Abstract
To better understand the distinct functional roles of the 220- and 130-kDa forms of myosin light chain kinase (MLCK), expression and intracellular localization were determined during development and in adult mouse tissues. Northern blot, Western blot, and histochemical studies show that the 220-kDa MLCK is widely expressed during development as well as in several adult smooth muscle and nonmuscle tissues. The 130-kDa MLCK is highly expressed in all adult tissues examined and is also detectable during embryonic development. Colocalization studies examining the distribution of 130- and 220-kDa mouse MLCKs revealed that the 130-kDa MLCK colocalizes with nonmuscle myosin IIA but not with myosin IIB or F-actin. In contrast, the 220-kDa MLCK did not colocalize with either nonmuscle myosin II isoform but instead colocalizes with thick interconnected bundles of F-actin. These results suggest that in vivo, the physiological functions of the 220- and 130-kDa MLCKs are likely to be regulated by their intracellular trafficking and distribution.
Keywords: contraction, actin, myosin, myosin light chain kinase, smooth muscle, nonmuscle myosin light chain kinase, endothelium, fibroblast
Regulation of myosin motor activities is accomplished through the activation of several calcium-dependent and independent serine/threonine protein kinases that can phosphorylate myosin II regulatory light chain (RLC). The protein kinases that have been shown to phosphorylate RLC in vitro or in vivo include protein kinase C (PKC), protein kinase A (PKA), p21-activated kinase (PAK), death-associated protein kinase (DAPK), Rho-associated protein kinase (RhoK), and myosin light chain kinase (MLCK) (1, 3, 4, 11, 27, 37, 41). The best characterized of these protein kinases is the conventional calcium-calmodulin (Ca2+/CaM)-dependent MLCK, which has been shown to associate with both actin and myosin in vitro and actin in vivo (5, 14, 26, 28, 29, 32, 35, 36, 40). Cloning of MLCKs has shown that the sequences of the conventional MLCKs are highly conserved and that the masses of the proteins encoded by these cDNAs fall into two general sizes that vary slightly depending on the species. The larger or “nonmuscle” MLCKs, range in size between 206 and 220 kDa. The smaller or “smooth muscle” MLCKs range in size from 125 to 155 kDa (24). The sequence of the larger MLCKs includes the entire coding region of the smaller MLCK, and the mass difference is the result of a large (~995 residues) extension of the amino terminus (11). Although not extensively characterized, the in vitro biochemical properties of the 206- to 220-kDa MLCKs appear to be indistinguishable from those of the smaller 125- to 155-kDa MLCKs (2, 26, 31).
Despite the extensive overlap in sequence and the indistinguishable biochemical properties, recent reports have suggested that these highly related kinases may have distinct functional roles in cells (24). For example, a recent report demonstrated that the large and small MLCKs are differentially distributed during cytokinesis and that they associate with actin via a repeated (DXRXXL) motif that spans the junction of the long and short forms (31). In addition to the distinctions in intracellular trafficking, the expression pattern during development and in adult tissues of these kinases has been examined (6, 7, 30, 39). Although these studies have provided some insight as to potential distinctions due to differences in expression between these highly related enzymes, it is not clear from these studies whether the larger-mass MLCK is a developmentally regulated form, an endothelium-specific form, or a form that is widely expressed during development and in adult tissues. In addition, the intracellular localization of either form of MLCK with respect to its substrate myosin II has not been determined, and a direct comparison between MLCK forms derived from the same species has been not made. To address these issues we used immunolocalization and in situ immunohistochemistry to characterize the expression and distribution of the mouse 220- and 130-kDa MLCKs. These studies reveal that although the mouse 220- and 130-kDa MLCKs have identical biochemical properties in vitro, the intracellular localization and tissue expression pattern of these enzymes are distinct.
MATERIALS AND METHODS
Antibodies and cultured cells
To detect MLCKs, the following antibodies were used: K36, a monoclonal anti-chicken gizzard MLCK (Sigma), a polyclonal antibody against 130- to 150-kDa MLCK produced by immunization of rabbits with tissue-purified bovine tracheal MLCK (R57; Ref. 23), the previously described D119 Repeat antibody (8), and an affinity-purified polyclonal antibody specific for residues 1–170 of the 220-kDa mouse MLCK (anti-220MLCK1–170). The affinity-purified polyclonal antibodies against nonmuscle myosin heavy chain (NMHC)-IIA and -IIB have been previously described (13). The anti-Flag epitope (M2) antibody was obtained from Sigma.
Bovine aortic, lung, and brain microvessel endothelial cells were obtained from Dr. Peter DelVecchio (VEC Technologies, Rensselaer, NY). Human umbilical vein endothelial cells (HUVECs) were harvested from umbilical cords and maintained in culture as described previously (18). Bovine pulmonary artery endothelial cells (BPAECs) were isolated from bovine pulmonary arteries and purified by Percoll gradient centrifugation based on a previous procedure (19). The freshly isolated cells were either lysed without plating and then analyzed by Western blotting or plated and maintained in culture for several passages before lysis and Western blotting analysis. Primary cells and cell lines including rat embryo fibroblasts (REF-52), rat embryonic thoracic aorta smooth muscle cells (A10), and mouse cardiomyocytes (AT-2) were maintained in Dulbecco’s minimal essential medium (DMEM) supplemented with 2 mM glutamine, 10% FCS, 50 U/ml penicillin, and 50 μg/ml streptomycin.
Immunoprecipitation and activity measurements
Transfected cells expressing 220-kDa MLCK were washed with phosphate-buffered saline (PBS), and lysates were prepared in extraction buffer [25 mM Tris · HCl pH 6.8, 150 mM NaCl, 50 mM MgCl2, 1.0 mM EGTA, 1.0 mM EDTA, 1% NP-40, 0.5% Triton X-100, 0.2 mM phenylmethylsulfonyl fluoride, 100 μg/ml benzamidine, 100 μg/ml soybean trypsin inhibitor, and 10 μg/ml each of N-tosyl-L-phenylalanine chloromethyl ketone (TPCK), Nα-p-tosyl-L-lysine chloromethyl ketone (TLCK), aprotinin, leupeptin, and pepstatin]. MLCK was immunoprecipitated, and assays to determine the specific activity of the recombinant murine 220- and 130-kDa MLCKs were performed as described previously (12). Specific activity was estimated by using purified 150-kDa bovine tracheal MLCK as a standard. The MLCK proteins were detected with the anti-MLCK K36 monoclonal antibody, and densitometric scans were used to generate a standard curve and to compute the amount of the recombinant 220-kDa MLCK present in the extract. RLC phosphorylation assays were performed as described previously (18).
Northern and Western blotting
Northern (21) and Western (13) blotting were performed as described previously. Equivalent amounts of total cellular protein or immunoprecipitates were fractionated by electrophoresis through an SDS-polyacrylamide gel and transferred to nitrocellulose. Immunoreactive proteins on Western blots were visualized with the Pierce West Pico or West Dura detection systems according to the manufacturer’s directions.
