Abstract
Detection of a sensory stimulus depends on its psychophysical saliency; the higher the saliency, the easier the detection. But it is not known whether sensory relay nuclei differ in their ability to detect low salient whisker stimuli. We found that reversible lesions of either the somatosensory thalamus or superior colliculus blocked detection of a low salience whisker conditioned stimulus (WCS) in an active avoidance task, without affecting detection of a high salience WCS. Thus, thalamic and tectal sensory relays work synergistically to detect low salient stimuli during avoidance behavior, but are redundant during detection of highly salient stimuli. We also recorded electrophysiological responses evoked by high and low salience stimuli in the superior colliculus and barrel cortex of freely behaving animals during active exploration, awake immobility, and sensory detection in the active avoidance task. Field potential (FP) responses evoked in barrel cortex and superior colliculus by high intensity stimuli are larger and adapt more to frequency than those evoked by low-intensity stimuli. FP responses are also more suppressed and adapt less during active exploration, and become further suppressed in barrel cortex during successful detection of either high or low salient stimuli in the active avoidance task. In addition, unit recordings revealed that firing rate increases in superior colliculus during active exploration and especially during successful detection of either high or low salient stimuli in the active avoidance task. We conclude that detection of low salient stimuli is achieved by a sparse neural code distributed through multiple sensory relays.
Introduction
Sensory stimuli (e.g., vibrissa stimulation in rodents) are transduced by specialized receptors that send neural signals to primary sensory neurons (trigeminal complex), which in turn relay those signals via two main ascending pathways to the superior colliculus (trigeminotectal pathway) in the midbrain and to the thalamus (trigeminothalamic pathway) in the forebrain (Killackey and Erzurumlu, 1981; Veazey and Severin, 1982; Huerta et al., 1983; Bruce et al., 1987; Rhoades et al., 1989; Veinante et al., 2000). A primary role of these sensory networks is to detect sensory stimuli that signal relevant events.
The superior colliculus is an early sensory hub well suited to mediate sensory detection of stimuli that require immediate action (Sprague and Meikle, 1965; Schneider, 1969; Sparks, 1986; Dean et al., 1989; Westby et al., 1990; Redgrave et al., 1993; Stein and Meredith, 1993; McHaffie et al., 2005; Redgrave and Gurney, 2006; Cohen and Castro-Alamancos, 2007). The thalamus is the main relay station to the cortex, which through primary and higher order thalamocortical loops (Sherman and Guillery, 1996; Castro-Alamancos and Connors, 1997) can represent (code) complex sensory stimuli (Johnson and Hsiao, 1992; Mountcastle, 1998; Parker and Newsome, 1998; de Lafuente and Romo, 2005; Kleinfeld et al., 2006). Interestingly, trigeminotectal and trigeminothalamic ascending pathways are both capable of independently (i.e., in the absence of the other) detecting sensory stimuli that signal impending danger (Cohen and Castro-Alamancos, 2007). However, the ability to detect behaviorally relevant stimuli is a function of psychophysical salience (Mountcastle et al., 1969; Mountcastle, 1998; Parker and Newsome, 1998; Treue, 2003; Connor et al., 2004; Knudsen, 2007). A stimulus exhibits a certain psychophysical salience that is best described as how well it is perceived by the subject. To identify the sensory stimulus detection capabilities of the trigeminothalamic and trigeminotectal pathways, we varied the psychophysical salience of a whisker conditioned stimulus (WCS) during performance in an active avoidance task and tested the impact of reversible lesions of these two pathways on the ability to detect the stimuli. Moreover, electrophysiological recordings in superior colliculus and somatosensory (barrel) cortex of freely behaving animals during spontaneous behaviors and during performance of the active avoidance task revealed the neural responses evoked by stimuli of different psychophysical salience, and their dependence on behavioral state and on successful sensory detection.
Materials and Methods
Adult male Spraque-Dawley rats (225–250 g) were used for the lesion experiments (n = 12) and chronic electrophysiology (n = 9). Animals were cared for in accordance with National Institutes of Health guidelines for laboratory animal welfare. All experiments were approved by the Drexel University Institutional Animal Care and Use Committee. At all times, food and water was available ad libitum. All animals were initially housed in groups of three for the first block of active avoidance training sessions. Once the animals were assigned to an experimental group, they were individually housed for the remainder of the experimental protocol.
Surgical procedures.
For all recovery surgeries, animals were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and placed in a stereotaxic frame. All skin incisions and frame contacts with the skin were injected with lidocaine (2%). Throughout the surgery, body temperature was automatically maintained constant with a heating pad (Harvard Apparatus). During recovery from surgery, animals received a dose of buprenorphine (0.03 mg/kg, i.m.) to reduce pain. Recovery from whisker pad electrode, guide cannula, and microelectrode implantations involved 5–7 d before retesting.
Whisker pad electrode implantation.
Before training in the WCS task (see below), an insulated stainless-steel bipolar electrode was placed in the left whisker pad subcutaneously to stimulate the whisker pad (Castro-Alamancos, 2004b). Electrode pole separation was ∼1 mm. The wires were normally placed around whisker C2–C4. All electrodes and connectors were held in place using miniscrews and dental cement on the skull.
Active avoidance training.
Animals were trained in the active avoidance task using procedures similar to those described previously (Castro-Alamancos, 2004b; Cohen and Castro-Alamancos, 2007). We use three versions of the active avoidance task depending on the CS that is used. The auditory version uses an auditory CS (ACS), the normal whisker version uses a High whisker CS (WCS), and the High/Low whisker version uses both a High and a Low WCS presented pseudo-randomly. Animals are placed in a standard shuttle box controlled using MedPC software (Med Associates) that consists of two compartments separated by a partition extending up from the grid floor that the animal has to traverse to shuttle between compartments. A single training trial consisted of a 7 s avoidance interval followed by a 10 s escape interval. During the avoidance interval, a CS was presented for the duration of the interval or until the animal produced a conditioned response by moving to the adjacent compartment (avoid), whichever occurred first. If the animal avoided, the CS was terminated and no escape interval was presented. However, if the animal did not avoid, then during the escape interval, a mild scrambled electric footshock (unconditioned stimulus, 0.3–0.6 mA) was delivered through the grid floor of the occupied half of the shuttle box. This mild electrical footshock motivates the animal to move readily to the adjacent compartment (escape), at which point the footshock and CS are coterminated ending the trial. According to this terminology, “avoids” are trials when the animal runs away (i.e., escapes) from the CS, while “escapes” are trials when the animal runs away from the footshock. During the intertrial interval (ITI), the animal awaited the next trial and is free to cross between compartments at will. These spontaneous responses are called intertrial crossings (ITC). The duration of the ITI in the avoidance task was determined by the investigator. Trials were triggered by the experimenter to assure that the animal was not “distracted” during the presentation of the CS. In particular, the investigator would start a trial as long as the animal was not grooming or producing an ITC, and at least 15 s had passed since the previous trial. The recorded variables that represent task performance are as follows: the number of avoids and the latency of avoids (from the CS onset).
For all animals, training began with the auditory version of the task that employs an ACS (8 kHz, 82 dB tone). Training was conducted over three or four 50 trial sessions (one session per day) until a high level of consistent ACS-mediated avoidance behavior was produced (>70% avoidance rate). Afterward and before training in the WCS version of the task, animals were subjected to unilateral implantation of a whisker pad-stimulating electrode as described above. All animals were further trained in the High WCS version of the active avoidance task. The High WCS consisted of a 10 Hz (1 ms duration) electrical stimulus train delivered through two wires implanted under the skin of the whisker pad (Castro-Alamancos, 2004b; Cohen and Castro-Alamancos, 2007). The High WCS stimulus was set at intensity just below that resulting in subtle movement of a few (3–5) whiskers but no elicitation of muscle twitches (0.25–0.6 mA). On the first day of training in the WCS task, animals were placed in the training apparatus for an acclimation session, during which the appropriate High WCS intensity was determined for each animal. Training was conducted over three or four 50 trial sessions until a high rate of avoidance was achieved (>70%). Following successful performance on the High WCS task, animals were trained on the High/Low WCS task. A session on the High/Low WCS task consists of 40 High and 40 Low WCS trials (80 trials per day) presented in a pseudo-random order. The intensity of the Low WCS was determined by reducing the intensity of the High WCS by 55%. At this point, animals avoid successfully at a rate of ∼50%.
For the drug infusion experiments, the trials were separated in two blocks. During the first block, 40 High/Low WCS trials are presented. Afterward, the animal is removed from the shuttle box and subjected to either a sham drug infusion or a TTX drug infusion through an implanted cannula, as described below. The animals were then placed back in the shuttle box and subjected to the second block of 40 High/Low WCS trials. The drug infusion sessions were repeated twice in most animals and the data were combined.
Cannula implantations and TTX infusions.
Animals subjected to reversible lesions were implanted with a guide cannula (Plastics One) that allowed the infusion of drugs to reversibly inactivate the target structure, as previously described (Cohen and Castro-Alamancos, 2007). Each animal had one guide cannula lowered over the somatosensory thalamus [bregma: anterior (A)/posterior (P): −3.5, medial (M)/lateral (L): 2.7, dorsal (D)/ventral (V): 4.5] or the superior colliculus (bregma: A/P: −6.5, M/L: 2.0, D/V: 4.0) contralateral to the whisker pad-stimulating electrode. The tip of the cannula was placed 1 mm dorsal to the intended depth. This was required to accommodate the injection cannula, which inserts into the guide cannula and extends 1 mm beyond it. The guide cannula was held in place by miniscrews and dental cement and was fitted with a dummy cannula. A Na+ channel blocker, tetrodotoxin (TTX, 10 μm), was used to reversibly inactivate the target structure. Previous results have demonstrated that in urethane anesthetized rats, the spread of TTX (1 μl, 10 μm) abolished neural activity in electrodes located 1 mm from the cannula tip (Cohen and Castro-Alamancos, 2007). Thus, we estimate that the diffusion radius for TTX is 1 mm. For the infusions, the animals were removed from the shuttle box and held by the experimenter. The injection cannula attached to a Hamilton syringe was lowered into the guide cannula and fastened securely. During a period of 2 min, the drugs (1 μl) were infused into the target area. The injection probe was left in place for an additional 3 min to ensure successful infusion within the region of interest, following which, the probe was removed and the drug was allowed to diffuse for ∼10 min before subsequent testing. A recovery from drug session was conducted on the following day. During sham infusions the same procedures were followed except that no drug was injected. The placement of the cannulas was verified using histological procedures and electrophysiological procedures, as previously described [Cohen and Castro-Alamancos (2007), their supplemental information].
