Abstract
Death-associated protein kinases (DAPK) are serine/threonine protein kinases that have an important role in regulating cell death. In this study two antisense approaches were employed to down-regulate expression of the endogenous DAPK-α and DAPK-β proteins. Transient expression of an antisense DAPK cDNA or antisense morpholino oligonucleotides in HeLa, 3T3, or primary human vascular smooth muscle cells demonstrate that decreased DAPK expression promotes a spontaneous, caspase-mediated apoptosis as evidenced by increased activities of caspases-3 and -9. Clonal HeLa cell lines with attenuated levels of DAPK expression, obtained following selection in the presence of antisense DAPK cDNA, are more sensitive to tumor necrosis factor-induced caspase-mediated apoptosis, and their sensitivity is inversely related to DAPK expression. In contrast, HeLa cells with reduced DAPK expression are moderately resistant to cell death induced by interfer-on-γ. This finding is consistent with previous studies showing that DAPK has a role in promoting caspase-independent cell death. Together, these studies demonstrate that the cellular activities of DAPK are critical for antagonizing caspase-dependent apoptosis to promote cell survival under normal cell growth conditions.
Death-associated protein kinase (DAPK)1 is a multi-domain Ser/Thr protein kinase that is regulated by calcium and cal-modulin and autophosphorylation (1–3). One human (4) and two mouse DAPK forms DAPK-α and DAPK-β have been described (1). DAPK-β is an alternatively spliced isoform of DAPK-α, with a carboxyl-terminal 12-amino acid extension. The human DAPK, which is the counterpart to mouse DAPK-α, was originally cloned using a technical knockout approach that involved expression of an antisense cDNA library in HeLa cells treated for 28 days with interferon-γ (IFN-γ) (4). This selection was used to identify novel pro-apoptotic factors and one of the antisense cDNA fragments encoded DAPK. These results and others have lead to a paradigm in which DAPK plays a central role as a pro-apoptotic factor (2–7).
In contrast to the studies supporting the role of DAPK is a pro-apoptotic factor, the results of other studies challenge this paradigm and raise the possibility that DAPK can also act as an anti-apoptotic factor, depending on the apoptotic stimuli. For example, the original antisense DAPK cDNA fragment that protected cells from IFN-γ-induced cell death has not been found to be effective in protecting cells from TNF-induced apoptosis (4, 8). Similarly we have shown previously (1) that over-expression of DAPK-α has a weak survival effect whereas DAPK-β confers a strong protective effect to rescue Madin-Darby canine kidney and HeLa cells from TNF-induced apoptosis (1). DAPK is also found to be highly expressed in an active form in many non-apoptotic tissues such as brain cortex and hippocampus (9–11), and DAPK has been suggested to have other functional roles such as regulating exocytosis of neurotransmitter release by phosphorylation of syntaxin-1 (12) and protecting neurons during development or recovery from hypoxic-ischemic injury (11). In addition, DAPK expression is induced in a rat seizure model in regions of the brain that were not undergoing apoptosis (13).
Many of the studies supporting a pro-apoptotic paradigm for DAPK rely on the use of over-expression of wild type or mutant forms of DAPK (2–4, 7, 8, 14–17), but there are also examples where under normal growth conditions the over-expression of DAPK does not induce a caspase-dependent apoptosis (1, 6). Previously, in our own studies we utilized either a transient or an inducible promoter system in which the expression of DAPK could be turned on or off and fine-tuned, but concerns about the effects of over-expression, as well as the potential for compensatory cellular alterations to occur during extended culture for selection of cell lines, prompted the current study where two antisense gene-silencing approaches were used to attenuate expression of the endogenous DAPK. In this study, we utilized both antisense cDNA transfection and morpholino oligonucleotides (M-oligonucleotides) to deplete expression of DAPK in a variety of cells, including primary smooth muscle cells. These studies show that depletion of DAPK expression promotes a caspase-dependent apoptosis in the absence of any overt apoptotic stimuli and thus suggests that the activities of DAPK are necessary for cell survival.
MATERIALS AND METHODS
Cell Culture, Antibodies, and Reagents
HeLa cells and NIH3T3 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% (v/v) fetal bovine serum, 2 mM glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. Primary human aortic smooth muscle cells (HASMC) were purchased from Cascade Biologics (Portland, OR). These cells were cultured in medium 231 supplemented with smooth muscle growth supplement (Cascade Biologics) and differentiated by culturing in medium 231 supplemented with smooth muscle differentiation supplement (Cascade Biologics) for 3 days. The HASMC were used no later than passage five and maintained in differentiated state for 72 h before each experiment, to ensure the differentiated phenotype. Smooth muscle α-actin was detected using a monoclonal antibody (clone 1A4; Sigma) and served as a marker of smooth muscle cell differentiation.
Total DAPK (DAPK-α and DAPK-β) was detected with a monoclonal (clone 17; BD Biosciences) antibody against human DAPK. Another antibody to specifically detect DAPK-β was produced by immunization of rabbits with a keyhole limpet hemocyanin-coupled synthetic peptide comprised of the sequence unique to the carboxyl terminus of DAPK-β (CRDSHAWTPLTDL). This antibody was shown by Western blotting and immunoprecipitation (not shown) to be specific for the β form and does not react with the α form of DAPK. Poly(ADP)ribose polymerase (PARP) antibody that recognizes both the full-length (116 kDa) and the caspase-cleaved fragment (89 kDa) was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Human and mouse recombinant TNF were purchased from Calbiochem.