Expression of MLCK in cultured cells
For transient expression, cells were plated at a density of 4 × 105 per 60-mm dish and incubated at 37°C for 24 h. Cells were transfected with 5 μg of DNA containing vector alone (pCDNA3) or vectors for the expression of 220-kDa MLCK, 220-kDa MLCK with a carboxyl-terminal Flag epitope, or 220-kDa MLCK with an amino-terminal green fluorescent protein (GFP) fusion (pCDNA-220MLCK, pCDNA-220MLCK-Fl, pEGFP-220MLCK) using Superfect (Qiagen) or FuGene (Roche) and following the manufacturer’s suggested protocol. Cells were harvested after 40–48 h, and 220-kDa MLCK was immunoprecipitated for analysis with K36, Flag (M2), R57, or 227423, all with similar results. Where indicated, cells were microinjected with cDNAs for immunolocalization studies. At 24 h after plating, cells were rinsed in PBS, flooded with DMEM without phenol red containing 20 mM HEPES-0.25% BSA, and placed into a Harvard Apparatus model PDMI-2 Micro-Incubator and maintained at 37°C. Nuclear microinjections were performed with an Eppendorf FemtoJet at an injection pressure of 0.9 psi, injection time of 0.3 s, and compensation pressure of 0.5 psi. BPAEC and REF-52 nuclei were injected with 15 ng/ml MLCK cDNAs (GFP-220-MLCK, Fl-220-MLCK, and Fl-130-MLCK) in sterile buffer (in mM: 50 HEPES, 100 KCl, 100 NaH2PO4, pH 7.3). After injection, cells were washed in PBS, refed with complete medium, and incubated at 37°C in 5% CO2 for 12 h before analysis.
One-dimensional tryptic peptide mapping and phosphoamino acid analysis
32P-phosphorylated RLCs were electrophoresed on 7.5–15% gradient SDS-polyacrylamide gels that were fixed in 50% methanol-10% acetic acid, dried at 70°C, and exposed to X-ray film. Labeled bands corresponding to the RLC were cut from the SDS-polyacrylamide gel, and tryptic peptide mapping and phosphoamino acid analysis were performed as described previously (17).
Immunolocalization of MLCK in cells and embryos
Monolayers of microinjected cell cultures were washed with PBS, fixed in 3% formaldehyde in stabilization buffer for 1 h, and permeabilized with 0.1% Triton X-100 for 10 min at room temperature. For localization of F-actin, cells were stained with rhodamine phalloidin as described previously (18). Cultures expressing Fl-130-MLCK were stained with 1.5 μg/ml of mouse anti-Flag M2 antibody (Sigma) and then Alexa 488-conjugated goat anti-mouse IgG antibody (Molecular Probes). Where indicated, cells were incubated in 0.5 μg/ml affinity rabbit NMHC-IIA or NMHC-IIB antibodies and then with Alexa 568-conjugated goat anti-rabbit IgG antibody. A Bio-Rad MRC 1024 confocal microscope was used to collect Z-series data images from both excitation channels simultaneously, and composite micrographs were constructed.
Immunohistochemistry of mouse adult tissues or embryos was as described previously (21). Serial control sections were reacted with nonimmune serum (nonimmune IgG) or appropriate marker antibodies to facilitate identification of the tissue layers. The embryo figures each represent a compilation of ~170 individual images that were assembled by eye using overlap features in Photoshop. The images reacted with immune and preimmune sera were treated identically.
RESULTS
Cloning, expression, and detection of mouse 220-kDa MLCK
A cDNA library prepared from mouse AT-2 cardiomyocytes was screened with a probe corresponding to bp 421–2963 of the previously described mouse 130-kDa MLCK (bp 3170–5712 of 220-kDa MLCK) (21). Thirty-eight positive lambda clones were obtained, purified, and sequenced to determine overlap and orientation of the fragments. Four overlapping clones were assembled to form a 6,270-bp cDNA. The largest open reading frame encoded a Ser/Thr protein kinase with a calculated molecular mass of 220 kDa. This cDNA differed from the mouse 130-kDa MLCK in that it has an additional 955 residues that extend the amino terminus (21). An alignment with the previously cloned human endothelial MLCK revealed a high degree (~85%) of identity between the mouse and human sequences and a lower identity (~67%) between the mouse and chicken sequences. Please refer to the Supplementary Material1 for this article (published online at the American Journal of Physiology-Cell Physiology web site) for the mouse 220-kDa MLCK, human endothelial MLCK, and chicken nonmuscle MLCK sequences. This alignment also revealed that the predicted open reading frame encoded by the mouse 220-kDa MLCK cDNA is extended at the amino terminus by an additional 44 residues compared with human and chicken sequences. The full-length cDNA for 220-kDa MLCK with a Flag epitope tag was expressed in cells and shown to comigrate with the endogenous 220-kDa MLCK, confirming that this cDNA encodes a full-length 220-kDa MLCK (Fig. 1A).
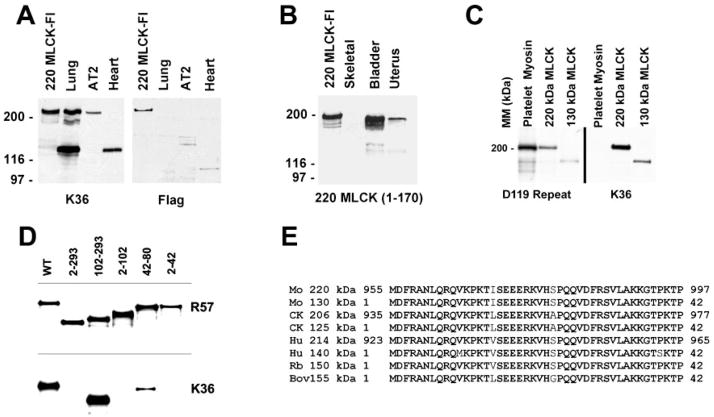
Fig. 1.
Expression and detection of 220-kDa myosin light chain kinase (MLCK). A: Western blot detecting expression of recombinant 220-kDa MLCK with the Flag epitope tag (220-kDa MLCK-Fl). Recombinant 220-kDa MLCK-Fl expressed in COS cells comigrates with 220-kDa MLCK expressed in lung and AT2 cardiomyocytes. The 220-kDa MLCK detected in COS cells represents both the recombinant 220-kDa MLCK-Fl and the endogenous 220-kDa MLCK and appears as a doublet on the original film of the Western blot reacted with the K36 monoclonal antibody. B: specificity of the 220MLCK1–170 antibody. Western blotting was used to characterize the specific reaction of an antibody to residues 1–107 of the 220-kDa MLCK with recombinant 220-kDa MLCK expressed in cells and the endogenous 220-kDa MLCK present in adult mouse bladder and uterine tissues. This antibody does not detect 130-kDa MLCK present in bladder and uterus. The lower-molecular-mass bands in some samples represent proteolytic degradation products. C: examination of the specificity of D119 and K36 antibodies for MLCK. Purified platelet myosin (NMHC-IIA) and COS cell extracts expressing either recombinant 220- or 130-kDa mouse MLCKs were analyzed by Western blotting to determine whether D119 repeat antibody and K36 antibodies specifically detect only MLCK. D119 cross-reacts with NMHC-IIA, and K36 is highly specific for MLCK. MM, molecular mass. D: mapping the epitope for monoclonal antibody K36. COS cells transiently expressing mutant 150-kDa rabbit MLCK (numbers indicate residues deleted) were examined to determine reactivity by Western blotting of the K36 monoclonal antibody. The smallest region analyzed that is undetectable by K36 is a deletion of residues 2–42. WT, wild type. E: alignment of residues corresponding to those between 2 and 42 of the 150-kDa rabbit MLCK and all cloned 210- to 220-kDa and 130- to 150-kDa MLCKs. Mo, mouse; CK, chicken; Hu, human; Rb, rabbit; Bov, bovine.