Chronic electrophysiology.
Animals were implanted with a whisker pad-stimulating electrode and two recording electrodes at the same time. The recording electrodes were placed contralateral to the whisker pad-stimulating electrode. The recording electrodes were aligned with the whisker pad-stimulating electrode by recording evoked responses during surgery and moving the recording electrodes. Once in place, the electrodes attached to head connectors were fixed to the skull by screws and dental cement. In all animals, a FP electrode was implanted in the barrel cortex (bregma: A/P: −2.7, M/L: 5.0, D/V: 0.5–1.0). The FP electrodes consisted of blunt insulated stainless steel wires (100 μm outer diameter, ∼0.5 MΩ). Concurrently, either a FP electrode or a multiunit activity (MUA) electrode was implanted in the superior colliculus (lambda: A/P: 2.2, M/L: 2.2, D/V: 4–4.5). The MUA electrode consisted of a higher impedance insulated tungsten electrode edged to a fine tip (100 μm outer shaft diameter, 2–7 MΩ). The reference electrode consisted of a FP electrode placed above the superior colliculus, and the ground was attached to skull screws. In some recording sessions, clear single-unit activity was recorded through the MUA electrode, but we combined these cases with MUA sessions. Also, in some cases, FP activity was also collected in the superior colliculus through the MUA recording electrode.
During recording sessions, the head connector was attached to a headstage operational amplifier with unity gain that lead through fine tether cables to an electrical swivel that terminated in the amplifiers and recording system. Electrophysiological recording sessions (one per day) were of two types. In the first type, the animal was allowed to freely behave in a 35 × 25 cm Plexiglas cage. In the second type, the animal was subjected to training in the High/Low active avoidance task in a shuttle box as described above. During all sessions, electrophysiological activity was continuously recorded in synchrony with digital video of the behavior (Cineplex, Plexon). This allowed offline verification of the behavioral state. For the present study, the spontaneous behavior of the animal was classified as active exploration or awake immobility by watching the behavior on video and scoring each period. During active exploration (active) the animal moves about the cage or stands still while moving its head and whiskers to explore the environment. During awake immobility (immobile) the animal is standing or resting (not laying) with eyes open and generally fixed, and there are no active whisker movements.
Measures and statistical analyses.
For behavioral results obtained from the reversible lesion experiments, we performed repeated-measures ANOVAs on the number of avoids. To determine the effect of the reversible lesions on Low and High WCS detection, we conducted a two-factor repeated-measures ANOVA for Low WCS and another for High WCS, where the within-subjects factor was the effect of TTX (pre- vs post-TTX) and the between-subjects factor was the type of lesion (superior colliculus vs somatosensory thalamus). Individual pairwise comparisons were done with paired t tests. All results are presented as mean ± SD unless otherwise stated.
For electrophysiological results during spontaneous behavior or the High/Low WCS active avoidance task, we measured different FP and MUA responses, and data points correspond to recording sessions on different days (usually two sessions per animal). In barrel cortex, we measured the peak amplitude and time to peak (peak latency) of FP responses in barrel cortex during a 5–30 ms window poststimulus, which we have previously studied (Castro-Alamancos, 2004b). In superior colliculus, we measured the peak amplitude and time to peak of two different FP responses that encompass different time windows: peak1 (3–8 ms) and peak2 (9–20 ms), which we have previously studied (Cohen et al., 2008). We also measured the peak amplitude of peak3 (∼30 ms) by overlaying average traces for each session to find the largest peak3, typically evoked by High stimuli during awake immobility, and then obtaining the amplitude at that time point for the other three traces. Also in superior colliculus, we measured the number of spikes evoked per stimulus for three time windows corresponding to peak1 (3–8 ms), peak2 (9–20 ms), and peak3 (21–90 ms), or we used a large time window that encompasses the sum of the three peaks (3–90 ms poststimulus), which we call peak4. The border between peak1 and peak 2 was slightly adjusted per animal (range: 1–3 ms) based on the evoked responses. Responses were the average of at least 30 stimulus trials per session. Spontaneous firing was measured for each stimulus trial during a window lasting 1 s before the stimulus. To correct the evoked responses with the spontaneous firing, the spontaneous firing value was first adjusted by the duration of the response window and then subtracted from the response.
For each electrophysiological response measured during spontaneous behavior, we began by conducting two-factor repeated-measures ANOVAs of the effect of stimulus saliency (High vs Low) and of the effect of behavioral state (active vs immobile) for single whisker pad stimuli delivered every 2 s or for trains of 10 stimuli delivered every 5 s. Significant main effects were decomposed by pairwise comparisons that were either parametric (Tukey), if the two groups involved were normally distributed (according to the Shapiro–Wilk normality test), or nonparametric (Wilcoxon signed ranks, corrected by the number of comparisons) otherwise.
For each electrophysiological response measured during the High/Low active avoidance task, we began by conducting two-factor repeated-measures ANOVAs of the effect of stimulus saliency (High vs Low) or the effect of avoidance (avoids vs escapes) with the effect of stimulus number from WCS onset (first 10 stimuli; S1-S10) or from WCS offset (last 10 stimuli before avoid; E10–E1). Note that for the escapes, the last 10 stimuli from WCS offset correspond to the 10 last stimuli before the foot-shock. Significant main effects were decomposed by pairwise comparisons that were either parametric (Tukey), if the two groups involved were normally distributed (according to the Shapiro–Wilk normality test), or nonparametric (Wilcoxon signed ranks, corrected by the number of comparisons) otherwise.
Results
Effects of reversible lesions on detection of Low and High stimuli
Previous work has shown that the trigeminothalamic and trigeminotectal pathways serve as alternative routes for WCS detection during active avoidance behavior (Cohen and Castro-Alamancos, 2007). However, those results were determined using a highly salient (High) WCS that is easily detectable. Therefore, it is plausible that sensory pathways differ in their capacity to detect less salient sensory stimuli. In the present study, we compared the sensory detection capabilities of the trigeminothalamic and trigeminotectal pathways by presenting a High- or Low-intensity WCS. A group of animals (n = 12) were trained in the ACS (auditory) version of the active avoidance task for three or four sessions until a consistent rate of avoids was achieved (∼70%). These animals were implanted with a guide cannula in either the somatosensory thalamus (n = 6) or the superior colliculus (n = 6) contralateral to a whisker pad electrode. After a 1 week recovery period from surgery, animals were trained on the normal WCS (whisker) version of the avoidance task for three sessions, using a High intensity WCS, during which a consistent rate of avoids was achieved (∼70%). On the fourth and subsequent sessions, each animal was subjected to the High/Low version of the WCS task during which High and Low WCS trials were presented pseudo-randomly before and after sham or TTX infusion through the guide cannula (see Materials and Methods).
During the High/Low WCS task, the Low WCS was harder to detect than the High WCS. Thus, during the first block of trials, the Low WCS elicited significantly fewer avoids (52.8 ± 10.1% vs 75.3 ± 11.1%; High vs Low avoids, n = 12, p < 0.01) and slower avoid latencies (3.2 ± 0.58 vs 3.6 ± 0.77 s; High vs Low avoids, n = 12, p < 0.05) than the High WCS. These results indicate that the Low WCS is psychophysically less salient to the animal.
A sham drug infusion had no effect on the ability of the animals to detect the High or Low WCS. Thus, the rate of avoids (Fig. 1A) (p = 0.3) and the avoid latencies (p = 0.67) for High and Low stimuli were not affected by the sham infusion. TTX infusion into the somatosensory thalamus or superior colliculus had no significant impact on the ability to detect the High WCS (Avoids pre- vs post-TTX, p = 0.8) but robustly impaired the ability to detect the Low WCS (Avoids pre- vs post-TTX, p < 0.01). Thus, the rate of avoids for Low WCS was significantly reduced by TTX infusion into the superior colliculus (58 ± 12.5% vs 21 ± 5.2%; pre- vs post-TTX; n = 10 sessions, p < 0.01) or into the somatosensory thalamus (51.1 ± 7.9% vs 16.8 ± 6.7%; pre- vs post-TTX; n = 14 sessions, p < 0.01) to a level comparable to the behavior of animals that are not presented a CS at all (Cohen and Castro-Alamancos, 2007). Moreover, the effect of TTX did not depend on the infusion site (p = 0.4), which indicates that both the superior colliculus and somatosensory thalamus inactivation were similarly effective at impairing Low WCS detection.
Figure 1.