Transfection and Generation of Stable Cell Lines
HeLa or 3T3 cells were transfected with a pcDNA3 plasmid (Invitrogen) encoding either mouse DAPK-α cDNA (GenBank™ accession number gi:29825682) or inverted, antisense orientation (bp 4910 to bp 1) mouse DAPK-α cDNA, using FuGENE 6 transfection reagents (Roche Applied Science) according to the manufacture’s protocol. The mRNA transcribed from the inverted DAPK-α is complementary to the endogenous DAPK mRNA, thus blocking translation of both DAPK-α and DAPK-β. Where indicated, pEGFP plasmid (Clontech) was co-transfected at a ratio of 1:5 to serve as a visual selection for transfected cells, which were identified by the expression of green fluorescent protein (GFP). Stable cell lines were cloned by seeding transfected cells at low density in medium containing G418 at concentrations of 500 μg/ml. Individual colonies were amplified and tested for DAPK expression by Western blotting.
Antisense DAPK Oligonucleotides
DAPK-α/β antisense M-oligonucleotides (GeneTools LLC, Philomath, OR) (18, 19) were designed based upon the published mouse (5′-CCACGTTTTCCTGCCTGAACACAGT-3′) and human (5′-CGTTTTCCTGAACACGGTCAT-3′) DAPK cDNA sequences and correspond to the region near the 5′ translational start site of the DAPK open reading frame for each species. A random, scrambled M-oligonucleotide was used as control. For experiments, cells were seeded in 6-well dishes and 24 h later were treated with or without the DAPK-α/β antisense M-oligonucleotides. The M-oligonucleotides were mixed with ethoxylated polyethylenimine (Gene Tools) and delivered into cells according to the manufacture’s protocol, which resulted in >98% transfection efficiency as determined by including control fluorescinated oligonucleotides. Dose response experiments were performed by varying the amount of DAPK and control M-oligonucleotides to achieve a final concentration of 2 μM. DAPK expression levels were evaluated by Western blotting to determine the optimal M-oligonucleotide concentration necessary to achieve maximum gene silencing at 24 h after transfection by collecting floating and adherent cells and preparing cell lysates for Western blotting analysis.
Analysis of Apoptosis
Quantification of apoptotic cell death was performed as described previously (1). Briefly, at the indicated times, viable, attached cells were identified using trypan blue exclusion and counted. The extent of apoptosis is defined as (the number of control cells – the number of treated cells)/(the number of control cells). Apoptosis was also quantified by fluorescence-activated cell sorting (FACS) analysis of DNA fragmentation in cells fixed with 95% ethanol and 5% acetic acid at −20 °C and stained with 50 μg/ml propidium iodide (Sigma) on a FACStar plus flow cytometer (BD Biosciences).
Caspase Activity Assays
For measurement of caspase activity in vivo, both adherent and floating cells were collected and lysed with CHAPS lysis buffer (0.1% CHAPS, 100 mM NaCl, 100 μM EDTA, 10 mM dithiothreitol, and 50 mM HEPES, pH 7.4). After centrifugation, equal amounts of total cellular proteins were incubated at 37 °C, and the assay was initiated by addition of 200 μM Ac-IETD-pNA (caspase-8), 200 μM Ac-DEVD-pNA (caspase-3), or 200 μM Ac-LEHD-pNA (caspase-9). Change in absorbance at 405 nm versus time was monitored by spectrophotometry, and caspase activities (pmol/min/mg total protein) were calculated after subtraction of background, using pure p-nitroanilline for calibration of a standard curve. For every cell sample, the background was determined by adding the caspase-specific inhibitors, Ac-IETD-CHO (caspase-8), Ac-DEVD-CHO (caspase-3), or Ac-LEHD-CHO (caspase-9) as negative control.
Western Blotting and Immunofluorescence
Western blotting and indirect immunofluorescence were performed as described previously (20). Where indicated, vinculin was detected and used as control for loading. All densitometry analysis of Western blotting data was normalized to vinculin levels.
Data Analysis
All experiments were performed at least three times. All statistic analysis and graphs were created using GraphPad Prism software (GraphPad Software, San Diego, CA). Western blotting and immunofluorescence images are representative of the repeated experiments, and all panels have been treated identically. Significance (*) as determined by Student’s t test (GraphPad Prism Software) was accepted at p ≤ 0.01.