To specifically detect the 220-kDa MLCK, a polyclonal antibody was produced in rabbits against purified recombinant protein representing residues 1–170 of the 220-kDa MLCK (220MLCK1–170). Western blotting was used to confirm that the antibody reacts only with recombinant and tissue-expressed 220-kDa MLCK (Fig. 1B). In addition, no reactivity to the high level of 130-kDa MLCK present in mouse bladder and uterine smooth muscle tissues was detected (compare Fig. 1B with Fig. 2). Consistent with a previous study, the expression of 220-kDa MLCK in adult skeletal muscle is not detected (21). Two commonly used anti-MLCK antibodies were characterized to determine their ability to detect MLCKs from tissues and cell types of different species. An antibody generated against a peptide representing a repeated sequence present in all mammalian 220- and 130-kDa MLCKs (D119 Repeat; Ref. 8) recognized both 220- and 130-kDa MLCKs as expected but was also found to cross-react with purified platelet myosin IIA (Fig. 1C). To confirm this observation, REF-52 and BPAEC lysates were fractionated with a D119 antibody affinity column. The affinity-purified protein was identified as NMHC-IIA by direct amino acid sequencing (data not shown). Further support for this observation comes from an expression screen of a mouse AT-2 cDNA library in which D119 positive clones were sequenced and found to encode NMHC-IIA (data not shown). The epitope in MLCKs recognized by K36 monoclonal antibody was determined by using Western blotting of COS cell extracts in which deletion mutants of the 150-kDa rabbit MLCK that were transiently expressed. The results show that the K36 antibody binds between residues 2 and 42 of the rabbit 150-kDa MLCK (Fig. 1D), and alignment of the sequences of all cloned MLCKs revealed a high degree of identity between residues 2 and 42 (Fig. 1E). On the basis of this information, it is expected that the K36 antibody will detect both small- and large-molecular-mass MLCKs in human, chicken, mouse, or bovine tissues.
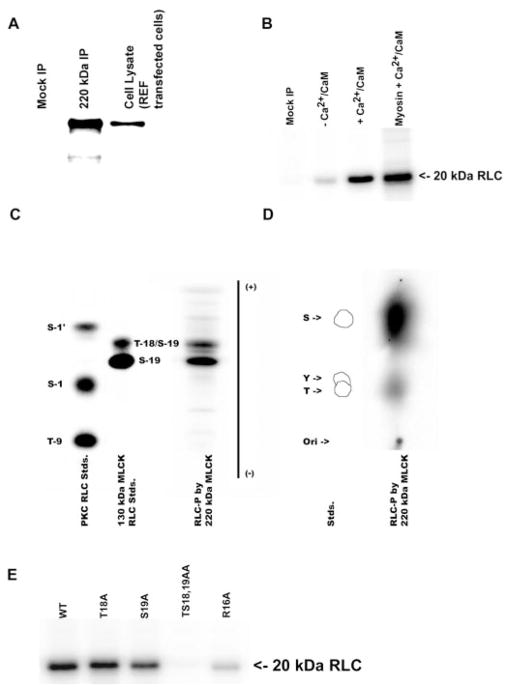
Fig. 2.
Phosphorylation of regulatory light chains (RLCs) by 220-kDa MLCK. A: immunoprecipitation and detection of the 220-kDa MLCK expressed in REF-52 cells with a plasmid encoding 220-kDa MLCK (220-kDa IP) or transfected with vector alone (Mock IP). The immunoprecipitated 220-kDa MLCK was detected by Western blotting using K36 monoclonal antibody. B: purified wild-type RLCs and platelet myosin [nonmuscle myosin heavy chain (NMHC)-IIA] were incubated with immunopurified 220-kDa MLCK in the presence (+) or absence (−) of Ca2+/calmodulin (Ca2+/CaM) for 30 min, and 32P-labeled light chains were analyzed by 12% SDS-PAGE and autoradiography. C: 1-dimensional tryptic peptide map of phosphorylation sites in RLCs after phosphorylation by immunoprecipitated 220-kDa MLCK. Control lanes are protein kinase C (PKC) phosphorylated RLC standards (Stds) and recombinant 150-kDa rabbit MLCK (MLCK) prepared as described in MATERIALS AND METHODS. S-1′, S-1, and T-9 are sites phosphorylated by PKC, and S-19 and T-18 are sites phosphorylated by MLCK. Two phosphopeptides corresponding to S-19 (monophosphorylated) and S-19/T-18 (diphosphorylated) RLCs are detected in the 220-kDa MLCK lane. D: phosphoamino acid analysis of 32P incorporation into RLCs by 220-kDa MLCK. The standards are phosphoserine (S), phosphothreonine (T), and phosphotyrosine (Y). Phosphoamino acid analysis demonstrates that both S and T residues are phosphorylated by 220-kDa MLCK. E: 220-kDa MLCK-catalyzed phosphorylation of WT and mutant recombinant RLCs. The immunoprecipitated 220-kDa MLCK phosphorylates WT and T18A, S19A, and R16A mutant RLCs and does not phosphorylate the TS1819AA mutant RLC.
Biochemical characterization of mouse 220-kDa MLCK
To confirm that the cDNA encoding the mouse 220-kDa MLCK is catalytically active as well as to compare its in vitro biochemical properties with the previously described mouse 130-kDa MLCK, the 220-kDa MLCK was immunoprecipitated and used in an in vitro kinase assay (Fig. 2A). Immunopurified 220-kDa MLCK was incubated with RLCs in the presence and absence of Ca2+/CaM for 30 min at 30°C and analyzed by SDS-PAGE, and in this assay the 220-kDa MLCK phosphorylated both isolated RLCs and RLCs associated with purified platelet myosin. Consistent with the Ca2+/CaM dependence of the 130-kDa MLCK, the 220-kDa MLCK has minimal light chain phosphorylation activity in the presence of EGTA (Fig. 2B).
One-dimensional tryptic peptide mapping and phosphoamino acid analysis were used to identify the site(s) in the RLC that are phosphorylated by 220-kDa MLCK. Figure 2C shows tryptic peptide maps of recombinant RLC phosphorylated in vitro by either PKC or 150-kDa rabbit MLCK. Tryptic peptide mapping of RLC phosphorylated by 220-kDa MLCK yielded two phosphopeptides that comigrate with the monophosphorylated S19 and diphosphorylated S19/18 standards. Phosphoamino acid analysis confirmed that both S and T residues are phosphorylated by 220-kDa MLCK, although at the low levels of 220-kDa MLCK used in the reaction, S19 predominates (Fig. 2D). RLCs with mutations in key consensus recognition elements were utilized (3) as substrates for in vitro phosphorylation by 220-kDa MLCK confirmed the specificity of phosphorylation at S19 and T18 (Fig. 2E). Mutation of T18 to A (T18A) resulted in a small effect on the level of phosphorylation, whereas mutation of S19 to A decreased phosphorylation by 12.5-fold. As expected, mutation of both S19 and T18 (TS18, 19AA) ablated RLC phosphorylation by 220-kDa MLCK and substitution of R16 with A (R16A) reduced the ability of 220-kDa MLCK to phosphorylate RLC by ~25-fold, consistent with the importance of the P-3 residue in the consensus recognition sequence.
Determination of the activity of the recombinant enzyme expressed in COS cells showed that the expressed protein was catalytically active and Ca2+/CaM dependent. The specific activity was estimated to be 21 pmol incorporated 32P · min−1 · ng enzyme−1 (data not shown). This estimated value is similar to previously reported values for the chicken gizzard (36.2 pmol · min−1 · ng−1) and recombinant rabbit smooth muscle MLCKs (39.0 pmol · min−1 · ng−1) (10, 25).