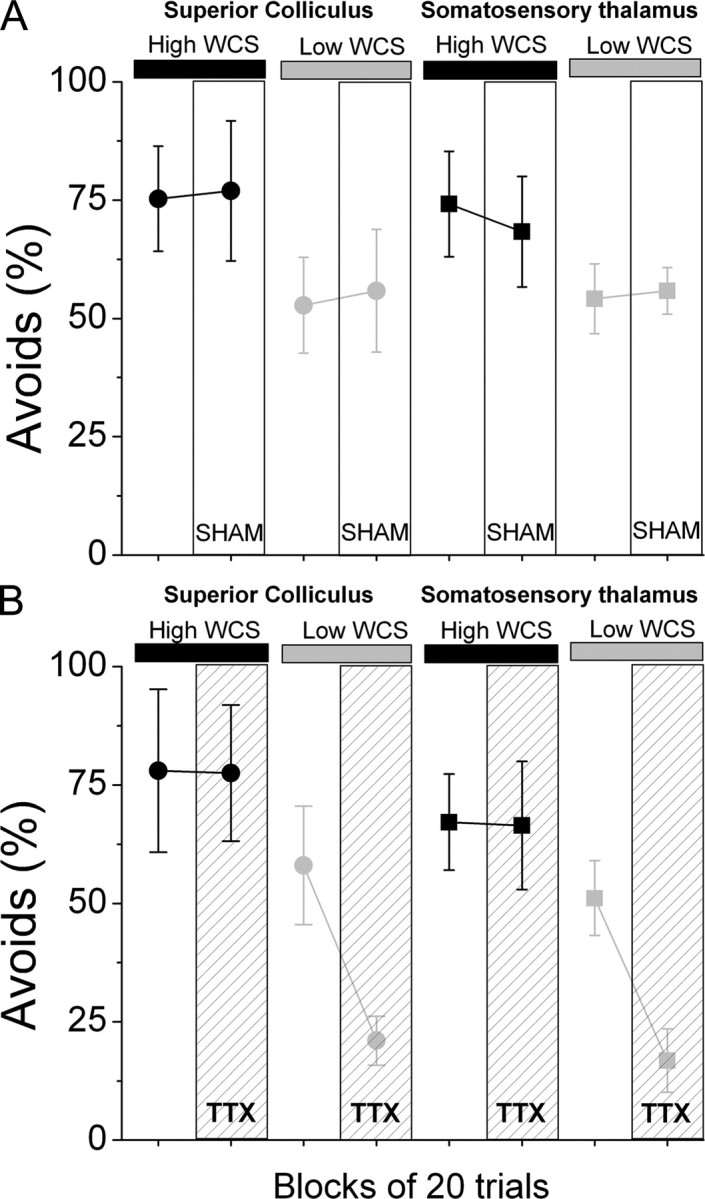
Effects of somatosensory thalamus or superior colliculus lesions on detection of a low salience WCS. A, Effect of sham injection (SHAM) on performance in the High/Low active avoidance task. Four groups are shown in pairs of data points. The first data point refers to 20 trials collected before SHAM injection. The second data point refers to 20 trials collected after SHAM injection. Note the decrease in task performance during the Low salience WCS trials and the lack of effect of the SHAM procedure on task performance. B, Effect of TTX inactivation (TTX) on performance in the High/Low active avoidance task. Data are shown as in A. Note the selective decrease in task performance during Low salience WCS trials as a consequence of the inactivation of the somatosensory thalamus or superior colliculus.
Together, these results support the previous finding that trigeminotectal or trigeminothalamic pathways can independently detect a High WCS during active avoidance behavior. Furthermore, the present results indicate that both of these pathways must work together to detect a Low WCS. Trigeminotectal and trigeminothalamic synergy is required for detection of stimuli that are of low psychophysical saliency.
Effects of High and Low stimuli on FP responses in barrel cortex during behavior
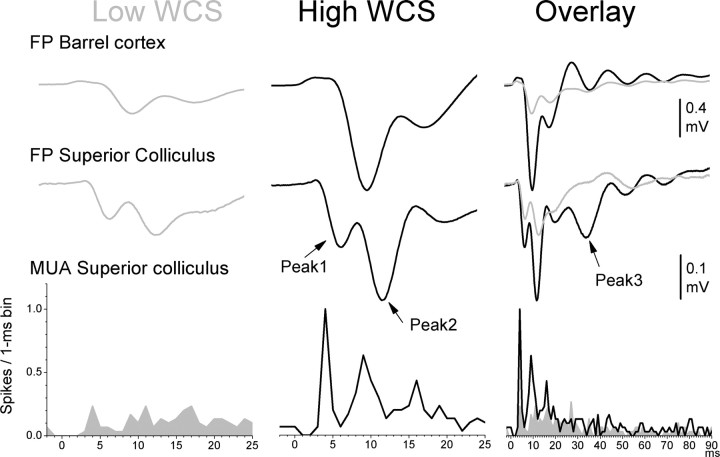
The previous results indicate that the trigeminothalamic and trigeminotectal pathways must both be available to detect a Low WCS during active avoidance behavior. To better understand the impact of the High- and Low-intensity stimuli on the early sensory circuits, we implanted a group of animals (n = 9) with a whisker pad-stimulating electrode, a FP recording electrode in the contralateral barrel cortex, and either a FP or a MUA recording electrode in the contralateral superior colliculus. Figure 2 shows an example of FP and MUA responses obtained during a recording session in which single High and Low stimuli were delivered to an awake animal (30 trials per condition). The High stimulus elicited large FP responses in both barrel cortex and superior colliculus, and a sharp MUA response characterized by a peak1 and peak2 components, which resemble multiwhisker responses in anesthetized animals (Cohen et al., 2008). A FP peak3 response at ∼30 ms was often obvious in the superior colliculus (Fig. 2, overlay). In contrast, the Low stimulus elicited smaller FP responses in both barrel cortex and superior colliculus, and a MUA response that was dispersed but greater than spontaneous activity.
Figure 2.
Representative FP and MUA responses in superior colliculus and barrel cortex to High and Low stimuli. Example of FP responses in barrel cortex and FP and MUA responses in superior colliculus evoked by Low (gray) and High (black) single stimuli. The right panel overlays the responses to High and Low stimuli. Each response is the average of 30 stimulus trials. The arrows point to the peaks of peak1, peak2, and peak3 FP responses in superior colliculus.
During the recording sessions, the animals displayed many behaviors and we distinguished between two clearly differentiated awake states during which the WCS is usually delivered in animals performing the High/Low active avoidance task; active exploration (active) and awake-immobility (immobile). Since some behavioral states are easily distinguishable by comparing FP activity in cortex (e.g., slow-wave sleep vs awake), we tested whether active exploration and awake immobility are also distinguishable by comparing FFT power spectrums from spontaneous FP activity in barrel cortex (i.e., excluding periods of sensory stimulation). Figure 3A shows that these awake states are not differentiable by the level of cortical FP activation (n = 21; p = 0.8), but are obviously different at the behavioral level. Importantly, both of these awake states are clearly differentiable from slow-wave sleep periods recorded during the same sessions (p < 0.01) (Fig. 3A).
Figure 3.
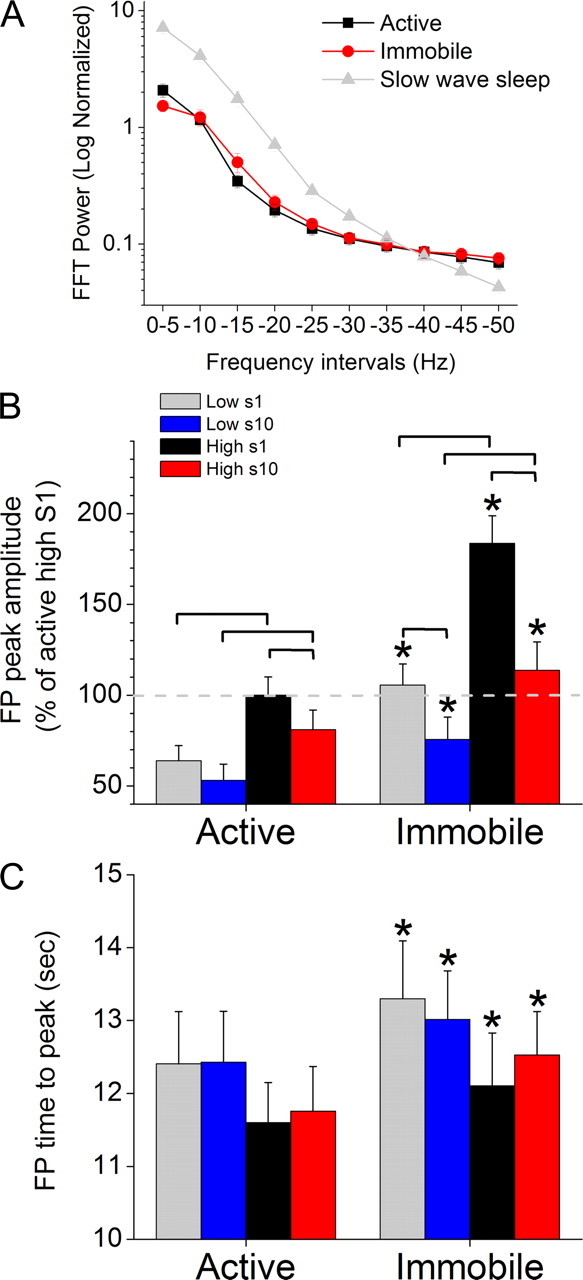
FP activity in barrel cortex evoked by High and Low stimuli during different behavioral states. A, FFT power spectrums taken from spontaneous FP activity in barrel cortex, excluding periods of sensory stimulation, during active exploration, awake immobility and slow-wave sleep. B, C, Peak amplitude (B) and time to peak (C) of FP responses evoked in barrel cortex by 10 Hz trains of High and Low stimuli during either active exploration (left) or awake immobility (right). Statistically significant (p < 0.05) differences between the groups within a behavioral state are marked by brackets, and significant differences between the two behavioral states are marked with asterisks in the right columns. The first (s1) and the last (s10) responses in the 10 Hz train (10 stimuli) are measured.
Using these methods, we determined the effect of stimulus intensity (Low vs High), frequency adaptation (S1 vs S10 stimulus in a 10 Hz train), awake behavioral states (Active vs Immobile) and their interactions on FP responses in barrel cortex (Fig. 3), and on FP (Fig. 4) and MUA (Figs. 5, 6) responses in superior colliculus. Responses were evoked by either single-stimuli protocols (one stimulus every 2 s) or 10 Hz protocols (10 Hz train of 10 stimuli every 5 s) as animals freely moved upon a cage (see Materials and Methods). In some cases, we found slight differences between the two stimulus protocols on low-frequency responses (single stimuli vs S1 in a 10 Hz train) and those are noted. Otherwise, only data from the 10 Hz protocols is presented for brevity. In Figures 3–6, statistically significant differences between the groups within a behavioral state are marked by brackets (i.e., Low S1, Low S10, High S1, High S10), and significant differences between the two behavioral states are marked with asterisks (over the immobile columns).
Figure 4.