RESULTS
Antisense DAPK cDNA Reduces Expression of Endogenous DAPK
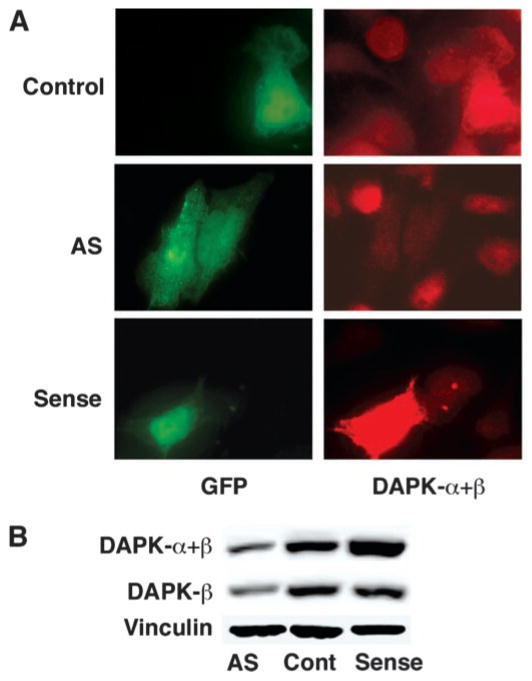
To examine the function of endogenous DAPK, expression vectors with DAPK-α either in the sense (DAPK) or anti-sense (AS-DAPK-α) orientation were generated and transfected into HeLa or 3T3 cells, together with the pEGFP plasmid encoding GFP, to identify transfected cells. Control cells were also transfected with a vector encoding β-galactosidase (LacZ). The RNA transcribed from the AS-DAPK-α plasmid was expected to block the translation of the total endogenous pool of DAPK, including both DAPK-α and DAPK-β. DAPK-β represents an alternative splice variant of DAPK-α with an additional 36-bp exon, which extends the extreme carboxyl terminus of the protein by 12 residues but is otherwise identical to DAPK-α (1). Fig. 1A shows representative immunofluorescence micrographs of HeLa cells transfected with plasmids encoding LacZ (Control), sense DAPK, or AS-DAPK. The monoclonal anti-DAPK antibody used to detect the expression of DAPK recognizes both the recombinant DAPK-α and the endogenous DAPK, which includes both DAPK-α and DAPK-β. GFP-positive cells transfected with AS-DAPK-α plasmid have a visible reduction in the intensity of DAPK fluorescence confirming that the AS-DAPK-α plasmid was able to decrease the endogenous DAPK level, whereas the sense DAPK plasmid increased the overall level of expression of DAPK. Similar results were seen in 3T3 cells (not shown). In another experiment, HeLa cells were transfected with vectors for expression of sense DAPK, AS-DAPK-α, or a LacZ expression vector. At 24 h following transfection, the cells were treated with G418 for 1 week to enrich for transfected cells. Western blotting of whole cell extracts was performed to detect the expression levels of total DAPK (DAPK-α+β) or DAPK-β prepared from this enriched cell population, and a representative blot is shown in Fig. 1B. In HeLa cells transfected with AS-DAPK-α plasmid, both total DAPK and DAPK-β expression levels were reduced, suggesting that as expected this AS-DAPK-α plasmid can deplete expression of both forms of DAPK. In cells transfected with sense DAPK-α plasmid, the total DAPK level increased, reflecting over-expression of DAPK-α, and the expression of DAPK-β was not affected. Although there is no antibody that is specific for DAPK-α, our previous studies using RNase protection analysis have determined that both 3T3 and HeLa cells have similar mRNA levels of each isoform (1). Based on these results it is reasonable to conclude that the antisense RNA produced from the AS-DAPK-α plasmid is equally effective for blocking the expression of both DAPK-α and DAPK-β.
Fig. 1. Reduction of endogenous DAPK by antisense DAPK cDNA.
A, fluorescent micrographs showing representative HeLa cells co-transfected with GFP (green) and sense DAPK-α or antisense (AS) DAPK-α cDNA plasmid, at ratios of 1:5. Control cells were co-transfected with GFP and LacZ. A monoclonal antibody that recognizes both α and β forms of DAPK was used to examine the levels of expression of DAPK (red). B, Western blotting to examine total DAPK (DAPK-α+β) or DAPK-β expression levels in HeLa cells transfected with sense DAPK-α or antisense (AS) DAPK-α plasmid and selected with G418 for 7 days. A monoclonal anti-DAPK antibody was used to detect the total expression level of DAPK (upper panel), and a polyclonal anti-DAPK-β was used to specifically detect DAPK-β (middle panel). The same blot was reacted with an anti-vinculin antibody to control (Cont) for sample loading (lower panel).
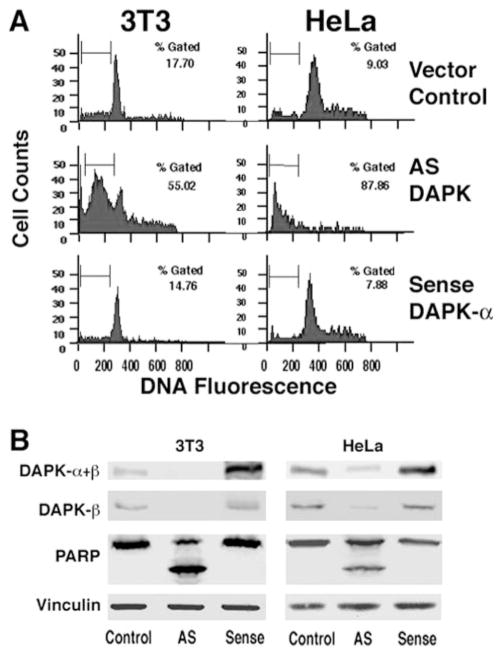
Because our previous data suggested that DAPK has a cyto-protective role, we asked whether the expression of this kinase is essential for cell survival. If this is true, then depletion of DAPK below a critical level might spontaneously induce apoptosis. This would, however, make the selection of cells with very low levels of DAPK impossible unless compensatory events had occurred. To circumvent this possibility, FACS was used to enrich for cells transiently expressing AS-DAPK-α cDNA, while simultaneously analyzing DNA fragmentation as a measure of caspase-dependent apoptosis. For this experiment, 3T3 and HeLa cells were co-transfected with pEGFP and either sense or antisense DAPK-α plasmid, at a ratio of 1:5. A vector for expression of LacZ was used in the control transfections. At 16 h following transfection all the floating and attached cells were collected, and the GFP-positive cells were analyzed to determine the levels of DNA fragmentation using propidium iodide staining of DNA. Fig. 2A shows DNA content histograms of GFP-positive cells. Cells with sub-G1 DNA content (indicated by the gated region) representing apoptotic cells with fragmented DNA were quantified. This experiment shows that in the absence of apoptotic stimulus, GFP-positive cells transfected with AS-DAPK-α have dramatically increased levels of apoptosis (55% for HeLa and 88% for 3T3), compared with control cells (18% for HeLa and 9% for 3T3). Consistent with our previous results, expression of DAPK from the sense plasmid does not induce apoptosis in transfected cells.