Northern and Western blotting to determine expression profile of 220- and 130-kDa mouse MLCKs
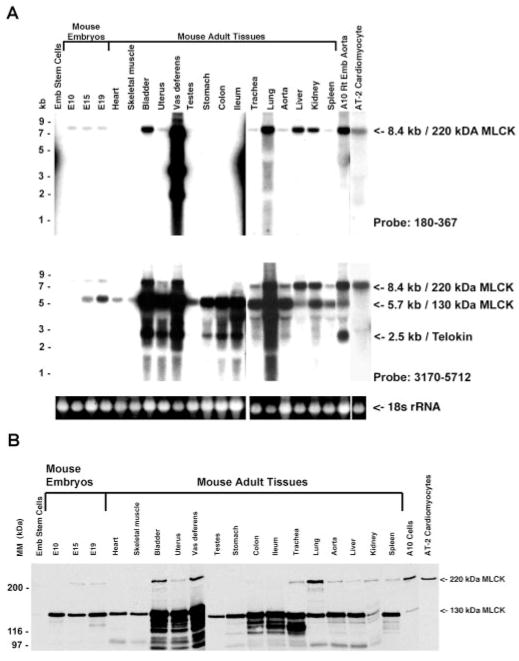
Northern blots reacted with a cRNA probe corresponding to bp 180–367 (residues 1–63) of the mouse 220-kDa MLCK cDNA hybridized to a single mRNA of ~8.4 kb in various mouse embryos, adult tissues, and cell lines (Fig. 3A). Among the group of adult tissues examined, the highest mRNA levels were detected in bladder, vas deferens, lung, liver, and kidney. With independently isolated samples of vas deferens mRNA, only an 8.4-kb mRNA is consistently found to react with the 220-kDa MLCK-specific probe, suggesting that the 4.0- and 2.4-kb mRNAs detected may be genomic DNA contamination of this sample. A second probe corresponding to bp 3170–5712 (residues 998–1865) of the mouse 220-kDa MLCK cDNA hybridized to mRNAs of 8.4 and 5.7 kb, which encode the 220- and 130-kDa MLCKs, respectively. The 5.7-kb mRNA is detectable in all tissues represented on this Northern blot, including both smooth muscle and nonmuscle tissues (Fig. 3A). In addition, this probe detected the 2.4-kb mRNA corresponding to the smooth muscle-specific telokin protein (9, 22, 34). Western blotting with the K36 monoclonal antibody detected a 220-kDa protein in many smooth muscle tissues including bladder, uterus, vas deferens, trachea, lung, and aorta (Fig. 3B).
Fig. 3.
Northern and Western blotting to detect MLCKs. A: Northern blots in which 15 μg of total RNA, isolated from the indicated mouse tissues, was separated on a 1.4% agarose gel and probed with a cRNA probe corresponding to bp 180–367 (top) or bp 3170–5712 (bottom) of the mouse 220-kDa MLCK. Top, a single, 8-kb mRNA corresponding to the 220-kDa MLCK is detected. The smaller mRNAs detected in vas deferens are not reproducible and may represent genomic contamination. Bottom, 3 messages are detected: 8.4- and 5.7-kb mRNAs corresponding to the 220- and 130-kDa MLCKs and a 2.7-kb mRNA corresponding to telokin. Shown below the Northern blots is a photograph of 18s ribosomal RNAs to indicate the relative loading of the total RNA samples used in this analysis. B: Western blot analysis of extracts from various mouse tissues and cell lines, reacted with the K36 monoclonal antibody. A 130-kDa protein corresponding to smooth muscle MLCK is detected in most tissues and cells; a 220-kDa protein corresponding to nonmuscle MLCK is detected in several smooth muscle tissues and some cell lines. The lower-molecular-mass bands in some samples represent proteolytic degradation products.
In situ immunohistochemical detection of 220-kDa MLCK in mouse embryos and adult tissues
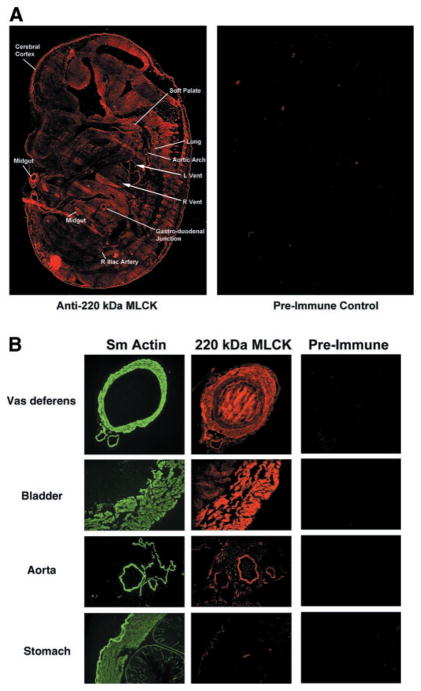
To determine whether the 220-kDa MLCK is expressed in a tissue-specific manner, the pattern of expression of this MLCK was examined during mouse development and in adult mouse tissues. In situ immunohistochemical localization performed on mouse embryos at day 14.5 revealed that the expression of the 220-kDa MLCK is detectable throughout the entire embryo, suggesting that this form of MLCK is ubiquitously expressed (Fig. 4A). However, in some areas of the developing brain and the lower gastrointestinal tract, as well as various blood vessels, there appeared to be a higher level of expression, a result consistent with the expression pattern of MLCK found during chicken embyrogenesis (7, 30). In contrast, the expression of the 220-kDa MLCK was enriched in some but not all adult tissues with smooth muscle components (Fig. 4B), although in these tissues, the expression of the 220-kDa MLCK was detectable in both the smooth muscle and non-muscle layers (Fig. 4B).
Fig. 4.
Localization of 220-kDa MLCK in mouse embryos and adult tissues. A: serial sections of mouse day 14.5 embryos were reacted with affinity-purified anti-220-kDa (1–170) antibody or preimmune control serum, followed by rhodamine-conjugated goat anti-rabbit IgG. The 220-kDa MLCK is detected in all tissues in the mouse day 14.5 embryo. The right and left ventricles of the heart (R vent, L vent) are indicated by arrows. The lines point to specific tissues that consistently had higher levels of expression of 220-kDa MLCK. B: localization of 220-kDa MLCK in adult tissues. Selected mouse adult tissues (vas deferens, bladder, aorta, and stomach) were fixed and reacted with antibodies to detect α-smooth muscle actin (fluorescence conjugated 1A4 antibody, Sm Actin), 220-kDa MLCK (1–170), or preimmune control serum followed by rhodamine-conjugated second antibody. Center panels, localization of 220-kDa MLCK with respect to the smooth muscle layers of the tissues detected in left panels with the α-smooth muscle actin antibody.