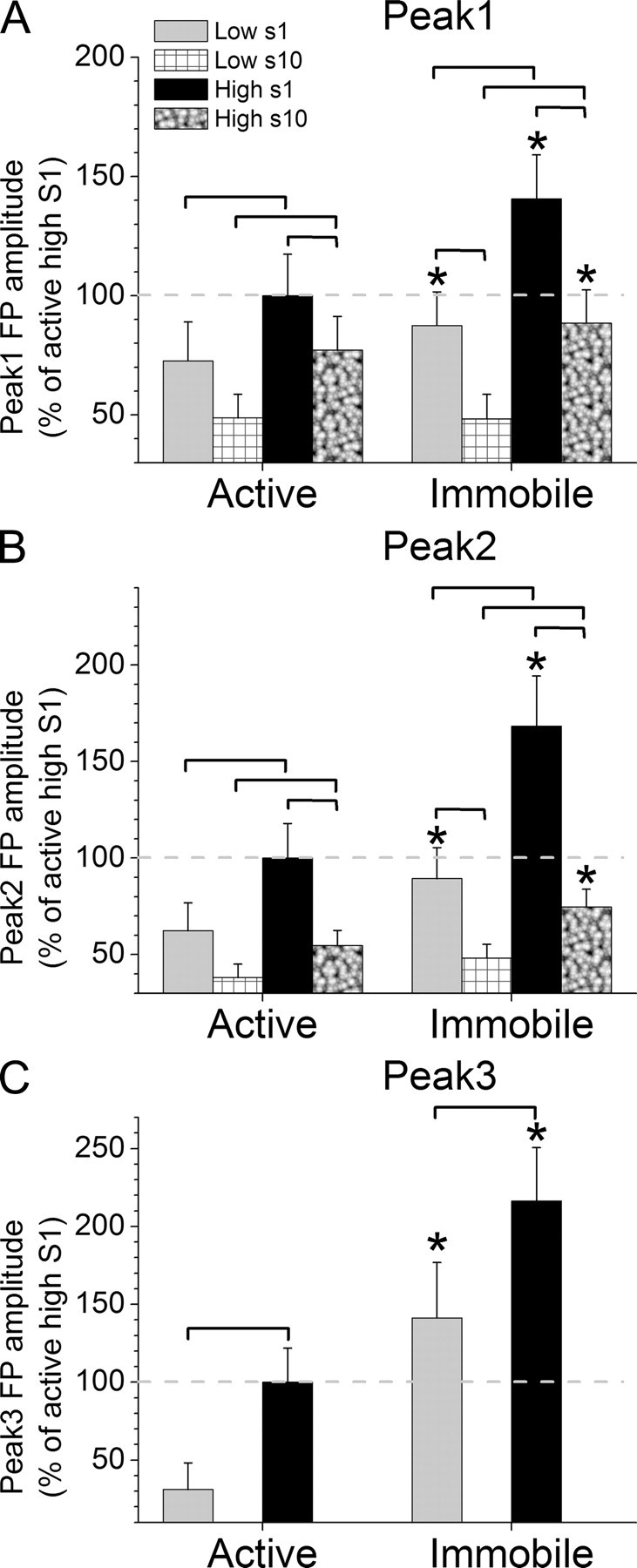
FP activity in superior colliculus evoked by High and Low stimuli during different behavioral states. A–C, Peak amplitude of peak1 (A), peak2 (B), and peak3 (C) FP responses evoked in superior colliculus by 10 Hz trains of High and Low stimuli during either active exploration (left) or awake immobility (right). Statistically significant (p < 0.05) differences between the groups within a behavioral state are marked by brackets, and significant differences between the two behavioral states are marked with asterisks in the right columns. The first (s1) and the last (s10) responses in the 10 Hz train (10 stimuli) are measured, except for peak3 where only s1 is measured.
Figure 5.
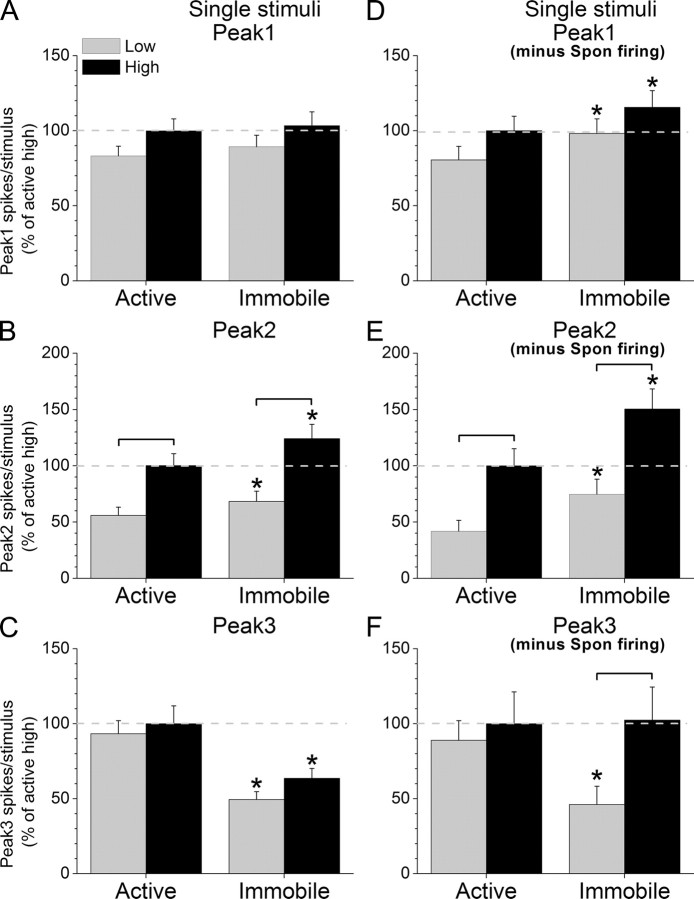
MUA in superior colliculus evoked by single High and Low stimuli during different behavioral states. A–F, Spikes per stimulus for peak1 (A, D), peak2 (B, E) and peak3 (C, F) MUA responses evoked in superior colliculus by single High and Low stimuli during either active exploration (left) or awake immobility (right) uncorrected (A–C) or corrected (D–F) by the spontaneous firing rate. Statistically significant (p < 0.05) differences between the groups within a behavioral state are marked by brackets, and significant differences between the two behavioral states are marked with asterisks in the right columns.
Figure 6.
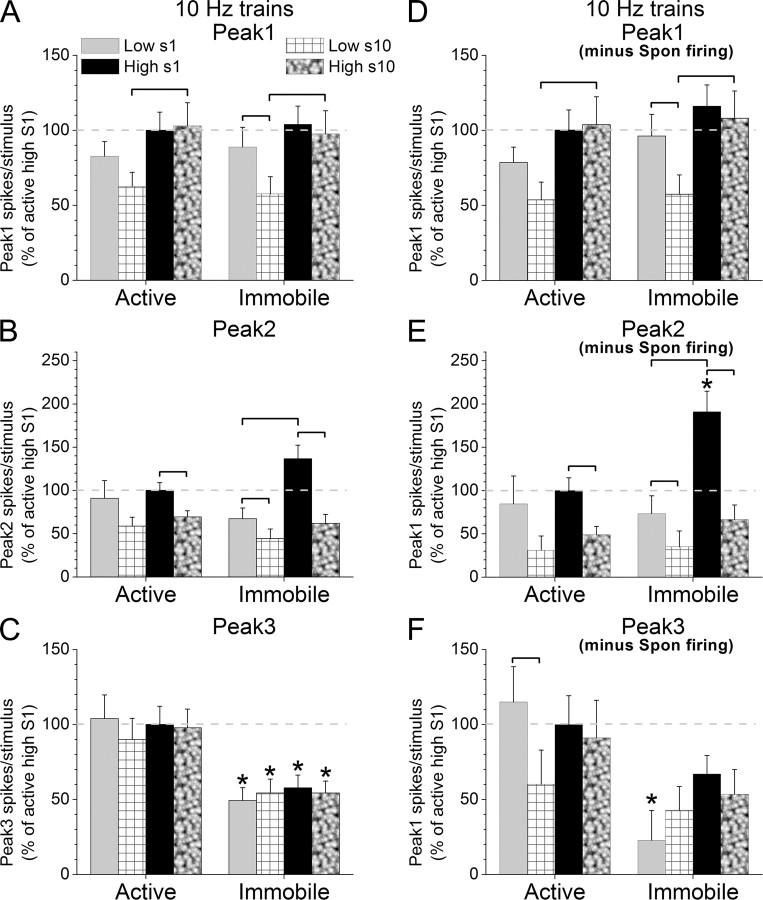
MUA in superior colliculus evoked by 10 Hz High and Low stimulus trains during different behavioral states. A–D, Spikes per stimulus for peak1 (A, D), peak2 (B, E) and peak3 (C, F) MUA responses evoked in superior colliculus by 10 Hz High and Low stimuli during either active exploration (left) or awake immobility (right) uncorrected (A–C) or corrected (D–F) by the spontaneous firing rate. Statistically significant (p < 0.05) differences between the groups within a behavioral state are marked by brackets, and significant differences between the two behavioral states are marked with asterisks in the right columns. The first (s1) and the last (s10) responses in the 10 Hz train (10 stimuli) are measured.
Figure 3, B and C, shows population data (n = 21) of FP responses evoked in barrel cortex by 10 Hz trains of High and Low stimuli during either active exploration or awake immobility. The peak amplitude of FP responses in barrel cortex (Fig. 3B) was significantly affected by stimulus intensity (p < 0.01), stimulus frequency (p < 0.01) and behavioral state (p < 0.01). In particular, the Low FP responses evoked by either S1 or S10 in a 10 Hz train were significantly smaller than the High FP responses, during both active exploration (p < 0.01) or awake immobility (p < 0.01). In addition, sensory adaptation between S1 and S10 in a 10 Hz train depended on the stimulus intensity and the state of the animal. The response to the Low stimulus depressed significantly only during awake immobility (p < 0.01) but not during active exploration (p = 0.1). In contrast, the responses to the High stimulus depressed during both active exploration (p < 0.05) and awake immobility (p < 0.01), although adaptation of High responses was much less pronounced during active exploration (18%) than during awake immobility (38%). Finally, the High and the Low FP responses evoked by either S1 or S10 in a 10 Hz train increased significantly (p < 0.01) as animals transition from active exploration to awake immobility.
The time to peak (peak latency) of barrel cortex FP responses (Fig. 3C) was significantly affected by behavioral state (p < 0.01) but not by stimulus intensity (p = 0.1) or stimulus frequency (p = 0.8). Thus, both the High and the Low FP responses evoked by either S1 or S10 in a 10 Hz train became significantly slower (p < 0.01) as animals transition from active exploration to awake immobility.
In conclusion, High intensity FP responses in barrel cortex are larger than Low-intensity responses, and responses decrease and become faster during active exploration. The effect of frequency on sensory adaptation depends on the intensity of the stimulus and behavioral state. Thus, High responses always adapt, albeit much less during active exploration, but Low responses adapt only during awake immobility.
