Abstract
Digit and interdigit (D/ID) development is one of the important research fields in molecular developmental biology. Interdigital cell death (ICD) is a morphogenetic event which has been considered as an essential process for D/ID formation. Although, some growth factors including Bmp and Fgf signaling can modulate ICD, growth factor crosstalk regulating ICD is poorly understood. Wnt canonical pathway and Bmp signal crosstalk has been considered as the essential growth factor crosstalk in organogenesis. To elucidate the crosstalk to regulate the D/ID formation, we analyzed conditional mutant mice with limb bud ectoderm expressing constitutively activated β-catenin signaling. We showed that modulation of Wnt/β–catenin signal in the limb ectoderm including the AER regulates ID apoptosis. We also demonstrated that Wnt/β–catenin signaling in the ectoderm can positively regulate Fgf8 possibly antagonizing the epithelial derived Bmp signaling. Human birth defects for digit abnormalities have been known to be affected by multiple parameters. Elucidation of the potential mechanisms underlying such D/ID development is an urgent medical issue to be solved. This work would be one of the first studies showing essential growth factor cascades in the D/ID formation.
Keywords: Bone morphogenetic protein (Bmp), Wnt/β–catenin, Fgf8, digit, interdigit, limb
Introduction
Studies on digit and interdigit (D/ID) development is one of the important research fields in molecular developmental biology and medicine. Interdigital cell death (ICD) is a morphogenetic event that leads to the individualization of digits necessary for proper limb development. Regulation of ICD has been considered as an important developmental process for several decades [1]. The involvement of growth factors for its regulation has been suggested in the case of Bone morphogenetic protein (Bmp) [2]. Growth factor crosstalk is one of the frequently studied growth factor cascades during organogenesis including limb development [3; 4]. However, the involvement of growth factor crosstalk for organogenesis including the Bmp signal has been only recently shown by utilizing conditional compound genetics [5; 6]. This is because of the recent innovation of modulating growth factor responses by conditional loss or gain of function mutations [7]. In fact, the development of several Cre driver mice lines has opened a way for such conditional compound genetics applied for organogenesis [8; 9]. Bmp signaling can modulate ICD by modulating the Fgf signals [5]. Several studies have shown that Fgf signaling is necessary for cell survival and outgrowth during organogenesis [10; 11]. Studies on compound mutants revealed the crosstalk between Fgf/Bmp signaling. However, multiple signal cascades remain unelucidated for the regulation of D/ID formation. Wnt canonical pathway and Bmp signal crosstalk has been considered as one of the essential growth factor crosstalk in other organogenesis. The involvement of such crosstalk to regulate the D/ID formation has been unexplored and thus investigated by the current study.
Developmental abnormalities for D/ID formation have been well recognized as human birth defects among young infants [12]. Numerous phenotypes including polydactyly, syndactyly have been described [13]. Human birth defects for digit abnormalities have been known to be affected by multiple parameters. However, the majority of those mechanisms as underlying such defects are still unelucidated. Analyzing the variety of human digit abnormalities based on the pathogenetic process of ICD would be an essential strategy to understand human birth defects. Hence, elucidation of the potential mechanisms underlying such D/ID development is an urgent medical issue to be solved. In the current study, we utilized a constitutively activated β–catenin mouse model by utilizing a conditional Cre driver mouse line. We asked a question whether the modulation of Wnt/β–catenin signal in the limb ectoderm including the AER (Apical Ectodermal Ridge) regulates Fgf's signaling and ID apoptosis. We also found that K5Cre-Catnb(ex3)fl/+BmprIAfl/fl (with additional mutation of Bmp signaling) mutant embryos display more severe defects of ID regression. Our data utilizing a compound mutant suggests that Wnt/β–catenin signaling in ectoderm can positively regulate Fgf8 possibly antagonizing the Bmp signaling within AER. This work would be one of the first studies showing essential growth factor crosstalk in the D/ID formation. These results provide solid mass of data to understand the mechanisms underlying the D/ID abnormalities.