Expression of MLCK is downregulated in cultured primary cells
In culture, primary cells utilize new and unique types of contractile processes such as those required for adherence, migration, and proliferation, and thus it is reasonable to predict that if the 220- and 130-kDa MLCKs have distinct roles in regulating these functions their levels of expression may be altered when isolated primary cells are placed in culture. To examine this possibility, the expression level of both MLCK forms was examined by Western blotting with primary BPAECs. This study revealed that both 220-and 130-kDa MLCKs are highly expressed in purified primary BPAECs and that on culture the only detectable MLCK is the 220-kDa form (Fig. 5A). To determine whether the purified primary BPAECs were contaminated with smooth muscle cells, the blot was probed with anti-smooth muscle α-actin monoclonal antibody (1A4). This blot shows that smooth muscle α-actin is not detectable in extracts of these Percoll gradient purified cells. Similarly, cell extracts prepared from cultures of primary bovine endothelial cells isolated from large (umbilical vein, aorta) and small (lung, brain) vessels revealed that only the 220-kDa MLCK is detectable after passage in culture (Fig. 5B). Figure 5C shows that parallel results were obtained with primary cultures of smooth muscle cells. This Western blot shows that both 220- and 130-kDa MLCKs are detectable in extracts of bovine tracheal smooth muscle tissues and uncultured as well as cultured cells, with the 130-kDa MLCK being the predominant form. On culture, the 130-kDa MLCK exhibits a significant decrease in expression (10-fold) compared with the expression of the 220-kDa MLCK, which remained fairly constant between the initial (P0) and third (P3) passages in culture. Consistent with previous results, the decrease in expression of MLCKs also parallels the decrease in expression of smooth muscle myosin heavy chain-2 (Sm MHC-2) (8, 10).
Fig. 5.
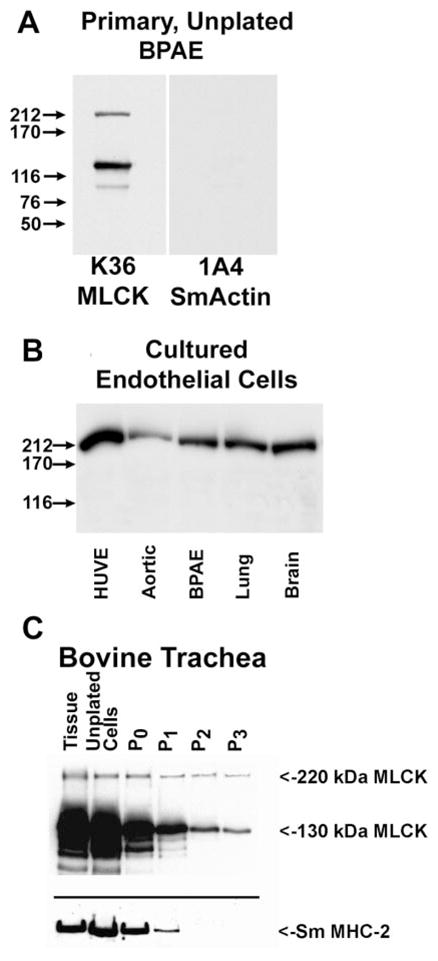
Changes in expression of MLCKs in cultured cells. Western blotting to detect MLCKs: primary uncultured bovine pulmonary artery (BPAE) cells (A), endothelial cells passed in culture (B), and bovine tracheal smooth muscle tissue and primary tracheal cells during passage in culture (C). HUVE, primary cultures of human umbilical vein endothelial cells. Aortic, BPAE, lung, and brain are all primary bovine endothelial cells that have been maintained in culture for several passages. In C, extracts of tracheal tissue (tissue), unpassaged freshly isolated tracheal smooth muscle cells, and cultured, passaged tracheal smooth muscle cells are compared. The passage (P) number for the bovine tracheal cells is indicated.
Intracellular distribution of 220- and 130-kDa MLCKs in cultured cells
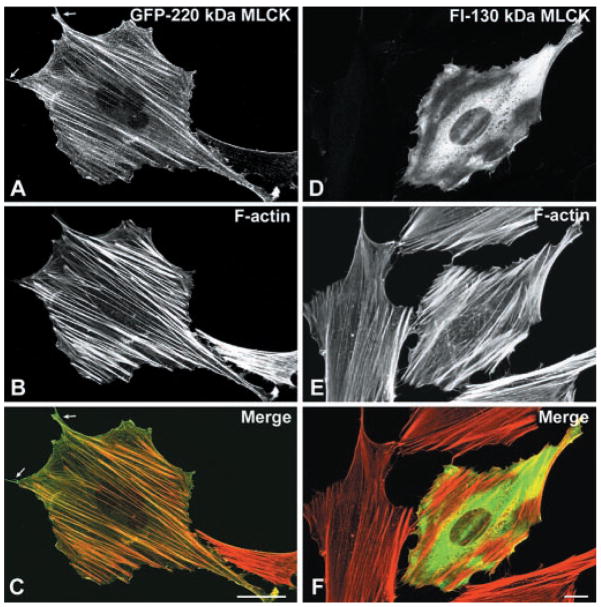
To further determine whether the 220-and 130-kDa MLCKs may have unique intracellular functions, we examined the intracellular distributions of the recombinant forms of these kinases with respect to both actin and nonmuscle myosin. For these experiments, REF-52 cells, which do not express detectable levels of either 220- or 130-kDa MLCKs as determined by either Northern (B. P. Herring, unpublished observation) or Western (8, 10) blotting, were injected with MLCK expression plasmid constructs and used for immunolocalization studies. Expressed GFP-220-kDa MLCK (Fig. 6, A–C) in REF-52 cells colocalized with thick actin bundles, and the expressed 130-kDa MLCK exhibited a diffuse localization throughout the cytoplasm with a few fine filamentous structures that do not appear to strongly colocalize with F-actin filaments (Fig. 6, D–F). Similar results were obtained using BPAECs transiently expressing these MLCKs (data not shown).
Fig. 6.
Intracellular localization of 220- and 130-kDa MLCKs in REF-52 cells. REF-52 cells transiently expressing either green fluorescent protein (GFP)-220-kDa MLCK or Fl-130-kDa MLCK were fixed, and MLCKs were detected by direct visualization of GFP or by staining cells with a mouse anti-Flag antibody and Alexa 488-conjugated goat-anti-mouse secondary antibody. Each vertical row represents the same cell expressing 220 (A–C)- or 130 (D–F)-kDa MLCK. GFP or Alexa 488 images are shown in A and D; the actin is visualized with rhodamine phalloidin in B and E. Merged images are shown in C and F (MLCK in green, C; F-actin in red, F). The 220-kDa MLCK colocalizes with thick actin stress fibers and is excluded from the peripheral tips of the filaments. The 130-kDa MLCK is concentrated in the perinuclear region and periphery of the cell and does not colocalize with F-actin.