Fig. 2. Antisense depletion of DAPK induces spontaneous apoptosis.
A, flow cytometry analysis of DNA content (propidium iodide staining) in antisense DAPK transfected cells. HeLa or 3T3 cells were co-transfected with GFP and sense DAPK-α or antisense (AS) DAPK-α plasmid at ratios of 1:5. Control cells were co-transfected with GFP and LacZ. Only GFP-positive cells were selected and analyzed. Percentages of cells gated with sub-G1 DNA were quantified. B, Western blot of total DAPK (DAPK-α+β) or DAPK-β, PARP, and vinculin (loading control) in transfected 3T3 or HeLa cells as described for A. GFP-positive cells were enriched by FACS of transfected cells. Data shown are representative of four independent experiments.
Total protein extracts from the GFP-positive cells were prepared by collecting and lysing the sorted cells directly into SDS lysis buffer. Lysates were separated by SDS-PAGE and analyzed by Western blotting to determine the expression levels of DAPK (Fig. 2B). Antisense DAPK-α transfected 3T3 cells have undetectable levels of DAPK, either total DAPK (DAPK-α+β) or DAPK-β. Activation of effector caspases such as caspase-3 was determined by examining the levels of full-length PARP and the caspase-cleaved form having a mass of 89 kDa. As shown in Fig. 2B, antisense depletion of DAPK resulted in increased levels of PARP cleavage suggesting that caspases (e.g. caspase-3) that cleave PARP have been activated in both 3T3 and HeLa cells. In contrast for both cell lines, transfection with sense DAPK-α increased total DAPK-α protein but not DAPK-β levels, with no resulting, detectable cleavage of PARP. This is consistent with our previous studies, which showed that expression of DAPK-α does not initiate a caspase-dependent apoptosis in the absence of pro-apoptotic stimuli (1).
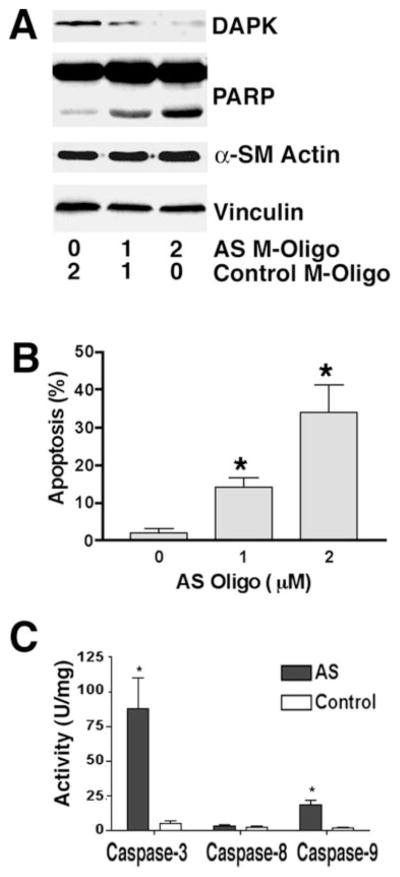
To examine the effect of reduced DAPK expression on apoptosis in primary cultured smooth muscle cells, M-oligonucleotides complementary to the mRNA sequence flanking the start AUG codon of human DAPK were synthesized and delivered into differentiated HASMC using an ethoxylated polyethylenimine transfection reagent (21). Similar to the antisense plasmids described above, the antisense M-oligonucleotides were predicted to block translation of both DAPK-α and DAPK-β because of the overlap and identity of the sequences encoding these two proteins. Mixtures of antisense DAPK and random scrambled (control) M-oligonucleotides were prepared as indicated to result in a 2 μM final concentration. At 36 h post-transfection the adherent and floating HASMC were collected and examined by Western blotting to detect expression of DAPK. A representative blot, shown in Fig. 3A, revealed that the antisense M-oligonucleotides were effective in depleting expression of DAPK in HASMC in a dose-dependent manner. Treatment of primary HASMC with a mixture containing 2 μM antisense DAPK oligonucleotides reduced the DAPK expression level to ~10% of that found in cells treated with an M-oligonucleotide mixture containing only control oligonucleotides. In addition there was an increase in non-adherent floating cells, as well as an increase in the number of adherent HASMC with apoptotic morphology that included blebbing cells, and cells with condensed cytoplasm and nuclei (not shown). The levels of spontaneous apoptosis in the cells treated with 1 or 2 μM antisense M-oligonucleotides were 14 ± 3 and 34 ± 7%, respectively, and were significantly higher than control M-oligonucleotide-treated HASMC (2 ± 1%; see Fig. 3B). Consistent with this being a caspase-dependent apoptosis, there was also a significant, dose-dependent increase in PARP cleavage (Fig. 3A). To confirm this, HASMC were treated with 2 μM antisense DAPK M-oligonucleotides. Control cells were treated with 2 μM control M-oligonucleotides. Following treatment for 24 h, both adherent and floating cells were collected, and caspase activities were measured. Antisense M-oligonucleotide-treated HASMC have significantly higher levels of caspase-3 (88 ± 22 versus 5 ± 2 units/mg) and caspase-9 (18 ± 2 versus 1.5 ± 1 units/mg) activities as compared with control cells. Caspase-8 activities are not significantly different in control cells and antisense M-oligonucleotide-transfected cells, suggesting the depletion of DAPK activates signaling events downstream of caspase-8 that lead to the activation of caspase-9 and caspase-3.