Materials and Methods
Mice
The mutant alleles used herein were Catnb(ex3)fl/+ [14], BmprIA [15], K5Cre [16], R26R [17] and BATLacZ [18]. Experimental procedures and protocols for mouse studies were approved by the Committee on the Animal Research of the Kumamoto University. Embryos for each experiment were collected from more than three independent pregnant females. The day on which a vaginal plug was detected was designated as E0.5.
Histology, LacZ, LysoTracker staining, and Whole mount in situ hybridization for gene expression analysis
Hematoxylin and eosin staining and LacZ staining were performed by standard procedures as previously described [19]. LysoTracker Red staining was done as suggested by the manufacturer's protocol (Molecular Probes). RNA expression analysis by whole mount in situ hybridization was performed as previously described [20]. Riboprobes were synthesized using the DIG RNA labeling kit (Roche) according to the manufacturer's recommendations. The probes used were Fgf8 and Bmp2 (provided by Dr. B. L. Hogan), Bmp4 [21], Bmp7 (provided by Dr. M. Yoshida), and Msx2 (provided by Dr. Y. Liu).
Results
Dynamic activation of Wnt/β–catenin signaling during limb development
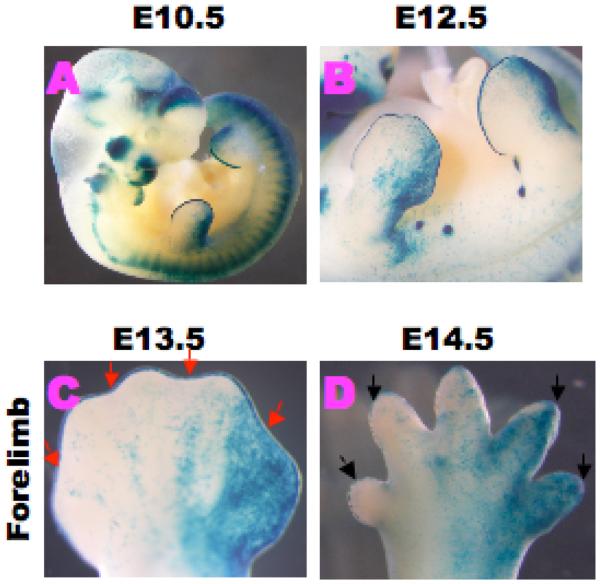
Dynamic activation of Wnt/β–catenin signaling during limb formation has been reported [22; 23]. In order to elucidate the activation status of Wnt/β–catenin signaling, we utilized canonical Wnt/β–catenin signaling indicator mice to monitor the activities during D/ID formation (Fig.1). BATLacZ is the mouse strain to monitor Wnt/β–catenin signaling [6; 18]. LacZ activity detected by the BATLacZ monitoring mouse line indicated the presence of Wnt/β–catenin signaling in the AER during E10.5 and E12.5 (Fig. 1A,B). At E13.5, Wnt/β–catenin signaling in the AER adjacent to the ID region (AER /ID) was reduced (red arrows; Fig.1C). Later, prominent LacZ staining was still detected in the distal digit tip at E14.5 (black arrows; Fig.1D).
Figure 1. Dynamic expression of Wnt/β-catenin signaling during limb development.
LacZ activity is detected by BATLacZ monitoring mouse line in the AER of the limb buds. (A-D) BATLacZ activity is detected in the AER and posterior limb surface ectoderm from E10.5 to E14.5. (C) At E13.5, BATLacZ activity in the limb bud is reduced in the AER (red arrows). (D) At E14.5, BATLacZ activity is detected in the distal digit tip of the limb bud (black arrows).