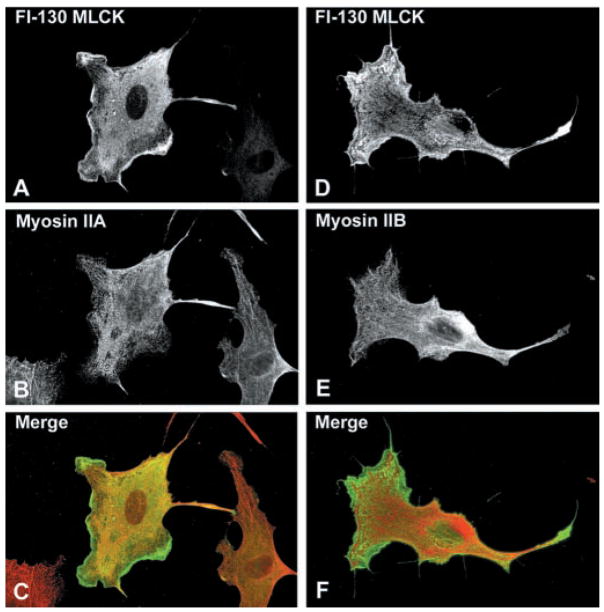
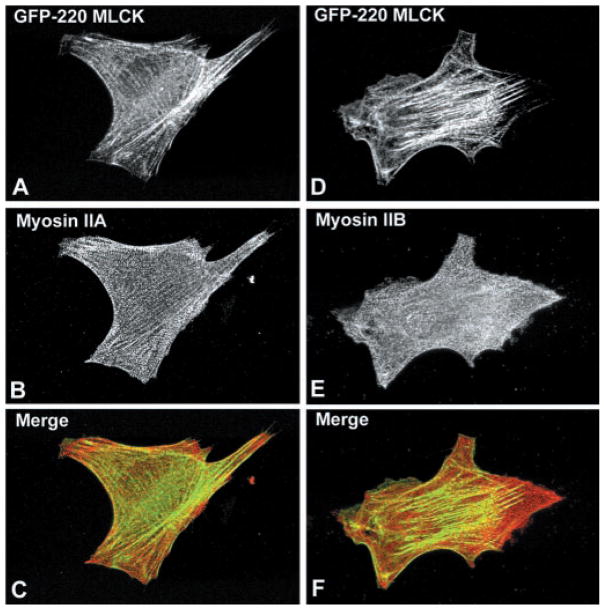
To determine whether the MLCKs colocalize with their substrate, nonmuscle myosin II, the distribution of NMHC-IIA and -IIB was examined in BPAECs expressing either Fl-130-kDa (Fig. 7) or GFP-220-kDa (Fig. 8) MLCK. In BPAECs, the filamentous structures corresponding to NMHC-IIA (Figs. 7B and 8B) are distinct from those of NMHC-IIB (Figs. 7E and 8E), with the NMHC-IIA consistently having thicker filaments and a more apparent periodic distribution. The distribution of the 130-kDa MLCK is primarily concentrated at the cell periphery and in the perinuclear region, although a small amount of the expressed protein does exhibit a filamentous pattern (Fig. 8, A and D) that overlaps with NMHC-IIA (Fig. 8, B and C), although this overlap may be coincidental because some of the 130-kDa MLCK has a diffuse cytoplasmic distribution. However, it is clear that the intracellular distribution of the 130-kDa MLCK does not overlap with NMHC-IIB filaments, which appear in a different plane in the cell (Fig. 8F). Unexpectedly, the 220-kDa MLCK does not colocalize with either NMHC-IIA or -IIB (Fig. 8, A–F).
Fig. 7.
Intracellular localization of 130-kDa MLCK in BPAE cells. BPAE cells transiently expressing Fl-130-kDa MLCK were fixed and reacted with mouse anti-Flag antibody and Alexa 488-conjugated goat-anti-mouse secondary antibody to detect MLCK or with affinity-purified anti-NMHC-IIA or -IIB antibodies and Alexa 568-conjugated goat anti-rabbit IgG as indicated. Each vertical row represents the same cell expressing 130-kDa MLCK and merged images are shown in C and F (MLCK in green; NMHC-IIA or -IIB in red; overlap is yellow-orange). The staining pattern for the 130-kDa MLCK is predominantly cytoplasmic, although fine filaments are detectable that colocalize with NMHC-IIA but do not coincide with NMHC-IIB.
Fig. 8.
Intracellular localization of 220-kDa MLCK in BPAE cells. BPAE cells transiently expressing GFP-220-kDa MLCK were fixed and incubated antibodies to detect NMHC-IIA or NMHC-IIB. Each vertical row represents the same cell expressing 220-kDa MLCK, and merged images are shown in C and F (MLCK in green; NMHC-IIA or -IIB in red). The filamentous staining pattern of the 220-kDa MLCK does not colocalize with either NMHC-IIA or NMHC-IIB.
DISCUSSION
A comparison of the deduced amino acid sequence of the mouse 220-kDa MLCK to the 214-kDa human and 210-kDa chicken MLCKs revealed a high degree of identity. In particular, there was a higher degree of sequence identity (84%) between the mouse and human cDNAs than between either the mouse and chicken (67%) or human and chicken (65%) sequences. This high degree of sequence conservation has suggested that regions amino- and carboxyl-terminal of the kinase domain and regulatory regions may have important and specific functions. Proposed functions for these regions include protein-protein interactions such as those required for intracellular localization (14, 31, 36) and sites for regulation of signal-transducing activities (2). Our studies extensively compare for the first time the properties of the 130- and 220-kDa forms of the mouse MLCKs both in vitro and in vivo.
Our in vitro biochemical studies have shown that the 220-kDa MLCK, like the 130-kDa MLCK, specifically phosphorylates only S19 and T18 in the myosin II RLC and that this activity is dependent on Ca2+/CaM. Northern and Western blotting and in situ immunohistochemistry demonstrated that the expression of 220-kDa MLCK is not restricted to a specific cell or tissue type in embryos. Similarly, in adult tissues the 220-kDa MLCK is expressed in both the nonmuscle as well as smooth muscle components of many mouse tissues, although it was not detectable in heart, skeletal muscle, testes, or tissues derived from the upper gastrointestinal tract. However, it is possible that the level of expression of the mouse 220-kDa MLCK in these tissues is below the sensitivity of detection by the techniques used in these studies. We noted some discordance between our Northern and Western blotting results, which show that in undifferentiated embryonic stem cells or day 10 (E10) embryos an 8.4-kb mRNA is present but no 220-kDa MLCK is detected by Western blotting. There also appears to be a lower level of expression of the 220-kDa MLCK in adult liver, lung, and kidney and E10 whole embryos than would be predicted from the mRNA levels. One possibility to account for this is that in these tissues the mRNAs and proteins are regulated either by posttranscriptional or posttranslational mechanisms. In contrast to the 220-kDa MLCK, expression of the 130-kDa MLCK is detected in day 10, 15, and 19 embryos as well as all adult tissues (8, 10) with only embryonic stem and AT2 cells having undetectable levels of 130-kDa MLCK. Together these results show that the 220- and the 130-kDa MLCKs, which have been descriptively referred to as nonmuscle and smooth muscle MLCKs, respectively, are not tissue-specific proteins, although higher relative levels of expression of both of these MLCKs are detected in smooth muscle tissues. This finding, together with our identification of at least one additional lower (~100 kDa)-molecular-mass MLCK derived from the MYLK gene (B. P. Herring and P. J. Gallagher, unpublished observation), leads us to suggest the use of molecular mass, rather than tissue-specific (non-muscle/smooth muscle) or long/short nomenclature, to refer to MLCKs.
The coincidental expression of the 130- and 220-kDa MLCKs during development and in adult tissues raises the question as whether or not these closely related protein kinases have distinctive functional or regulatory roles to account for their redundant expression. This prompted a closer examination of the intracellular distribution of these kinases with respect to actin and myosin filaments in cells. The overlap in protein structure between the 220- and 130-kDa MLCKs excludes the possibility of an antibody that reacts exclusively with the 130-kDa MLCK, and thus we utilized epitope-tagged versions of these kinases for these studies. These immunolocalization studies revealed that the intracellular distribution of the overexpressed recombinant 130-kDa MLCK does not overlap with F-actin filaments or with NMHC-IIB but may partially overlap with NMHC-IIA filaments that extend beyond the perinuclear region and flow toward the leading edges of the cell. In contrast, the immunofluorescence pattern of the 220-kDa MLCK showed a high degree of coincidence with thick actin cables but did not overlap with either of the two nonmuscle myosin forms (NMHC-IIA and NMHC-IIB) found in endothelial cells (BPAECs; Fig. 8) or in smooth muscle cells (A10) or fibroblasts (REF-52) (data not shown). This result is consistent with and extends those of Poperechnaya et al. (31), who showed that the 220-kDa MLCK does not appear to directly associate with either NMHC-IIA or NMHC-IIB in unstimulated interphase cells.
