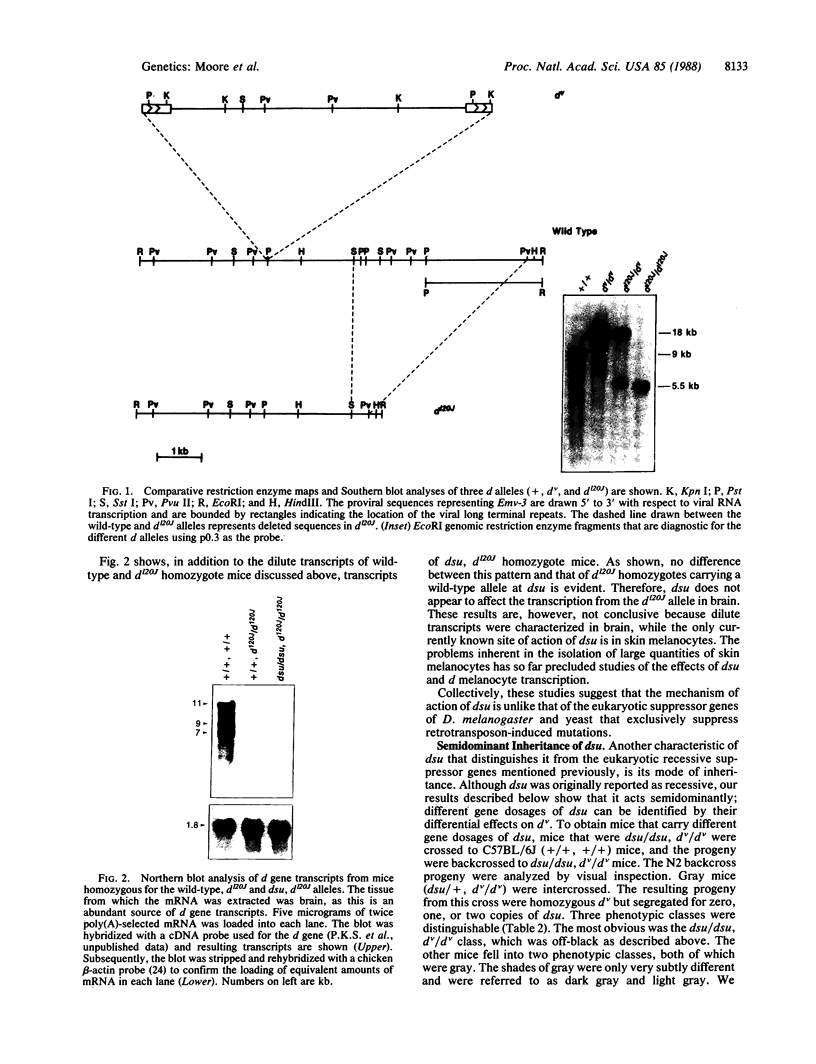
The murine dilute suppressor (dsu) gene is the only unlinked trans-acting suppressor identified in mammals. dsu, which was originally reported to be recessive, was recognized by its ability to suppress the coat color phenotype of a retroviral insertion mutation, dv, of the murine dilute (d) locus. This insertion mutation resulted from the integration of an ecotropic murine leukemia virus into noncoding sequences of the dilute gene. Therefore, dsu may act like other allele-specific recessive suppressors identified in Drosophila melanogaster and yeast that suppress mutations induced by retrotransposon insertions. To investigate this possibility, we have examined whether dsu could suppress a spontaneously arising allele of d, dl20J, which is shown here to result from a 3.5-kilobase deletion. These studies indicate that dsu does not function like other eukaryotic suppressor genes that suppress retrotransposon-induced mutations. We also show that dsu is not, as originally reported, a recessive gene but is semidominantly inherited. Collectively, these results allow us to propose a mechanism for the suppressor activity of dsu.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Copeland N. G., Hutchison K. W., Jenkins N. A. Excision of the DBA ecotropic provirus in dilute coat-color revertants of mice occurs by homologous recombination involving the viral LTRs. Cell. 1983 Jun;33(2):379–387. doi: 10.1016/0092-8674(83)90419-1. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Engstrom J., Wong A., Maurer R. Interaction of DNA polymerase III gamma and beta subunits in vivo in Salmonella typhimurium. Genetics. 1986 Jul;113(3):499–515. doi: 10.1093/genetics/113.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HU F., LESNEY P. F. THE ISOLATION AND CYTOLOGY OF TWO PIGMENT CELL STRAINS FROM B-16 MOUSE MELANOMAS. Cancer Res. 1964 Oct;24:1634–1643. [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Dilute (d) coat colour mutation of DBA/2J mice is associated with the site of integration of an ecotropic MuLV genome. Nature. 1981 Oct 1;293(5831):370–374. doi: 10.1038/293370a0. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982 Jul;43(1):26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Pfeifer-Ohlsson S., Kato M., Larsson E., Rydnert J., Ohlsson R., Cohen M. Tissue-specific expression of human provirus ERV3 mRNA in human placenta: two of the three ERV3 mRNAs contain human cellular sequences. J Virol. 1987 Jul;61(7):2182–2191. doi: 10.1128/jvi.61.7.2182-2191.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost T. A., Theodorakis N., Hughes S. H. The nucleotide sequence of the chick cytoplasmic beta-actin gene. Nucleic Acids Res. 1983 Dec 10;11(23):8287–8301. doi: 10.1093/nar/11.23.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert C L, Silvers W K. The Effects of Genotype and Cell Environment on Melanoblast Differentiation in the House Mouse. Genetics. 1956 May;41(3):429–450. doi: 10.1093/genetics/41.