Digit and interdigit abnormalities induced by β–catenin stabilization by the conditional mutation of K5Cre-Catnb(ex3)fl/+
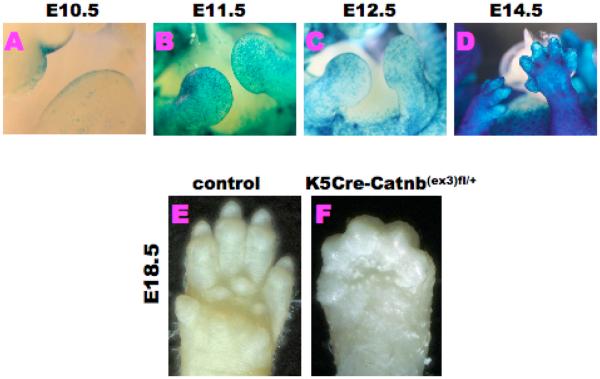
In order to examine the function and phenotype of altered Wnt/β–catenin signaling in D/ID formation, we examined the phenotype of Wnt/β–catenin gain of function (GOF) mutant embryos using constitutively activated β-catenin signal allele (Catnb(ex3)fl/+) [14]. To perform such conditional gene mutation, we utilized Keratin 5 (K5) Cre mouse line to achieve epithelial specific target gene recombination [16]. Cre activities were detected in the limb AER and in the limb surface ectoderm during E10.5-E14.5 (Fig. 2A-D). We then next examined the resultant phenotype of K5Cre-Catnb(ex3)fl/+ embryos (Fig. 2F). Intriguingly, prominent digit fusion and abnormal ID phenotype was observed in such conditional mutants both in the forelimbs (FL) (Fig. 2F) and hindlimbs (HL; data not shown) at E18.5. The overall phenotype of such digital fusion was more severe in the case of forelimb rather than in the case of hindlimb.
Figure 2. Limb phenotypes of K5Cre-Catnb(ex3)fl/+ embryos.
(A) K5Cre LacZ activity at E10.5 is observed in the AER. (B-D) At E11.5 to E14.5, K5Cre activity is broadly detected in the limb surface ectoderm. (E,F) At E18.5, forelimb phenotypes of K5Cre-Catnb(ex3)fl/+ embryos show prominent digit fusion and failure of ID regression. Digital fusion is more prominent in the forelimb as compared to the hindlimb at E18.5 (data not shown).
Dynamic change of Wnt/β–catenin activities and alteration of cell death in the K5Cre-Catnb(ex3)fl/+ mutant limb buds
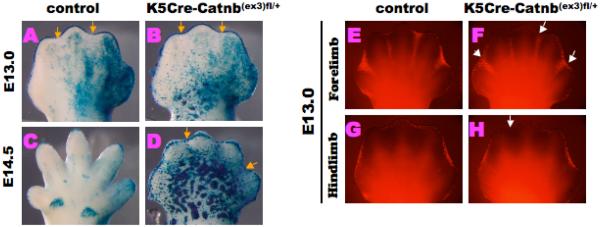
We next examined the kinetics of Wnt/β–catenin activities during the limb development in the control and K5Cre-Catnb(ex3)fl/+ mutant embryos. In the control embryos, the LacZ activity for the Wnt/β–catenin signaling in the AER/ID was reduced at E13.0 (yellow arrows; Fig. 3A). In contrast, the Wnt/β–catenin signaling in the AER/ID in the mutant limb was still activated at E13.0 and its activity was sustained at E14.5 (yellow arrows; Fig. 3B,D). In order to further investigate the mechanisms underlying the digit abnormality of the conditional mutants, we examined the status of apoptosis in the mutant mice limb buds. The conditional mutant mice displayed reduced rate of interdigital mesenchymal apoptosis adjacent to the AER at E13.0 (white arrows; Fig. 3F). The control embryos displayed already complete loss of ID region at E14.5. In contrast, the K5Cre-Catnb(ex3)fl/+ mutant limb buds displayed still prominent apoptosis at E14.5 in the ID region (data not shown). These results indicate that the elevated epithelial Wnt/β–catenin signaling affect the interdigital apoptosis.
Figure 3. Dynamic change of Wnt/β-catenin activities and alteration of cell death in the K5Cre-Catnb(ex3)fl/+ mutant limb buds.