Fig. 3. Loss of DAPK expression induces apoptosis in differentiated HASMC.
A, Western blot showing the levels of total DAPK (DAPK-α+β) and PARP in HASMC treated with varying concentration of antisense DAPK M-oligonucleotides (AS M-Oligo). A total of 2 μM oligonucleotides was transfected in each experiment by addition of control M-oligonucleotides. The blot is representative of three independent experiments. B, quantification of apoptosis in HASMC treated with AS or control M-oligonucleotides. Levels of apoptosis were determined as described under “Materials and Methods.” C, analysis of caspase activity. For each experiment, at 24 h post-transfection with AS or control M-oligonucleotides (2 μM) cells were lysed, and caspase activity was determined by quantitating cleavage of IETD-pNA (Caspase-8), LEHD-pNA (Caspase-9), or DEVD-pNA (Caspase-3).
Clonal Cell Lines Expressing Antisense DAPK cDNA
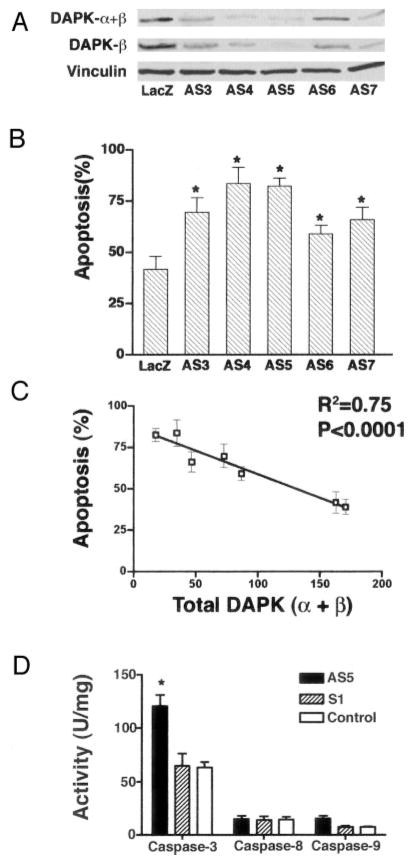
Mouse 3T3 cells transfected with control LacZ or a sense or antisense plasmid encoding DAPK-α were subjected to G418 selection for 7 days. Isolated G418-positive clones were amplified and examined for DAPK expression. Fig. 4A shows Western blots of total DAPK (DAPK-α+β) and DAPK-β in five independent 3T3 clones, which have variable expression levels of DAPK. The five clonal cell lines (AS3 through AS7) expressing an antisense DAPK-α cDNA all have reduced levels of total DAPK expression ranging from 11–69% of the levels in the parental LacZ-positive control (Cont) clonal cell line. As expected for this antisense cDNA, the decrease in total DAPK was paralleled by an equivalent decrease in the expression level of DAPK-β. To assess the effect of reduced endogenous DAPK on apoptosis, these clonal cell lines were treated with TNF (10 ng/ml) and cycloheximide (10 μg/ml) to induce apoptosis. Control cells were treated with cycloheximide only. After 4 h, apoptosis was measured by cell counting as described under “Materials and Methods.” The control cell line that expresses LacZ has 41% apoptosis, a level that is comparable with apoptosis measured in parental 3T3 cells (Fig. 4B). Cells with reduced expression of DAPK, because of expression of antisense DAPK-α, had a significantly increased sensitivity to TNF as evidenced by an increased level of apoptotic cells (ranging from 59 to 84%) after 4 h of TNF treatment. A correlation between the relative levels of total DAPK or DAPK-β and the TNF apoptotic sensitivity reveals that there was an inverse relationship between DAPK expression and apoptosis (Fig. 4C). This result is consistent with our previous studies (1) and suggests that DAPK is protective against TNF-induced apoptosis.
Fig. 4. Relationship between DAPK expression level and sensitivity to TNF-induced apoptosis.
Clonal 3T3 cell lines incorporating sense DAPK-α (S1) or antisense DAPK-α (AS3 through AS7) plasmid were selected with G418 for 7 days and then expanded. β-Galactosidase was expressed in G418-resistant control cells (LacZ). A, Western blotting examining the levels of total DAPK (DAPK-α+β) or DAPK-β in these cell lines. Western blots were also reacted with an anti-vinculin antibody to control for sample loading. B, levels of apoptosis determined for cell lines expressing either sense or antisense DAPK cDNAs following treatment with TNF and cycloheximide (CHX) at 10 ng/ml and 10 μg/ml, respectively, for 4 h. Levels of apoptosis were calculated as described under “Materials and Methods” after counting the number of trypan blue-excluding viable cells in TNF/CHX-treated and CHX-only-treated control experiments. C, linear regression dot plot of levels of apoptosis (y axis) compared with total DAPK levels (x axis; determined by densitometry measurement of Western blots to detect total DAPK). Both an R2 value of 0.74 and a p value of <0.0001 are indicative of a high degree of correlation. D, analysis of caspase activity. For each experiment, the cells were treated with TNF (10 ng/ml) and CHX (10 μg/ml) for 4 h and lysed, and caspase activity was determined by quantitating cleavage of IETD-pNA (Caspase-8), LEHD-pNA (Caspase-9), or DEVD-pNA (Caspase-3).