Effects of High and Low stimuli on FP responses in superior colliculus during behavior
Figure 4 shows population data (n = 18) of FP responses in superior colliculus evoked by 10 Hz trains (10 stimuli) of High and Low stimuli during either active exploration or awake immobility. We measured the peak amplitude and latency of peak1 (3–8 ms after stimulus) and peak2 (9–20 ms poststimulus). We also measured the peak amplitude of peak3 (∼30 ms) by overlaying average traces for each session to find the largest peak3, typically evoked by High stimuli during awake immobility, and then obtaining the amplitude at that time point for the other three traces. Peak3 was only measured for single (low-frequency) stimuli because it can be difficult to recognize during high-frequency stimuli.
The peak amplitude of peak1 FP responses in superior colliculus (Fig. 4A) was significantly affected by stimulus intensity (p < 0.01), stimulus frequency (p < 0.01), and behavioral state (p < 0.01). In particular, the Low peak1 FP responses evoked by either S1 or S10 in a 10 Hz train were significantly smaller than the High peak1 FP responses, during both active exploration (p < 0.05) or awake immobility (p < 0.01). In addition, sensory adaptation between S1 and S10 in a 10 Hz train depended on the stimulus intensity and the state of the animal. The peak1 response to the Low stimulus depressed significantly only during awake immobility (p < 0.01) but not during active exploration (p = 0.1). In contrast, the peak1 response to the High stimulus depressed during both active exploration (p < 0.01) and awake immobility (p < 0.01). Finally, the High peak1 FP responses evoked by either S1 or S10 in a 10 Hz train increased significantly (p < 0.01and p < 0.05, respectively) as animals transition from active exploration to awake immobility. The Low peak1 FP responses evoked by S1 also increase significantly (p < 0.05) as animals transition from active exploration to immobility, but those evoked by S10 do not (p = 0.9).
The peak amplitude of peak2 superior colliculus FP responses (Fig. 4B) was significantly affected by stimulus intensity (p < 0.01), stimulus frequency (p < 0.01), and behavioral state (p < 0.01). In particular, the Low peak2 FP responses evoked by either S1 or S10 in a 10 Hz train were significantly smaller than the High peak2 FP responses, during both active exploration (p < 0.05 and p < 0.05, respectively) or awake immobility (p < 0.01 and p < 0.05, respectively). In addition, sensory adaptation between S1 and S10 in a 10 Hz train depended on the stimulus intensity and the state of the animal. The peak2 response to the Low stimulus depressed significantly only during awake immobility (p < 0.01) but not during active exploration (p = 0.1). In contrast, the peak2 response to the High stimulus depressed during both active exploration (p < 0.01) and awake immobility (p < 0.01). Finally, the High peak2 FP responses evoked by either S1 or S10 in a 10 Hz train increased significantly (p < 0.01and p < 0.05, respectively) as animals transition from active exploration to awake immobility. The Low peak2 FP responses evoked by S1 also increase significantly (p < 0.05) as animals transition from active exploration to immobility, but those evoked by S10 do not (p = 0.2).
The peak amplitude of peak3 superior colliculus FP responses (Fig. 4C) was significantly affected by stimulus intensity (p < 0.01) and behavioral state (p < 0.01). In particular, the Low peak3 FP responses evoked by single stimuli were significantly smaller than the High peak3 FP responses, during both active exploration (p < 0.01) or awake immobility (p < 0.01). Finally, the High and Low peak3 FP responses evoked by single stimuli increased significantly (p < 0.01) as animals transition from active exploration to awake immobility.
Regarding peak latency, peak1 responses peaked faster as they increased due to intensity (p < 0.01; Low vs High). However, only low-frequency (S1) peak2 responses peaked faster as they increased due to intensity (p < 0.05), while high-frequency (S10) peak2 responses did not. High intensity peak1 responses peaked faster as they decreased in amplitude during frequency adaptation (p < 0.01), but Low-intensity peak1 responses did not change peak latency during adaptation. Peak2 responses peaked faster as they decreased in amplitude during frequency adaptation (p < 0.05), except for Low-intensity responses during active exploration, which did not change. The peak latency of either peak 1 or peak2 responses did not change significantly as a function of behavioral state.
In conclusion, peak1, peak2, and peak3 FP responses in superior colliculus were similarly affected by stimulus intensity, frequency and behavioral state. Basically, High intensity responses are larger and usually faster than Low responses. Responses usually decrease during active exploration, except for Low-intensity high-frequency responses (Low S10), which are unaffected by behavioral state (i.e., they are very small during either state). Also, the effect of frequency on peak1 and peak2 sensory adaptation depends on the intensity of the stimulus and the behavioral state; High responses always adapt but Low responses adapt only during awake immobility (i.e., when they are larger), and as responses adapt they usually become faster.
Effects of High and Low stimuli on MUA responses in superior colliculus during behavior
Figures 5 and 6 show population data of MUA responses in superior colliculus evoked by single stimuli protocols (Fig. 5) or 10 Hz protocols (Fig. 6). Data for both protocols are shown because the 10 Hz protocols tended to affect the MUA responses evoked by S1, and thus the single stimuli protocols are a better reflection of low-frequency stimuli. We first tested the effect of behavioral state on spontaneous MUA and found that firing rate doubled (96.8 ± 16% increase) as animals transition from awake immobility to active exploration (p < 0.01; n = 19). Because we found a significant effect of behavioral state on spontaneous firing, the evoked responses are presented either uncorrected (left panels) or corrected (right panels) for the effects of spontaneous firing. Thus, we measured spikes per stimulus for peak1 (3–8 ms poststimulus), peak2 (9–20 ms poststimulus), and peak3 (21–90 ms) time windows. Note that peak3 measured for MUA encompasses a larger time window than the FP peak3.
For single stimuli protocols (Fig. 5) (n = 19), peak1 responses were not significantly affected by stimulus intensity (p = 0.06) or behavioral state (p = 0.09) when the effect of spontaneous firing was not considered (Fig. 5A). When the effect of spontaneous firing was subtracted from peak1 responses (Fig. 5B), they were still not significantly affected by stimulus intensity (p = 0.06), but they were affected by behavioral state (p < 0.01) so that both High and Low peak1 responses increased as animals transitioned from active exploration to awake immobility (Fig. 5D). Peak2 responses were significantly affected by both stimulus intensity (p < 0.01) and behavioral state (p < 0.01) regardless of whether spontaneous firing was considered (Fig. 5E) or not (Fig. 5B). Thus, High peak2 responses were significantly larger than Low peak2 responses during active exploration (p < 0.01) or awake immobility (p < 0.01), and both High (p < 0.01) and Low (p < 0.01) peak2 responses increased significantly as animals transitioned from active exploration to awake immobility (Fig. 5E). Peak 3 responses uncorrected by spontaneous firing were not significantly affected by stimulus intensity (p = 0.06) but were significantly affected by behavioral state (p < 0.01), and this was in part due to the effect of spontaneous firing. Thus, when spontaneous firing was subtracted from peak3 responses, we found that the effect of stimulus intensity and behavioral state depended on each other, so that High peak3 responses were significantly larger than Low responses during awake immobility (p < 0.01) but not during active exploration (p = 0.55). Also, Low peak3 responses were enhanced by active exploration (p < 0.01), but High responses were not (p = 0.8).
For 10 Hz train protocols (Fig. 6), the results of peak1 responses were not significantly affected by subtracting the spontaneous firing. Peak1 responses in a train were not affected by behavioral state (p = 0.9) even when spontaneous firing was subtracted (p = 0.06). However, Peak1 responses were significantly affected by stimulus intensity (p < 0.01), and the effect of frequency adaptation between the first (S1) and 10th (S10) stimulus in a train depended on the intensity of the stimulus (p < 0.05). Thus, the effect of stimulus intensity was only significant for S10 (p < 0.01) and not for S1 (p = 0.2), and only Low-intensity responses during awake immobility (p < 0.01) adapted significantly.
Peak2 responses from 10 Hz trains were mostly not affected by subtracting the spontaneous firing, except for the effect of behavioral state. Therefore, Peak2 responses in a train were significantly affected by stimulus intensity (p < 0.05) and frequency adaptation (p < 0.01), and the effect of behavioral state was evident only after spontaneous firing was subtracted from the responses (p = 0.7 vs p = 0.02). Moreover, the effect of stimulus intensity depended on the state of the animal (p < 0.01) and the level of sensory adaptation (p < 0.05). Thus, High responses were significantly larger than Low responses during awake immobility (p < 0.01), but not during active exploration, and only for S1unadapted responses and not for S10 adapted responses. In other words, awake immobility increased unadapted High intensity peak2 responses. All peak 2 responses adapted significantly between S1and S10 except for Low-intensity responses during active exploration. This effect seems to be due to the larger variability of S1 low-intensity responses compared with S10 responses during active exploration, which may be caused by repetition of the stimulus trains since Low responses evoked by single stimuli protocols were not as variable during active exploration.
Peak3 responses in 10 Hz trains were significantly influenced by spontaneous firing. In particular, Low and High intensity peak3 responses evoked by either S1 or S10 increased during active exploration, but after spontaneous firing was subtracted only Low-intensity responses evoked by S1 were significantly enhanced by active exploration (p < 0.01). None of the peak 3 responses adapted significantly between S1and S10, except for Low-intensity responses during active exploration after subtraction of spontaneous firing (p = 0.04).
Because superior colliculus spontaneous firing rate increased in active animals, our conclusions are based on evoked responses corrected by subtracting the spontaneous firing. Moreover, the conclusions about low-frequency stimuli are taken from single stimuli protocols because 10 Hz trains tended to affect the S1 response in a train. We found that Low-frequency peak1 MUA responses were enhanced by awake immobility but not by intensity, while high-frequency peak1 responses were enhanced by intensity but not by awake immobility, and only low-intensity peak1 responses during awake immobility adapted. Low-frequency peak2 MUA responses were enhanced by awake immobility and by intensity, while high-frequency peak2 responses were not affected by either behavioral state or intensity, and most peak2 responses adapted. Active exploration enhanced only peak3 MUA responses evoked by Low-intensity and low-frequency stimuli, and intensity only enhanced low-frequency responses during awake immobility. Finally, only Low-intensity peak3 responses evoked during active exploration adapted.