(A) BATLacZ activity in the control is slightly reduced in the ID region (yellow arrows). (B) In contrast, its activity is still activated in the mutant AER and limb surface ectoderm at E13.0 (yellow arrows). (C, D) At E14.5, ID region is formed in the control limb but BATLacZ activity is sustained in the AER and its ectopic activation in the mutant limb is detected (yellow arrows). (E-H) At E13.0, apoptotic signal is observed in the ID region of the control and mutant limbs. (F-H) Apoptotic signal in the mutant AER is reduced as compared to the control (white arrows).
Alteration of Fgf8 and other marker genes upon dysregulated Wnt/β–catenin signaling
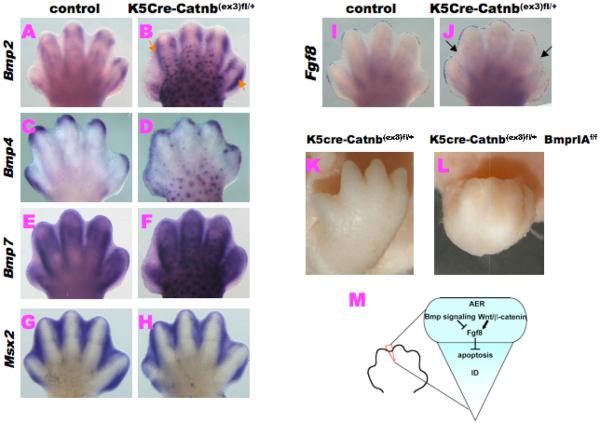
It has been suggested that several growth factors and marker genes can be regulated by the net readout of growth factor signaling. In order to analyze the consequences of altered Wnt/β–catenin signaling, we examined the expression of several marker genes during D/ID development (Fig. 4A-J). Bmp signaling is one of the essential growth factors for ID apoptosis. It has been known that several Bmp genes' expression is affected by the altered Wnt/β–catenin signaling [22]. Bmps 2, 4 and 7 are upregulated during ID regression, suggesting that these are candidate ligands for activating Bmp signaling [2; 5]. Therefore, we examined these expressions in the mutant limb buds. We found no significant differences between control and mutant ID region (Fig. 4A-F). The expression of Msx2, a downstream target of Bmp signaling was not changed in the mutant ID (Fig. 4G,H). Several Fgf genes have been suggested as playing major roles for the D/ID formation [24]. Among such Fgfs, Fgf8 expression status is examined in the GOF Wnt/β–catenin signaling. Intriguingly, sustained Fgf8 expression is still detected in the mutant ID region of the developing AER compared with the control embryos (black arrows; Fig. 4J). The functional interaction between Bmp and Wnt/β–catenin signaling is integrated in developmental processes [25]. To further analyze the signal crosstalk between Bmp and Wnt/β–catenin signaling within AER, we investigated the double mutant, K5Cre-Catnb(ex3)fl/+BmprIAfl/fl limbs. We found that the K5Cre-Catnb(ex3)fl/+BmprIAfl/fl limb displays more severe phenotype in the ID region as compared with the K5Cre-Catnb(ex3)fl/+ limbs (Fig. 4K,L).
Figure 4. Alteration of Fgf8 and other marker expression in K5Cre Catnb(ex3)fl/+ limbs.
Whole mount In situ hybridization of Bmp ligands, Msx2 and Fgf8 genes in the forelimbs at E13.5. (A-H) Bmps 2, 4 and 7 expressions in the mutant AER and ID regions do not show significant differences with those of the control limbs. (I) In control limb, Fgf8 expression is already lost in the ID region. (J) While in mutant limb, Fgf8 expression is sustained in the ID region (black arrows). (K, L) Double conditional mutant, K5Cre-Catnb(ex3)fl/+BmprIAf/f limbs, show more severe phenotype of ID compared with that of K5Cre-Catnb(ex3)fl/+ mutant limbs. (M) An illustration showing the possible interaction of Wnt/β-catenin, Bmp and Fgf signaling in the AER to regulate apoptosis in the ID.