Because depletion of DAPK in HASMC results in activation of caspases, we determined the caspase activities of caspase-3, caspase-8, and caspase-9 in DAPK-reduced 3T3 cell lines following 4 h of TNF treatment. For these measurements, all adherent and floating cells were collected, and caspase activities were measured using appropriate substrates. The results from one representative antisense DAPK cDNA expressing cell line (AS5) and a cell line expressing sense DAPK cDNA (S1), as well as the control cell line that expresses LacZ (Control), are shown in Fig. 4D. All three cell lines have very low basal caspase activities (less than 1 unit/mg; not shown) in the absence of TNF treatment. When treated with TNF, the cell line expressing antisense DAPK cDNA (AS5) has almost twice the caspase-3 activity (120 units/mg) of the control cell line expressing LacZ (63 ± 5 units/mg; Control). Caspase-3 activity in the cell line expressing sense DAPK (64 ± 11 units/mg; S1) is not significantly different from the control cell line. As with caspase-3, the cell line expressing antisense DAPK has higher caspase-9 activity (16 ± 3 units/mg) than control (7 ± 1 units/mg), whereas sense-DAPK expressing cells have the same caspase-9 activity (7 ± 1 units/mg). In contrast, all three cell lines have statistically indistinguishable caspase-8 activity (15 ± 1 units/mg), suggesting that the reduction of DAPK levels has no effect on TNF-induced caspase-8 activation.
Decreased Levels of DAPK Protects Cells from INF-γ-induced Cell Death
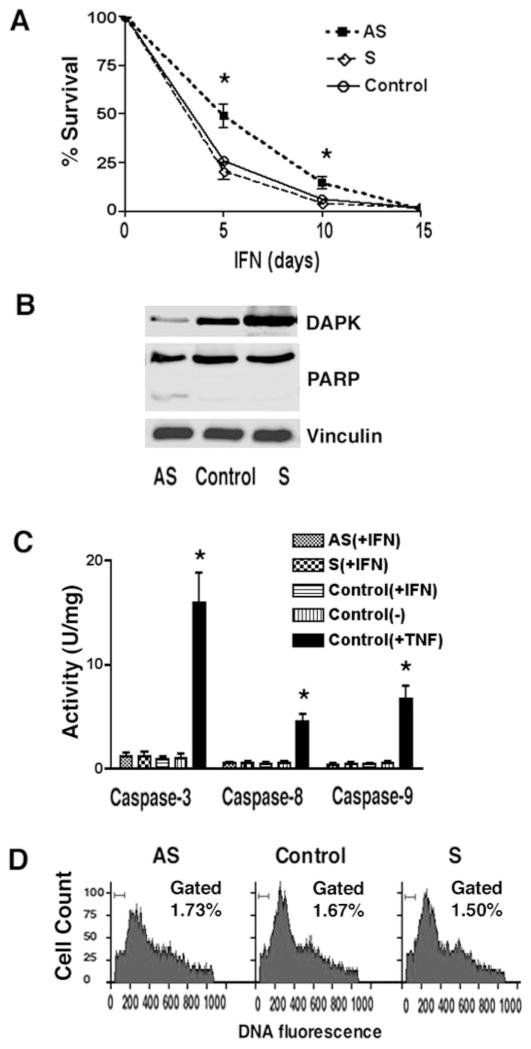
To resolve the discrepancy between our results suggesting that reduction of DAPK in stable cell lines enhances TNF-induced apoptosis and a previous study suggesting that depletion of DAPK rescued cells from IFN-γ-induced cell death, we examined the survival of HeLa cells that had been transfected either with antisense or sense DAPK cDNA or a LacZ vector (control) and treated with INF-γ. Two days after transfection, the cells were split into separate dishes and treated with either G418 only (500 μg/ml) or G418 and human IFN-γ (1000 units/ml), and the number of viable, trypan blue-excluding cells was determined following 5, 10, or 15 days of selection. These time points were used, because IFN-γ treatment does not kill cells rapidly but induces a slow reduction in the number of viable cells. The results reveal that HeLa cells transfected with the AS-DAPK vector have decreased cell death as compared with control cells (49 versus 26% at 5 days and 15 versus 6% at 10 days) (Fig. 5A). This suggests that reduction of DAPK can protect HeLa cells from IFN-γ-induced cell death. In addition, at 5 days both adherent and floating G418R/IFN-γ treated HeLa cells were collected and lysed, and the relative expression levels of DAPK were examined by Western blotting (Fig. 5B). As expected, cells transfected with an AS-DAPK construct have reduced (10-fold decrease) levels of endogenous DAPK compared with control cells expressing LacZ (Control), whereas cells expressing sense DAPK (S) have higher levels (3.5-fold increase) of expression of DAPK. To further examine the mechanism underlying this protective effect of decreased DAPK levels, PARP cleavage and caspase activities were measured. In control, DAPK-S, or AS transfected cells, after 5 days of IFN-γ selection, there was no significant increase in the presence of the 89-kDa PARP fragment (Fig. 5B), detected by Western blotting, nor was there any significant change in caspase-3, caspase-8, or caspase-9 activities (Fig. 5C) as compared with cells treated with vehicle only (−). Flow cytometric analysis was used to determine the level of sub-G1 fragmented DNA in HeLa cells transfected either with antisense DAPK (AS), sense DAPK (S), or control LacZ (Control) vectors that had been selected with G418 and treated with IFN-γ for 5 days. This analysis revealed that DNA fragmentation levels in these transfected cells were not increased compared with cells that were not treated with INF-γ (less than 2% in INF-γ- and vehicle-treated cells, (Fig. 5D) These results suggest that IFN-γ-induced cell death is caspase-independent and that depletion of DAPK can enhance survival in IFN-γ-treated cells, a result consistent with previous studies (4).