Effects of WCS on FP responses in barrel cortex during High/Low active avoidance task
We recorded neural activity within the barrel cortex and superior colliculus as animals performed the High/Low active avoidance task. Activity in barrel cortex was monitored with a FP electrode (n = 7 animals), and activity in superior colliculus was monitored with a FP electrode (n = 2 animals) or a MUA electrode (n = 5 animals) from which we also obtained FP data. The animals used in these experiments were previously trained in the High only avoidance task for 3–4 sessions (50–80 trials/session), during which they learned to successfully avoid the presentation of a mild aversive stimulus (footshock) by detecting the High WCS. A typical High/Low WCS session consisted of High and Low WCS trials (50–80 total trials distributed equally) presented pseudo-randomly. Similar to the behavioral data presented above, avoidance rates for the High WCS trials were significantly greater than for the low WCS trials (n = 28 sessions, High WCS avoidances: 71.6% vs Low WCS avoidances: 42.3%; p < 0.01).
We compared High and Low WCS-evoked responses between trials where the animal produced successful avoidance behavior (Avoids) with trials during which the animal did not produce the avoidance response, and thus escaped (Escapes). Since trials can last different periods of time depending on when and if the animal avoided, the responses evoked by the first 10 WCS in a trial (from WCS onset; S1–S10) and the responses evoked by the last 10 WCS during the avoidance interval in a trial (from WCS offset; E10–E1, where E1 is last) were measured. Response parameters were measured as in the previous sections (see Materials and Methods). For each measurement, we made three main statistical comparisons. First, we determined the effect of avoidance for High and Low stimuli by testing whether onset and offset evoked responses (first and last 10 stimuli) differed significantly between avoids and escapes. Second, we determined the effect of stimulus intensity for avoids and escapes by testing whether onset and offset evoked responses (first and last 10 stimuli) differed significantly between High and Low stimuli. Finally, since responses from WCS offset were generally already adapted, we determined the effect of frequency adaptation on responses from WCS onset by comparing the S1 and S10 responses for High, Low, avoids, and escapes.
Figure 7A shows population data (n = 28 sessions) of the peak amplitude of FP responses in barrel cortex during performance in the High/Low active avoidance task. Regarding the effect of avoidance, for the responses from WCS onset, there was a significant difference between avoids and escapes for Low stimuli (p < 0.01) but not for High stimuli (p = 0.2). Moreover, the avoidance effect depended on the stimulus number in the Low train (p < 0.01) so that only S9 and S10 Low responses were significantly different between avoids and escapes (p < 0.01). For the responses from WCS offset, there was a significant difference between avoids and escapes for Low stimuli (p < 0.01) and for High stimuli (p < 0.01). Thus, the peak amplitude of adapted barrel cortex responses are able to distinguish between avoids and escapes, so that barrel cortex FP responses adapt significantly more during avoids than during escapes. Regarding the effect of stimulus intensity, we found that High responses were always significantly larger than Low responses for either avoids or escapes from WCS onset or offset (p < 0.01). Finally, there was significant adaptation between S1 and S10 for High and Low avoids and escapes from WCS onset (p < 0.01).
Figure 7.
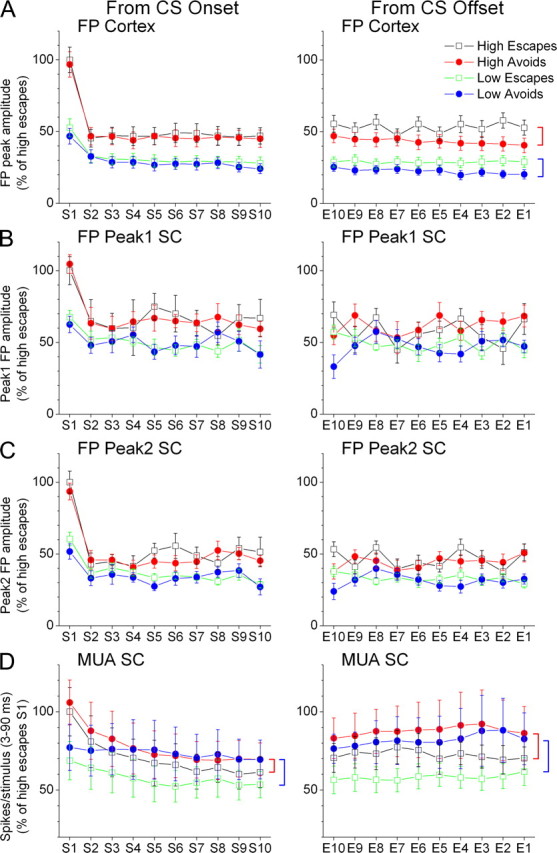
FP and MUA activity in barrel cortex and superior colliculus evoked by the WCS during performance in the High/Low active avoidance task. A–D, Cortex FP responses (A), and superior colliculus (SC) FP peak1 (B) and FP peak2 (C), and superior colliculus MUA (D) responses evoked by High and Low WCS (10 Hz) that lead to either avoids or escapes in the task. The responses are plotted from CS onset, which includes the first 10 stimuli in the WCS (s1–s10), and from WCS offset, which includes the last 10 stimuli in the WCS before avoids or before the onset of the escape interval for escapes (E10–E1, where E1 is the last stimulus). Statistically significant differences (p < 0.05) between avoids and escapes are marked with vertical brackets on the right of the figure. A red bracket indicates a significant difference between High avoids and escapes, and a blue bracket indicates a significant difference between Low avoids and escapes.
The time to peak of FP responses in barrel cortex was less affected than the peak amplitude. In particular, regarding the effect of avoidance, there was a significant difference between avoids and escapes for High responses from WCS onset (p < 0.01) and somewhat for High responses from WCS offset (p = 0.04). Thus, avoids evoked by High stimuli produced faster peaking FP responses than escapes evoked by High stimuli. Regarding the effect of stimulus intensity, High responses were faster than Low responses evoked from WCS onset (p < 0.05) but not for responses evoked from WCS offset. Thus, the peak latency of adapted FP responses does not code stimulus intensity. Regarding frequency adaptation, the time to peak of FP responses did not reflect significant adaptation.
In conclusion, WCS onset responses cannot distinguish avoids from escapes. However, the peak amplitude of adapted FP responses in barrel cortex, taken from WCS offset, readily distinguish between avoids and escapes because during avoids responses are more suppressed (adapted) than during escapes. Stimulus intensity is coded by the FP peak amplitude from WCS onset or offset.
Effects of WCS on FP responses in superior colliculus during High/Low active avoidance task
Figure 7B shows population data (n = 19 sessions) of peak1 FP responses in superior colliculus during performance in the High/Low active avoidance task. Regarding the effect of avoidance, there was no significant difference between avoids and escapes for Low or High stimuli from either WCS onset or offset. Thus, peak1 superior colliculus responses are unable to distinguish between avoids and escapes. Regarding the effect of stimulus intensity, we found that High responses were always significantly larger than Low responses for either avoids or escapes from WCS onset (p < 0.01) but only for avoids (p < 0.01) during WCS offset. Finally, there was significant adaptation between S1 and S10 for High and Low avoids and escapes from WCS onset (p < 0.05).
Figure 7C shows population data (n = 19 sessions) of peak2 FP responses in superior colliculus during performance in the High/Low active avoidance task. Regarding the effect of avoidance, there was no significant difference between avoids and escapes for Low or High stimuli from either WCS onset or offset. Thus, peak2 superior colliculus responses are unable to distinguish between avoids and escapes. Regarding the effect of stimulus intensity, we found that High responses were always significantly larger than Low responses for either avoids or escapes from WCS onset (p < 0.01) but only for avoids (p < 0.01) during WCS offset. Finally, there was significant adaptation between S1 and S10 for High and Low avoids and escapes from WCS onset (p < 0.05).
In conclusion, neither peak1 nor peak2 FP WCS responses in superior colliculus can distinguish between avoids and escapes. The inability of peak2 responses to differentiate between avoids and escapes contrasts with the ability of barrel cortex FP responses, which drive peak2 responses (Cohen et al., 2008), to distinguish between avoids and escapes. However, this may be due to the fact that cortical responses evoked during either escapes or avoids from WCS offset are very adapted; too suppressed to drive sharp peak2 responses in superior colliculus.
Effects of WCS on MUA in superior colliculus during High/Low active avoidance task
For the MUA superior colliculus analysis during the High/Low task, we combined together into one group the 10 evoked responses (n = 20 sessions from 5 animals) and measured peak4 responses (3–90 ms), a large time-window which encompasses peak1, 2, and peak3 responses. This was done because it appeared that the main effect of the task on MUA was on the overall firing rate, and not on individual responses (also based on the FP data described above). Figure 7D shows population data of MUA responses in superior colliculus during performance in the High/Low active avoidance task for peak4 (3–90 ms) responses. Thus, we determined the effect of avoidance, stimulus intensity, and their interaction. We found that MUA from either WCS onset or offset was larger for avoids than for escapes evoked by either Low (p < 0.01) or High (p < 0.01) WCS. Thus, superior colliculus activity ramps up during avoids compared with escapes. Moreover, the effect of intensity depended on avoidance, so that High WCS produced stronger MUA than Low WCS during escapes (p < 0.01) but not during avoids (p = 0.4). These results indicate that overall MUA in superior colliculus codes effective performance in the High/Low task by ramping up during avoids.
Discussion
The present results indicate that trigeminotectal and trigeminothalamic pathways can independently detect a High WCS during active avoidance behavior but must work together to detect a Low WCS. Thus, synergy between these neural pathways is required for the detection of stimuli of low psychophysical saliency. Electrophysiological recordings from the superior colliculus and barrel cortex (the target of the trigeminothalamic pathway) of freely behaving animals revealed how psychophysical salience, active exploration, awake immobility, and sensory detection in the active avoidance task affect the neural responses of these pathways. Briefly, the main findings are: (1) FP responses evoked in superior colliculus and barrel cortex by high intensity stimuli are larger and adapt more to frequency than those evoked by low-intensity stimuli, and as animals transition between awake immobility to active exploration FP responses become suppressed and adapt less. (2) Firing rate increase in superior colliculus during active exploration compared with awake immobility. (3) MUA responses evoked in superior colliculus are generally larger during active exploration than during awake immobility, but only high-frequency trigeminotectal (peak1) and low-frequency corticotectal responses (peak2) increase with stimulus intensity. (4) During active avoidance behavior, FP responses in barrel cortex taken from WCS offset are more suppressed (adapted) for avoids than for escapes, but FP response in superior colliculus are not different between avoids and escapes. However, superior colliculus firing rate ramps up during avoids compared with escapes. Thus, superior colliculus codes effective performance in the avoidance task by ramping up during avoids. Below, we discuss the requirement for synergy during detection of low saliency stimuli, the coding of active avoidance behavior in cortex and superior colliculus, and how transitions in behavioral state impact sensory responses in superior colliculus.