Discussion
D/ID development has been one of the central issues of modern developmental biology [26; 27]. Numerous growth factors as well as other developmental regulators have been studied for such developmental processes [4; 23]. Of interest is the growth factor crosstalk for such morphogenesis. One of the fundamental regulations for D/ID morphogenesis is the regulation of apoptosis [24; 28]. Proper apoptosis in the developing ID region is the prerequisite for normal D/ID development. It has been shown that the adequate amount of Bmp signaling plays a vital role for such regulation [2; 5]. More recently, growth factor crosstalks involving Bmp signaling have been suggested by utilizing compound conditional mutant [5; 29]. In addition to such regulation of apoptosis, developmental regulation by the neighboring ectoderm, AER, has been suggested as important for limb and digit development [30]. However, the effect of such neighboring ectoderm (AER) on ICD has been still unelucidated. Ectodermally (dorsally) regulated Wnt/β–catenin signaling has been playing an important role for the regulation of AER formation during limb development. In the current study, we found dynamic change of Wnt/β–catenin activity during D/ID formation. The ectodermal influence, particularly the influence of ectodermal Wnt/β–catenin signaling on the ICD has been unelucidated. During normal D/ID developmental processes, regulation of Fgf8 is suggested as an important process in addition to its functions during early limb bud outgrowth. The role of Fgf8 in ID region including apoptotic regulation is a complex event [5; 28]. It has been known that Fgf8 is negatively regulating or antagonizing Bmp signaling in the developing ID region, which is considered as a result of the growth factor relay of Bmp/Fgf. It has been also suggested that context dependent Fgf signal input is also necessary and prerequisite for the proper ID apoptosis [5]. In the current study, we have performed compound conditional genetic mutant analyses modulating ectoderm specific GOF mutation for Wnt/β-catenin signaling. The results clearly show that upregulated Wnt/β-catenin signaling leads to the sustained Fgf8 expression in the corresponding ectodermal (AER) region. This result indicates the role of Fgf8 and corresponding AER region adjacent to the ID mesenchyme. It suggests that a concerted interaction between epithelia and mesenchyme is necessary for the development of AER region adjacent to ID mesencyhme and apoptotic ID mesenchyme itself. The mechanism underlying such sustained Fgf8 expression remains unelucidated. The regulatory mechanism of Fgf8 expression in the AER has been under investigation [7; 31]. ID phenotype of K5Cre-Catnb(ex3)fl/+BmprIAfl/fl double mutant was more severe than that of the K5Cre-Catnb(ex3)fl/+ mutant limbs. It is possible that the combination of Bmp and Wnt/β-catenin signaling exerts synergistic regulation for the ectodermal regulation of Fgf8 adjacent to the developing D/ID. Further studies are necessary to elucidate such molecular mechanisms of ID regression and the associated Fgf8 regulation.
Digit abnormalities in the affected human newborn infants have been extensively studied genetically [12]. Several classes of genes have been identified as underlying such abnormalities. Among such birth defects, digit abnormalities due to the dysregulated ICD have been well studied thus drawing very much attention of researchers. Such dysregulation of the ID apoptosis often leads to polydactyly or syndactyly. It is becoming clear that developmental genes which possess vital functions during early embryonic development and other organogenesis can also affect such digit and ID region development such as the case of Bmp or Wnt/β-catenin signaling. Hence, due to the essential function of such genes during early organogenesis, null mutations of such genes are expected to lead to early embryonic lethality. Therefore, analysis of such developmental key regulators including Wnt/β-catenin or Bmp signaling is vital by utilizing compound conditional genetics. In the current study, integration of the above pathways contributes to develop the D/ID region (Fig. 4M). It will be also intriguing to examine the genetic interaction and crosstalk between Wnt/β-catenin and other regulators. Hox genes have been often analyzed and described as regulating and affecting limb and digit formation [32]. In fact, possible regulations of several Hox genes have been suggested as regulated by several key growth factor systems [33]. Bmp and Wnt/β-catenin have been also suggested as regulating Hox genes [29; 34; 35]. It would be intriguing to check the effect of signal crosstalk of the above genes for ID development.