We also observed that REF-52 cells expressing 220-kDa MLCK have highly organized actin stress fibers compared with mock-transfected cells or cells expressing the 130-kDa MLCK, a finding consistent with the suggestion that one function of the 220-kDa MLCK is to bind to and assemble F-actin filaments into thick bundles (20, 26, 31). One explanation for why this property is specific to the 220-kDa MLCK is that the larger number of DXRXXL actin binding motifs in the 220-kDa MLCK potentially increases its binding to actin and contributes to a bundling activity as suggested previously (31). Because REF cells used in these studies lack endogenous expression of both the 220-and 130- kDa MLCKs, the absence of colocalization of the recombinant 130-kDa MLCK with actin or the partial localization with NMHC-IIA filaments in these cells cannot be the result of a competition by the endogenous MLCKs for binding sites on these filaments, although it is possible that these cells may lack a binding site or localizing protein specific for the 130-but not the 220-kDa MLCK. Together these results support the proposal that the 220- and 130-kDa MLCKs have unique intracellular distributions, which may result in distinct regulatory functions. The finding that isolated primary endothelial and smooth muscle cells extensively downregulate the expression level of the 130-kDa MLCK when placed in culture suggests that the contractile functions regulated by the 130-kDa MLCK may be less essential in isolated, adherent, proliferating cells than those regulated by the 220-kDa MLCK and is supportive of unique functional roles for these kinases.
The finding that a previously described anti-MLCK antibody (D119) also cross-reacted with purified NMHC-IIA in addition to both the smaller and larger MLCK forms calls into question studies that have used this antibody for Western blotting to identify a putative novel embryonic MLCK (8). In addition, these results also suggest that the D119 antibody should be used with caution to detect the large 220-kDa MLCK, which has a molecular mass similar to that of non-muscle myosin IIA (15, 38, 39). The basis for this cross-reaction appears to result from an overlap in the peptide sequence (KPVGNAKPAETL) used to generate this antibody and a sequence present at the extreme carboxyl terminus of NMHC-IIA (KPAE residues 1948–1951) (33).
In summary, a full-length mouse 220-kDa MLCK has been cloned, expressed in cells, and found to have in vitro biochemical properties that are indistinguishable from those of the 130- to 150-kDa MLCKs. Like the 130-kDa MLCK, the 220-kDa MLCK does not appear to be an embryonic form and is not expressed in a tissue-specific manner, although its expression is undetectable in several adult tissues including cardiac, skeletal, and gastrointestinal tract. This study also revealed that the intracellular distributions of the 210-to 220-kDa and 130- to 150-kDa MLCKs are distinct, supporting the proposal that these highly related kinases may have specific and unique functions that regulate contractile processes in cells and tissues.
Acknowledgments
This work was supported by grants from the American Heart Association (AHA; Grant-In-Aid no. 95009230 to P. J. Gallagher) and the National Heart, Lung, and Blood Institute (HL-54118 to P. J. Gallagher, HL-58571 to B. P. Herring, and HL-45788 to R. B. Wysolmerski). Z. M. Goeckeler was supported in part by the Department of Anesthesiology Research Fund, St. Louis University School of Medicine. L. Hou was supported by an AHA postdoctoral fellowship, and Y. Jin was supported by an AHA predoctoral fellowship.
Footnotes
Supplemental Material to this article is available online at http://ajpcell/physiology.org/cgi/content/full/282/3/C451/DC1.
References
- 1.Adelstein RS, Pato MD, Sellers JR, de Lanerolle P, Conti MA. Regulation of contractile proteins by reversible phosphorylation of myosin and myosin kinase. Soc Gen Physiol Ser. 1982;37:273–281. [PubMed] [Google Scholar]
- 2.Birukov KG, Csortos C, Marzilli L, Dudek S, Ma SF, Bresnick AR, Verin AD, Cotter RJ, Garcia JG. Differential regulation of alternatively spliced endothelial cell myosin light chain kinase isoforms by p60(Src) J Biol Chem. 2000;276:8567–8573. doi: 10.1074/jbc.M005270200. [DOI] [PubMed] [Google Scholar]
- 3.Chew TL, Masaracchia RA, Goeckeler ZM, Wysolmerski RB. Phosphorylation of non-muscle myosin II regulatory light chain by p21- activated kinase (gamma-PAK) J Muscle Res Cell Motil. 1998;19:839–854. doi: 10.1023/a:1005417926585. [DOI] [PubMed] [Google Scholar]
- 4.Cohen O, Feinstein E, Kimchi A. DAP-kinase is a Ca2+/calmodulin-dependent, cytoskeletal-associated protein kinase, with cell death-inducing functions that depend on its catalytic activity. EMBO J. 1997;16:998–1008. doi: 10.1093/emboj/16.5.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dabrowska R, Hinkins S, Walsh MP, Hartshorne DJ. The binding of smooth muscle myosin light chain kinase to actin. Biochem Biophys Res Commun. 1982;107:1524–1531. doi: 10.1016/s0006-291x(82)80172-1. [DOI] [PubMed] [Google Scholar]
- 6.Dalla Libera L, Collins JH. Myosin light chain kinase expression during smooth muscle development. Biosci Rep. 1993;13:289–295. doi: 10.1007/BF01137965. [DOI] [PubMed] [Google Scholar]
- 7.Fisher SA, Ikebe M. Developmental and tissue distribution of expression of nonmuscle and smooth muscle isoforms of myosin light chain kinase. Biochem Biophys Res Commun. 1995;217:696–703. doi: 10.1006/bbrc.1995.2829. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher PJ, Garcia JG, Herring BP. Expression of a novel myosin light chain kinase in embryonic tissues and cultured cells. J Biol Chem. 1995;270:29090–29095. doi: 10.1074/jbc.270.49.29090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallagher PJ, Herring BP. The carboxyl terminus of the smooth muscle myosin light chain kinase is expressed as an independent protein, telokin. J Biol Chem. 1991;266:23945–23952. [PMC free article] [PubMed] [Google Scholar]
- 10.Gallagher PJ, Herring BP, Griffin SA, Stull JT. Molecular characterization of a mammalian smooth muscle myosin light chain kinase. J Biol Chem. 1991;266:23936–23944. [PMC free article] [PubMed] [Google Scholar]
- 11.Gallagher PJ, Herring BP, Stull JT. Myosin light chain kinases. J Muscle Res Cell Motil. 1997;18:1–16. doi: 10.1023/a:1018616814417. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher PJ, Herring BP, Trafny A, Sowadski J, Stull JT. A molecular mechanism for autoinhibition of myosin light chain kinases. J Biol Chem. 1993;268:26578–26582. [PMC free article] [PubMed] [Google Scholar]