Detection of low salience stimuli requires tectal and thalamic synergy
The reversible inactivation experiments revealed that while trigeminotectal or trigeminothalamic pathways can independently detect a highly salient sensory stimulus during performance of an active avoidance task, these pathways must work in synergy to detect a stimulus of low psychophysical saliency. The need for synergy may be due to different neural coding schemes in barrel cortex and superior colliculus during sensory detection. In particular, electrophysiological recordings during performance in the High/Low active avoidance task revealed that whereas barrel cortex FP responses code stimulus saliency and successful responses in the task (i.e., avoids), superior colliculus FP responses (peak1 and peak2) code stimulus saliency but not successful responses in the task. However, superior colliculus MUA coded successful responses in the task by enhancing the overall firing rate during avoids.
Low-intensity stimuli may not be detectable by the barrel cortex or superior colliculus alone because the neural responses they evoke are weak and dispersed. In the case of the superior colliculus, this sparse code is further reduced by blocking the cortical feedback during trigeminothalamic inactivation. Indeed, we previously showed that superior colliculus responses to single-whisker stimuli, which are comparable to low-intensity stimuli used here, are suppressed by cortical inactivation (Cohen et al., 2008). Therefore, the superior colliculus may require cortical feedback to enhance sensory responses for successful detection of low salient stimuli.
The reason why the cortex alone is incapable of detecting low-intensity stimuli is not so obvious because the barrel cortex codes both stimulus intensity and successful avoids, and it is not clear how superior colliculus inactivation may influence cortical processing. We speculate that superior colliculus and cortical outputs triggered by the sensory stimulus converge in another structure (see below) where they drive activity that mediates sensory detection and triggers the avoid locomotion. In the absence of neural activity in the superior colliculus (during trigeminotectal inactivation), the cortical activity produced by low-intensity stimuli may be ineffective at driving this structure, and hence the effective detection of the stimulus and successful avoids. Indeed, the enhanced firing tone in superior colliculus during avoids would fulfill this putative role effectively.
But what may be the structure where cortical and tectal outputs converge? One possibility is that the superior colliculus and cortical outputs converge in the basal ganglia. Both the superior colliculus and cortex form loops through the basal ganglia (McHaffie et al., 2005; Redgrave and Gurney, 2006), and the basal ganglia appears to be critically involved in active avoidance behavior (Delacour et al., 1977; Chavez-Martinez et al., 1987). Another possibility is that superior colliculus and cortex converge in the brainstem. The superior colliculus has robust descending projections to brainstem that give rise to orienting and escape responses (Sprague and Meikle, 1965; Schneider, 1969; Sparks, 1986; Dean et al., 1989; Westby et al., 1990; Redgrave et al., 1993; Stein and Meredith, 1993), and the cortex also projects extensively in the brainstem.
Previous studies in humans, macaques, and rats indicate that lesions of sensory cortices reduce the psychophysical salience (increase detection threshold) of stimuli compared with controls (Roland, 1987; Cooke et al., 2007; Hayes and Merigan, 2007). In other studies, superior colliculus lesions have been shown to disrupt orientation to low salient visual stimuli, while having little effect on the detection of highly salient stimuli (Midgley and Tees, 1981; Midgley et al., 1988). It is also known that lesions of the barrel cortex impair surface discrimination and gap crossing behaviors (Hutson and Masterton, 1986; Guic-Robles et al., 1992). However, the effects of cortical lesions must be taken cautiously because of the possibility that a “Sprague effect” occurs (Sprague, 1966). In this case, the cortical lesion actually interferes with the normal activity of the superior colliculus leading to the observed behavioral deficits (Loop and Sherman, 1977; Sherman, 1977; Ciaramitaro et al., 1997; Jiang et al., 2003). But a Sprague effect is not expected in our study, because lesions of the trigeminothalamic pathway were done in the thalamus, not the cortex. Our results further indicate that thalamic and tectal relays are not specialized for the detection of low salient stimuli. Instead, these regions must work in synergy to detect low salient stimuli. We conclude that detection of low salient stimuli requires a sparse neural code distributed along multiple sensory relays.
Barrel cortex and superior colliculus code active avoidance in different ways
Electrophysiological recordings during active avoidance revealed that FP responses in barrel cortex taken from WCS offset are more suppressed (adapted) during avoids. In barrel cortex, stronger sensory suppression occurs in vigilant and attentive animals (Castro-Alamancos, 2004a,b) and leads to focusing of cortical representations during states of cortical activation (Castro-Alamancos, 2002; Castro-Alamancos and Oldford, 2002). Thus, it is not surprising that effective performance in the task is associated with stronger sensory suppression in barrel cortex. However, in superior colliculus, FP responses did not differ between avoids and escapes indicating that phasic responses evoked by the WCS do not code performance in the task. Instead, in superior colliculus, the tonic MUA firing was consistently higher during avoids; MUA ramped up during avoids. The superior colliculus is well known to be involved in orienting, approach, and escape responses to stimuli from a wide range of modalities, including somatosensory, auditory, and visual (Sprague and Meikle, 1965; Schneider, 1969; Meredith and Stein, 1985; Sparks, 1986; Dean et al., 1989; Stein and Meredith, 1993; Stein, 1998; Redgrave and Gurney, 2006). In line with this model, tonic level of MUA in superior colliculus was enhanced during successful sensory detections (avoids) compared with unsuccessful detections (escapes). Such tonic firing may be useful for driving the target structure where barrel cortex and superior colliculus activity converge to drive avoidance responses. Together, these results indicate that a tonic level of superior colliculus firing rate may be critical for successful sensory detection and processing of behaviorally significant stimuli that require immediate action.
Behavioral state affects responses evoked by sensory stimuli
Electrophysiological recordings in freely behaving animals during spontaneous behavior allowed us to monitor high- and low-intensity sensory responses in barrel cortex and superior colliculus as animals transitioned between awake immobility and active exploration. These experiments revealed that barrel cortex and superior colliculus (peak1, peak2, peak3) FP responses code (i.e., represent) the psychophysical saliency of the stimulus (i.e., increase with intensity) and the state of the animal during wakefulness (i.e., decrease during active exploration). Also, FP responses adapt significantly during awake immobility, but are already adapted during active exploration (Castro-Alamancos, 2004b). Finally, while evoked FP responses effectively code the awake behavioral states, the power spectrum of spontaneous FP activity was not different between awake immobility and active exploration.
Evoked MUA responses in superior colliculus behaved somewhat different from FP responses. Spontaneous MUA in superior colliculus was enhanced during active exploration, and the coding of superior colliculus responses was quite complex. Low-frequency peak1 responses, which reflect a direct trigeminotectal input (Cohen et al., 2008), coded the state of the animal (i.e., decrease during active exploration) but not the psychophysical saliency of the stimulus, while high-frequency peak1 responses coded the psychophysical saliency of the stimulus (i.e., increase with intensity) but not the state of the animal. Low-frequency peak 2 responses, which reflect corticotectal feedback (Cohen et al., 2008), behaved like barrel cortex FP responses, coding the psychophysical saliency of the stimulus (i.e., increase with intensity) and the state of the animal during wakefulness (i.e., decrease during active exploration). Low-frequency peak3 MUA responses evoked by low-intensity stimuli were enhanced by active exploration. Regarding rapid sensory adaptation, superior colliculus (peak1 and peak2) MUA responses behaved like FP responses, adapting more during awake immobility than during active exploration because during active exploration responses are already adapted (Castro-Alamancos, 2004b).
Apparently, no previous studies have addressed the effect of behavioral state on superior colliculus neural responses in behaving rodents. Based on spontaneous firing, the superior colliculus appears to come online during active exploratory states and rapidly goes offline during quiescent awake periods, suggesting an important direct role in active sensory processing. An interesting finding is that different components of the superior colliculus response were affected differently by behavioral state, which argues that neural coding within the superior colliculus is rather complex. For instance, peak2 and peak3 MUA responses were affected by behavioral state in different directions. The suppressing effect of active exploration on peak2 responses is in line with our previous observation that peak2 responses are also suppressed during forebrain activation in anesthetized animals (Cohen et al., 2008). Thus, the strongest output of whisker-sensitive cells in the intermediate layers of the superior colliculus occurs for salient stimuli during quiescent states. This appears useful as a powerful alerting stimulus in an animal that is sleeping, drowsy, or unattentive, and an unknown moving object or animal makes contact with its whiskers. Because the target of these cells in deeper layers and in the brainstem drive orienting responses (Redgrave et al., 1987a; Dean et al., 1989; Westby et al., 1990), such a powerful alerting output makes good functional sense. Moreover, this enhanced output during quiescence may also serve to trigger forebrain activation in quiescent animals by impacting on neuromodulatory systems in the midbrain and brainstem that cause cortical activation (Castro-Alamancos, 2004a), which are well known targets of superior colliculus cells (Redgrave et al., 1987b, 1993; Dean et al., 1989). We also found that peak3 (long-latency) MUA responses evoked by Low-intensity stimuli were enhanced by active exploration. This indicates that the superior colliculus ability to detect low saliency signals is enhanced by active exploration, which further supports the tenet that the superior colliculus' ability to detect low saliency stimuli depends on higher-order activity feeding back to the superior colliculus (i.e., long latency responses), most likely from higher-order cortical areas. Thus, cortical feedback appears crucial for the superior colliculus to be able to detect low saliency stimuli, as we found in the lesion experiments.
Footnotes
This work was supported by the National Institutes of Health.