Fig. 5. Interferon-γ does not promote caspase-dependent apoptosis in DAPK-depleted cells.
A, analysis of survival efficiency in HeLa cells transfected with DAPK-AS, DAPK-S, or control plasmids and treated with IFN-γ. At 24 h following transfection cells were selected in the presence of G418 (500 μg/ml) and IFN-γ (1000 units/ml). Viable cells were quantified at the indicated times using trypan blue exclusion. B, Western blotting to examine DAPK expression and PARP cleavage in G418R cells following treatment with IFN-γ for 5 days. C, analysis of caspase activity. For each experiment, cells were treated with G418 and IFN-γ for 5 days and lysed, and caspase activity was determined by quantitating cleavage of IETD-pNA (Caspase-8), LEHD-pNA (Caspase-9), or DEVD-pNA (Caspase-3) as described under “Materials and Methods.” D, flow cytometry analysis of DNA content (propidium iodide staining) in transfected cells. HeLa cells were transfected with sense DAPK-α or antisense (AS) DAPK-α plasmid and selected with G418/IFN-γ for 5 days. Control cells were transfected with LacZ. Percentages of cells gated with sub-G1 DNA were quantified.
DISCUSSION
Acute Depletion of DAPK Induces Spontaneous Apoptosis
To better understand the role of DAPK in cells, two anti-sense approaches were designed based on the use of an anti-sense DAPK cDNA plasmid or antisense M-oligonucleotides. Both of these approaches were found to be able to significantly reduce the expression of DAPK in cultured cell lines and primary cells. The results of these studies show that depletion of DAPK-α/β expression in either HeLa cells or primary HASMC by AS-DAPK cDNA or antisense M-oligonucleotides resulted in a rapid, spontaneous apoptosis. Evidence that loss of DAPK expression induced caspase-dependent apoptosis included increased caspase activity, PARP cleavage, and enhanced DNA fragmentation. Based on these findings, we suggest that the role of the endogenous DAPK is to act as survival factor and that acute depletion of this cytoprotective kinase induces apoptosis in cells cultured under normal growth conditions.
Reduction of DAPK in Clonal Cell Lines Enhances Caspase-mediated TNF-induced Apoptosis
Despite the fact that decreasing DAPK expression induces spontaneous apoptosis, it is also clear that a viable cell population may be obtained following extended selection of cells transfected with AS-DAPK cDNA. Examination of these G418R/AS-DAPK HeLa cell lines revealed that each clonal cell line had stably reduced but detectable levels of DAPK expression. Treatment of these HeLa cell lines with TNF revealed that each clonal cell line had enhanced sensitivity to TNF-induced apoptosis that was inversely proportional to the relative expression level of DAPK. These results suggest that small decreases in DAPK expression levels result in increased sensitivity to TNF-induced apoptosis whereas a large decrease in DAPK expression results in spontaneous apoptosis. The increased sensitivity to TNF-induced apoptosis correlated with statistically significant increase in caspase-3 and caspase-9 but not caspase-8 activity. These data support the proposal that DAPK is an important survival factor that impacts the apoptotic pathway distal to the activation of caspase-8 in this death receptor cascade (1).
Reduced DAPK Expression Protects HeLa Cells from Caspase-independent Cell Death Induced by IFN-γ
When HeLa cells expressing AS-DAPK cDNA are challenged with IFN-γ, this population of cells is more resistant to cell death. However, in contrast to the caspase-dependent apoptosis induced by TNF or by the acute depletion of DAPK, IFN-γ induces a non-caspase-dependent cell death in HeLa cell lines as evidenced by the absence of any change in caspase activities or DNA fragmentation. This result is consistent with previous findings of other investigators that showed that cell lines with reduced levels of DAPK are more resistant to INF-γ induced cell death with morphological features consistent with autophagy (4, 22). These findings suggest that, in addition to being a critical anti-apoptotic survival factor, DAPK also promotes specific types of non-caspase-dependent cell death such as autophagy.
Overall, the studies summarized in this and our previous report (1) strongly argue for a cytoprotective role for DAPK in cellular homeostasis and provide an partial explanation for the discrepancy between our results and those of previous studies that challenge the paradigm that DAPK is a pro-apoptotic molecule. The data presented here show that although acute depletion of DAPK (including both DAPK-α and DAPK-β) to very low expression levels can induce spontaneous caspase-dependent apoptosis, in response to chronic INF-γ stimulation, DAPK can promote a caspase-independent cell death. Considering that DAPK has a complex multiple-domain structure that includes a Ser/Thr kinase domain, a Ca2+/calmodulin binding site, several ankyrin repeats, two P-loops and a death domain, and an alternatively spliced carboxyl terminus, it does not seem unreasonable to expect that DAPK has multiple functions in cells and may differentially participate in regulation of different types of cell death. Distinguishing and elucidating these seemingly contradictory roles of DAPK will be critical in understanding its role in programmed cell death and tumorigenesis.
Acknowledgments
We thank Shelley Dixon for expert technical support and Paul Herring and Andrew Jefcoat for helpful comments and suggestion.