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modolell J., Bender W., Meselson M. Drosophila melanogaster mutations suppressible by the suppressor of Hairy-wing are insertions of a 7.3-kilobase mobile element. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1678–1682. doi: 10.1073/pnas.80.6.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. J., Swing D. A., Rinchik E. M., Mucenski M. L., Buchberg A. M., Copeland N. G., Jenkins N. A. The murine dilute suppressor gene dsu suppresses the coat-color phenotype of three pigment mutations that alter melanocyte morphology, d, ash and ln. Genetics. 1988 Aug;119(4):933–941. doi: 10.1093/genetics/119.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst S. M., Corces V. G. Forked, gypsys, and suppressors in Drosophila. Cell. 1985 Jun;41(2):429–437. doi: 10.1016/s0092-8674(85)80016-7. [DOI] [PubMed] [Google Scholar]
- Parkhurst S. M., Corces V. G. Interactions among the gypsy transposable element and the yellow and the suppressor of hairy-wing loci in Drosophila melanogaster. Mol Cell Biol. 1986 Jan;6(1):47–53. doi: 10.1128/mcb.6.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinchik E. M., Russell L. B., Copeland N. G., Jenkins N. A. Molecular genetic analysis of the dilute-short ear (d-se) region of the mouse. Genetics. 1986 Feb;112(2):321–342. doi: 10.1093/genetics/112.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E S. A Quantitative Histological Study of the Pigment Found in the Coat Color Mutants of the House Mouse. II. Estimates of the Total Volume of Pigment. Genetics. 1948 May;33(3):228–236. doi: 10.1093/genetics/33.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. B. Definition of functional units in a small chromosomal segment of the mouse and its use in interpreting the nature of radiation-induced mutations. Mutat Res. 1971 Jan;11(1):107–123. doi: 10.1016/0027-5107(71)90036-4. [DOI] [PubMed] [Google Scholar]
- Siracusa L. D., Russell L. B., Jenkins N. A., Copeland N. G. Allelic variation within the Emv-15 locus defines genomic sequences closely linked to the agouti locus on mouse chromosome 2. Genetics. 1987 Sep;117(1):85–92. doi: 10.1093/genetics/117.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet H. O. Dilute suppressor, a new suppressor gene in the house mouse. J Hered. 1983 Jul-Aug;74(4):305–306. doi: 10.1093/oxfordjournals.jhered.a109794. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil C. F., Oakley C. E., Oakley B. R. Isolation of mip (microtubule-interacting protein) mutations of Aspergillus nidulans. Mol Cell Biol. 1986 Aug;6(8):2963–2968. doi: 10.1128/mcb.6.8.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachar Z., Davison D., Garza D., Bingham P. M. A detailed developmental and structural study of the transcriptional effects of insertion of the Copia transposon into the white locus of Drosophila melanogaster. Genetics. 1985 Nov;111(3):495–515. doi: 10.1093/genetics/111.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]