References
- Bruce LL, McHaffie JG, Stein BE. The organization of trigeminotectal and trigeminothalamic neurons in rodents: a double-labeling study with fluorescent dyes. J Comp Neurol. 1987;262:315–330. doi: 10.1002/cne.902620302. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Role of thalamocortical sensory suppression during arousal: focusing sensory inputs in neocortex. J Neurosci. 2002;22:9651–9655. doi: 10.1523/JNEUROSCI.22-22-09651.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Dynamics of sensory thalamocortical synaptic networks during information processing states. Prog Neurobiol. 2004a;74:213–247. doi: 10.1016/j.pneurobio.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Absence of rapid sensory adaptation in neocortex during information processing states. Neuron. 2004b;41:455–464. doi: 10.1016/s0896-6273(03)00853-5. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Connors BW. Thalamocortical synapses. Prog Neurobiol. 1997;51:581–606. doi: 10.1016/s0301-0082(97)00002-6. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Oldford E. Cortical sensory suppression during arousal is due to the activity-dependent depression of thalamocortical synapses. J Physiol. 2002;541:319–331. doi: 10.1113/jphysiol.2002.016857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez-Martínez ME, Reyes-Vázquez C, Brust-Carmona H. Lever pressing and active avoidance conditioning after electrolytic lesions of the entopeduncular nucleus in cats. Brain Res Bull. 1987;18:279–284. doi: 10.1016/0361-9230(87)90003-7. [DOI] [PubMed] [Google Scholar]
- Ciaramitaro VM, Todd WE, Rosenquist AC. Disinhibition of the superior colliculus restores orienting to visual stimuli in the hemianopic field of the cat. J Comp Neurol. 1997;387:568–587. doi: 10.1002/(sici)1096-9861(19971103)387:4<568::aid-cne7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Castro-Alamancos MA. Early sensory pathways for detection of fearful conditioned stimuli: tectal and thalamic relays. J Neurosci. 2007;27:7762–7776. doi: 10.1523/JNEUROSCI.1124-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Hirata A, Castro-Alamancos MA. Vibrissa sensation in superior colliculus: wide-field sensitivity and state-dependent cortical feedback. J Neurosci. 2008;28:11205–11220. doi: 10.1523/JNEUROSCI.2999-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor CE, Egeth HE, Yantis S. Visual attention: bottom-up versus top-down. Curr Biol. 2004;14:R850–R852. doi: 10.1016/j.cub.2004.09.041. [DOI] [PubMed] [Google Scholar]
- Cooke JE, Zhang H, Kelly JB. Detection of sinusoidal amplitude modulated sounds: deficits after bilateral lesions of auditory cortex in the rat. Hear Res. 2007;231:90–99. doi: 10.1016/j.heares.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Dean P, Redgrave P, Westby GW. Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci. 1989;12:137–147. doi: 10.1016/0166-2236(89)90052-0. [DOI] [PubMed] [Google Scholar]
- Delacour J, Echavarria MT, Senault B, Houcine O. Specificity of avoidance deficits produced by 6-hydroxydopamine lesions of the nigrostriatal system of the rat. J Comp Physiol Psychol. 1977;91:875–885. doi: 10.1037/h0077367. [DOI] [PubMed] [Google Scholar]
- de Lafuente V, Romo R. Neuronal correlates of subjective sensory experience. Nat Neurosci. 2005;8:1698–1703. doi: 10.1038/nn1587. [DOI] [PubMed] [Google Scholar]
- Guic-Robles E, Jenkins WM, Bravo H. Vibrissal roughness discrimination is barrelcortex-dependent. Behav Brain Res. 1992;48:145–152. doi: 10.1016/s0166-4328(05)80150-0. [DOI] [PubMed] [Google Scholar]
- Hayes RD, Merigan WH. Mechanisms of sensitivity loss due to visual cortex lesions in humans and macaques. Cereb Cortex. 2007;17:1117–1128. doi: 10.1093/cercor/bhl021. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Frankfurter A, Harting JK. Studies of the principal sensory and spinal trigeminal nuclei of the rat: projections to the superior colliculus, inferior olive, and cerebellum. J Comp Neurol. 1983;220:147–167. doi: 10.1002/cne.902200204. [DOI] [PubMed] [Google Scholar]
- Hutson KA, Masterton RB. The sensory contribution of a single vibrissa's cortical barrel. J Neurophysiol. 1986;56:1196–1223. doi: 10.1152/jn.1986.56.4.1196. [DOI] [PubMed] [Google Scholar]
- Jiang H, Stein BE, McHaffie JG. Opposing basal ganglia processes shape midbrain visuomotor activity bilaterally. Nature. 2003;423:982–986. doi: 10.1038/nature01698. [DOI] [PubMed] [Google Scholar]
- Johnson KO, Hsiao SS. Neural mechanisms of tactual form and texture perception. Annu Rev Neurosci. 1992;15:227–250. doi: 10.1146/annurev.ne.15.030192.001303. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Erzurumlu RS. Trigeminal projections to the superior colliculus of the rat. J Comp Neurol. 1981;201:221–242. doi: 10.1002/cne.902010207. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Ahissar E, Diamond ME. Active sensation: insights from the rodent vibrissa sensorimotor system. Curr Opin Neurobiol. 2006;16:435–444. doi: 10.1016/j.conb.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental components of attention. Annu Rev Neurosci. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Loop MS, Sherman SM. Visual discriminations of cats with cortical and tectal lesions. J Comp Neurol. 1977;174:79–88. doi: 10.1002/cne.901740106. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Stanford TR, Stein BE, Coizet V, Redgrave P. Subcortical loops through the basal ganglia. Trends Neurosci. 2005;28:401–407. doi: 10.1016/j.tins.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Descending efferents from the superior colliculus relay integrated multisensory information. Science. 1985;227:657–659. doi: 10.1126/science.3969558. [DOI] [PubMed] [Google Scholar]
- Midgley GC, Tees RC. Orienting behavior by rats with visual cortical and subcortical lesions. Exp Brain Res. 1981;41:316–328. doi: 10.1007/BF00238889. [DOI] [PubMed] [Google Scholar]
- Midgley GC, Wilkie DM, Tees RC. Effects of superior colliculus lesions on rats' orienting and detection of neglected visual cues. Behav Neurosci. 1988;102:93–100. doi: 10.1037//0735-7044.102.1.93. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. Perceptual neuroscience. The cerebral cortex. Cambridge, MA: Harvard UP; 1998. [Google Scholar]
- Mountcastle VB, Talbot WH, Sakata H, Hyvärinen J. Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J Neurophysiol. 1969;32:452–484. doi: 10.1152/jn.1969.32.3.452. [DOI] [PubMed] [Google Scholar]
- Parker AJ, Newsome WT. Sense and the single neuron: probing the physiology of perception. Annu Rev Neurosci. 1998;21:227–277. doi: 10.1146/annurev.neuro.21.1.227. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Gurney K. The short-latency dopamine signal: a role in discovering novel actions? Nat Rev Neurosci. 2006;7:967–975. doi: 10.1038/nrn2022. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Mitchell IJ, Dean P. Further evidence for segregated output channels from superior colliculus in rat: ipsilateral tecto-pontine and tecto-cuneiform projections have different cells of origin. Brain Res. 1987a;413:170–174. doi: 10.1016/0006-8993(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Mitchell IJ, Dean P. Descending projections from the superior colliculus in rat: a study using orthograde transport of wheatgerm-agglutinin conjugated horseradish peroxidase. Exp Brain Res. 1987b;68:147–167. doi: 10.1007/BF00255241. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Westby GW, Dean P. Functional architecture of rodent superior colliculus: relevance of multiple output channels. Prog Brain Res. 1993;95:69–77. doi: 10.1016/s0079-6123(08)60358-1. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Fish SE, Chiaia NL, Bennett-Clarke C, Mooney RD. Organization of the projections from the trigeminal brainstem complex to the superior colliculus in the rat and hamster: anterograde tracing with Phaseolus vulgaris leucoagglutinin and intra-axonal injection. J Comp Neurol. 1989;289:641–656. doi: 10.1002/cne.902890409. [DOI] [PubMed] [Google Scholar]
- Roland PE. Somatosensory detection in patients with circumscribed lesions of the brain. Exp Brain Res. 1987;66:303–317. doi: 10.1007/BF00243307. [DOI] [PubMed] [Google Scholar]
- Schneider GE. Two visual systems. Science. 1969;163:895–902. doi: 10.1126/science.163.3870.895. [DOI] [PubMed] [Google Scholar]
- Sherman SM. The effect of superior colliculus lesions upon the visual fields of cats with cortical ablations. J Comp Neurol. 1977;172:211–229. doi: 10.1002/cne.901720203. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Functional organization of thalamocortical relays. J Neurophysiol. 1996;76:1367–1395. doi: 10.1152/jn.1996.76.3.1367. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus. Physiol Rev. 1986;66:118–171. doi: 10.1152/physrev.1986.66.1.118. [DOI] [PubMed] [Google Scholar]
- Sprague JM. Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science. 1966;153:1544–1547. doi: 10.1126/science.153.3743.1544. [DOI] [PubMed] [Google Scholar]
- Sprague JM, Meikle TH., Jr The role of the superior colliculus in visually guided behavior. Exp Neurol. 1965;11:115–146. doi: 10.1016/0014-4886(65)90026-9. [DOI] [PubMed] [Google Scholar]
- Stein BE. Neural mechanisms for synthesizing sensory information and producing adaptive behaviors. Exp Brain Res. 1998;123:124–135. doi: 10.1007/s002210050553. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. The merging of the senses. Cambridge, MA: MIT; 1993. [Google Scholar]
- Treue S. Visual attention: the where, what, how and why of saliency. Curr Opin Neurobiol. 2003;13:428–432. doi: 10.1016/s0959-4388(03)00105-3. [DOI] [PubMed] [Google Scholar]
- Veazey RB, Severin CM. Afferent projections to the deep mesencephalic nucleus in the rat. J Comp Neurol. 1982;204:134–150. doi: 10.1002/cne.902040204. [DOI] [PubMed] [Google Scholar]
- Veinante P, Jacquin MF, Deschênes M. Thalamic projections from the whisker-sensitive regions of the spinal trigeminal complex in the rat. J Comp Neurol. 2000;420:233–243. doi: 10.1002/(sici)1096-9861(20000501)420:2<233::aid-cne6>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Westby GW, Keay KA, Redgrave P, Dean P, Bannister M. Output pathways from the rat superior colliculus mediating approach and avoidance have different sensory properties. Exp Brain Res. 1990;81:626–638. doi: 10.1007/BF02423513. [DOI] [PubMed] [Google Scholar]