- 13.Gallagher PJ, Jin Y, Killough G, Blue EK, Lindner V. Alterations in expression of myosin and myosin light chain kinases in response to vascular injury. Am J Physiol Cell Physiol. 2000;279:C1078–C1087. doi: 10.1152/ajpcell.2000.279.4.C1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallagher PJ, Stull JT. Localization of an actin binding domain in smooth muscle myosin light chain kinase. Mol Cell Biochem. 1997;173:51–57. doi: 10.1023/a:1006876318155. [DOI] [PubMed] [Google Scholar]
- 15.Garcia JG, Lazar V, Gilbert-McClain LI, Gallagher PJ, Verin AD. Myosin light chain kinase in endothelium: molecular cloning and regulation. Am J Respir Cell Mol Biol. 1997;16:489–494. doi: 10.1165/ajrcmb.16.5.9160829. [DOI] [PubMed] [Google Scholar]
- 17.Goeckeler ZM, Masaracchia RA, Zeng Q, Chew TL, Gallagher P, Wysolmerski RB. Phosphorylation of myosin light chain kinase by p21-activated kinase PAK2. J Biol Chem. 2000;275:18366–18374. doi: 10.1074/jbc.M001339200. [DOI] [PubMed] [Google Scholar]
- 18.Goeckeler ZM, Wysolmerski RB. Myosin light chain kinase-regulated endothelial cell contraction: the relationship between isometric tension, actin polymerization, and myosin phosphorylation. J Cell Biol. 1995;130:613–627. doi: 10.1083/jcb.130.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gospodarowicz D, Ill C. Extracellular matrix and control of proliferation of vascular endothelial cells. J Clin Invest. 1980;65:1351–1364. doi: 10.1172/JCI109799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayakawa K, Okagaki T, Higashi-Fujime S, Kohama K. Bundling of actin filaments by myosin light chain kinase from smooth muscle. Biochem Biophys Res Commun. 1994;199:786–791. doi: 10.1006/bbrc.1994.1298. [DOI] [PubMed] [Google Scholar]
- 21.Herring BP, Dixon S, Gallagher PJ. Smooth muscle myosin light chain kinase expression in cardiac and skeletal muscle. Am J Physiol Cell Physiol. 2000;279:C1656–C1664. doi: 10.1152/ajpcell.2000.279.5.C1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herring BP, Smith AF. Telokin expression is mediated by a smooth muscle cell-specific promoter. Am J Physiol Cell Physiol. 1996;270:C1656–C1665. doi: 10.1152/ajpcell.1996.270.6.C1656. [DOI] [PubMed] [Google Scholar]
- 23.Kamm KE, Leachman SA, Michnoff CH, Nunnally MH, Persechini A, Richardson AL, Stull JT. Myosin light chain kinases and kinetics of myosin phosphorylation in smooth muscle cells. Prog Clin Biol Res. 1987;245:183–193. [PubMed] [Google Scholar]
- 24.Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem. 2000;276:4527–4530. doi: 10.1074/jbc.R000028200. [DOI] [PubMed] [Google Scholar]
- 25.Kemp BE, Pearson RB, House C. Role of basic residues in the phosphorylation of synthetic peptides by myosin light chain kinase. Proc Natl Acad Sci USA. 1983;80:7471–7475. doi: 10.1073/pnas.80.24.7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kudryashov DS, Chibalina MV, Birukov KG, Lukas TJ, Sellers JR, Van Eldik LJ, Watterson DM, Shirinsky VP. Unique sequence of a high molecular weight myosin light chain kinase is involved in interaction with actin cytoskeleton. FEBS Lett. 1999;463:67–71. doi: 10.1016/s0014-5793(99)01591-4. [DOI] [PubMed] [Google Scholar]
- 27.Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, Kaibuchi K, Ito M. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1997;272:12257–12260. doi: 10.1074/jbc.272.19.12257. [DOI] [PubMed] [Google Scholar]
- 28.Lin P, Luby-Phelps K, Stull JT. Binding of myosin light chain kinase to cellular actin-myosin filaments. J Biol Chem. 1997;272:7412–7420. doi: 10.1074/jbc.272.11.7412. [DOI] [PubMed] [Google Scholar]
- 29.Lin P, Luby-Phelps K, Stull JT. Properties of filament-bound myosin light chain kinase. J Biol Chem. 1999;274:5987–5994. doi: 10.1074/jbc.274.9.5987. [DOI] [PubMed] [Google Scholar]
- 30.Paul ER, Ngai PK, Walsh MP, Groschel-Stewart U. Embryonic chicken gizzard: expression of the smooth muscle regulatory proteins caldesmon and myosin light chain kinase. Cell Tissue Res. 1995;279:331–337. doi: 10.1007/BF00318489. [DOI] [PubMed] [Google Scholar]
- 31.Poperechnaya A, Varlamova O, Lin P, Stull JT, Bresnick AR. Localization and activity of myosin light chain kinase isoforms during the cell cycle. J Cell Biol. 2000;151:697–708. doi: 10.1083/jcb.151.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sellers JR, Pato MD. The binding of smooth muscle myosin light chain kinase and phosphatases to actin and myosin. J Biol Chem. 1984;259:7740–7746. [PubMed] [Google Scholar]
- 33.Simons M, Wang M, McBride OW, Kawamoto S, Yamakawa K, Gdula D, Adelstein RS, Weir L. Human nonmuscle myosin heavy chains are encoded by two genes located on different chromosomes. Circ Res. 1991;69:530–539. doi: 10.1161/01.res.69.2.530. [DOI] [PubMed] [Google Scholar]
- 34.Smith AF, Bigsby RM, Word RA, Herring BP. A 310-bp minimal promoter mediates smooth muscle cell-specific expression of telokin. Am J Physiol Cell Physiol. 1998;274:C1188–C1195. doi: 10.1152/ajpcell.1998.274.5.C1188. [DOI] [PubMed] [Google Scholar]
- 35.Smith L, Stull JT. Myosin light chain kinase binding to actin filaments. FEBS Lett. 2000;480:298–300. doi: 10.1016/s0014-5793(00)01931-1. [DOI] [PubMed] [Google Scholar]
- 36.Smith L, Su X, Lin P, Zhi G, Stull JT. Identification of a novel actin binding motif in smooth muscle myosin light chain kinase. J Biol Chem. 1999;274:29433–29438. doi: 10.1074/jbc.274.41.29433. [DOI] [PubMed] [Google Scholar]
- 37.Turbedsky K, Pollard TD, Bresnick AR. A subset of protein kinase C phosphorylation sites on the myosin II regulatory light chain inhibits phosphorylation by myosin light chain kinase. Biochemistry. 1997;36:2063–2067. doi: 10.1021/bi9624651. [DOI] [PubMed] [Google Scholar]
- 38.Verin AD, Gilbert-McClain LI, Patterson CE, Garcia JG. Biochemical regulation of the nonmuscle myosin light chain kinase isoform in bovine endothelium. Am J Respir Cell Mol Biol. 1998;19:767–776. doi: 10.1165/ajrcmb.19.5.3126. [DOI] [PubMed] [Google Scholar]
- 39.Verin AD, Lazar V, Torry RJ, Labarrere CA, Patterson CE, Garcia JG. Expression of a novel high molecular-weight myosin light chain kinase in endothelium. Am J Respir Cell Mol Biol. 1998;19:758–766. doi: 10.1165/ajrcmb.19.5.3125. [DOI] [PubMed] [Google Scholar]
- 40.Ye LH, Hayakawa K, Lin Y, Okagaki T, Fujita K, Kohama K. The regulatory role of myosin light chain kinase as an actin-binding protein. J Biochem (Tokyo) 1994;116:1377–1382. doi: 10.1093/oxfordjournals.jbchem.a124690. [DOI] [PubMed] [Google Scholar]
- 41.Zeng Q, Lagunoff D, Masaracchia R, Goeckeler Z, Cote G, Wysolmerski R. Endothelial cell retraction is induced by PAK2 monophosphorylation of myosin II. J Cell Sci. 2000;113:471–482. doi: 10.1242/jcs.113.3.471. [DOI] [PubMed] [Google Scholar]