Footnotes
The abbreviations used are: DAPK, death-associated protein kinase; GFP, green fluorescent protein; HASMC, human aortic smooth muscle cells; IFN-γ, interferon-γ; M-oligonucleotide, morpholino oligonucleotide; PARP, poly(ADP-ribose) polymerase; TNF, tumor necrosis factor; FACS, fluorescence-activated cell sorting; CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid; AS, antisense; CHX, cycloheximide.
This work was supported in part by American Heart Association Grant-in-aid 0150578N (to P. J. G.) and National Institutes of Health Grant HL54118 (to P. J. G.).
References
- 1.Jin Y, Blue EK, Dixon S, Hou L, Wysolmerski RB, Gallagher PJ. J Biol Chem. 2001;276:39667–39678. doi: 10.1074/jbc.M101886200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen O, Feinstein E, Kimchi A. EMBO J. 1997;16:998–1008. doi: 10.1093/emboj/16.5.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shohat G, Spivak-Kroizman T, Cohen O, Bialik S, Shani G, Berrisi H, Eisenstein M, Kimchi A. J Biol Chem. 2001;276:47460–47467. doi: 10.1074/jbc.M105133200. [DOI] [PubMed] [Google Scholar]
- 4.Deiss LP, Feinstein E, Berissi H, Cohen O, Kimchi A. Genes Dev. 1995;9:15–30. doi: 10.1101/gad.9.1.15. [DOI] [PubMed] [Google Scholar]
- 5.Feinstein E, Druck T, Kastury K, Berissi H, Goodart SA, Overhauser J, Kimchi A, Huebner K. Genomics. 1995;29:305–307. doi: 10.1006/geno.1995.1255. [DOI] [PubMed] [Google Scholar]
- 6.Inbal B, Cohen O, Polak-Charcon S, Kopolovic J, Vadai E, Eisenbach L, Kimchi A. Nature. 1997;390:180–184. doi: 10.1038/36599. [DOI] [PubMed] [Google Scholar]
- 7.Shohat G, Spivak-Kroizman T, Eisenstein M, Kimchi A. Eur Cytokine Netw. 2002;13:398–400. [PubMed] [Google Scholar]
- 8.Jang CW, Chen CH, Chen CC, Chen JY, Su YH, Chen RH. Nat Cell Biol. 2002;4:51–58. doi: 10.1038/ncb731. [DOI] [PubMed] [Google Scholar]
- 9.Sakagami H, Kondo H. Brain Res Mol Brain Res. 1997;52:249–256. doi: 10.1016/s0169-328x(97)00268-4. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto M, Takahashi H, Nakamura T, Hioki T, Nagayama S, Ooashi N, Sun X, Ishii T, Kudo Y, Nakajima-Iijima S, Kimchi A, Uchino S. J Neurosci Res. 1999;58:674–683. doi: 10.1002/(sici)1097-4547(19991201)58:5<674::aid-jnr8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Schumacher AM, Velentza AV, Watterson DM, Wainwright MS. Biochim Biophys Acta. 2002;1600:128–137. doi: 10.1016/s1570-9639(02)00453-3. [DOI] [PubMed] [Google Scholar]
- 12.Tian JH, Das S, Sheng ZH. J Biol Chem. 2003 doi: 10.1074/jbc.M300492200. [DOI] [PubMed] [Google Scholar]
- 13.Henshall DC, Araki T, Schindler CK, Shinoda S, Lan JQ, Simon RP. J Neurochem. 2003;86:1260–1270. doi: 10.1046/j.1471-4159.2003.01934.x. [DOI] [PubMed] [Google Scholar]
- 14.Cohen O, Inbal B, Kissil JL, Raveh T, Berissi H, Spivak-Kroizaman T, Feinstein E, Kimchi A. J Cell Biol. 1999;146:141–148. doi: 10.1083/jcb.146.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raveh T, Berissi H, Eisenstein M, Spivak T, Kimchi A. Proc Natl Acad Sci U S A. 2000;97:1572–1577. doi: 10.1073/pnas.020519497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raveh T, Droguett G, Horwitz MS, DePinho RA, Kimchi A. Nat Cell Biol. 2001;3:1–7. doi: 10.1038/35050500. [DOI] [PubMed] [Google Scholar]
- 17.Cohen O, Kimchi A. Cell Death Differ. 2001;8:6–15. doi: 10.1038/sj.cdd.4400794. [DOI] [PubMed] [Google Scholar]
- 18.Summerton J, Weller D. Antisense Nucleic Acid Drug Dev. 1997;7:187–195. doi: 10.1089/oli.1.1997.7.187. [DOI] [PubMed] [Google Scholar]
- 19.Summerton J, Stein D, Huang SB, Matthews P, Weller D, Partridge M. Antisense Nucleic Acid Drug Dev. 1997;7:63–70. doi: 10.1089/oli.1.1997.7.63. [DOI] [PubMed] [Google Scholar]
- 20.Jin Y, Atkinson SJ, Marrs JA, Gallagher PJ. J Biol Chem. 2001;30:30. doi: 10.1074/jbc.M102404200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morcos PA. Genesis. 2001;30:94–102. doi: 10.1002/gene.1039. [DOI] [PubMed] [Google Scholar]
- 22.Inbal B, Bialik S, Sabanay I, Shani G, Kimchi A. J Cell Biol. 2002;157:455–468. doi: 10.1083/jcb.200109094. [DOI] [PMC free article] [PubMed] [Google Scholar]