Acknowledgements
We thank Drs. R. Zeller, R. Behringer, M. Lewandoski, Y. Mishina, B. Crenshaw, W. Birchmeier, A. McMahon, P. Chambon, T. Yamaguchi, A. Moon, J. Takeda, C. Takada, S. Fujikawa and S. Miyaji for materials and helps. This work was supported by Grant-in-Aid for Scientific Research B, Scientific Research on Priority Areas (General promotion of Cancer research in Japan), and the Global COE program Cell Fate Regulation Research and Education Unit from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, and a grant for Child Health and Development (17-2, 20-3) and Health Sciences Research Grant from the Ministry of Health, Labor, and Welfare, Japan. This work was also supported by National Institutes of Health Grant R01-ES016597-01A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li C, Xu X, Nelson DK, Williams T, Kuehn MR, Deng CX. FGFR1 function at the earliest stages of mouse limb development plays an indispensable role in subsequent autopod morphogenesis. Development. 2005;132:4755–64. doi: 10.1242/dev.02065. [DOI] [PubMed] [Google Scholar]
- 2.Yokouchi Y, Sakiyama J, Kameda T, Iba H, Suzuki A, Ueno N, Kuroiwa A. BMP-2/-4 mediate programmed cell death in chicken limb buds. Development. 1996;122:3725–34. doi: 10.1242/dev.122.12.3725. [DOI] [PubMed] [Google Scholar]
- 3.Panman L, Galli A, Lagarde N, Michos O, Soete G, Zuniga A, Zeller R. Differential regulation of gene expression in the digit forming area of the mouse limb bud by SHH and gremlin 1/FGF-mediated epithelial-mesenchymal signalling. Development. 2006;133:3419–28. doi: 10.1242/dev.02529. [DOI] [PubMed] [Google Scholar]
- 4.Francis PH, Richardson MK, Brickell PM, Tickle C. Bone morphogenetic proteins and a signalling pathway that controls patterning in the developing chick limb. Development. 1994;120:209–18. doi: 10.1242/dev.120.1.209. [DOI] [PubMed] [Google Scholar]
- 5.Pajni-Underwood S, Wilson CP, Elder C, Mishina Y, Lewandoski M. BMP signals control limb bud interdigital programmed cell death by regulating FGF signaling. Development. 2007;134:2359–68. doi: 10.1242/dev.001677. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki K, Yamaguchi Y, Villacorte M, Mihara K, Akiyama M, Shimizu H, Taketo MM, Nakagata N, Tsukiyama T, Yamaguchi TP, Birchmeier W, Kato S, Yamada G. Embryonic hair follicle fate change by augmented beta-catenin through Shh and Bmp signaling. Development. 2009;136:367–72. doi: 10.1242/dev.021295. [DOI] [PubMed] [Google Scholar]
- 7.Miyagawa S, Satoh Y, Haraguchi R, Suzuki K, Iguchi T, Taketo MM, Nakagata N, Matsumoto T, Takeyama K, Kato S, Yamada G. Genetic interactions of the androgen and Wnt/beta-catenin pathways for the masculinization of external genitalia. Mol Endocrinol. 2009;23:871–80. doi: 10.1210/me.2008-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki K, Economides A, Yanagita M, Graf D, Yamada G. New horizons at the caudal embryos: coordinated urogenital/reproductive organ formation by growth factor signaling. Current Opin Genet Dev. 2009 doi: 10.1016/j.gde.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshida T, Vivatbutsiri P, Morriss-Kay G, Saga Y, Iseki S. Cell lineage in mammalian craniofacial mesenchyme. Mech Dev. 2008;125:797–808. doi: 10.1016/j.mod.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Iseki S, Wilkie AO, Morriss-Kay GM. Fgfr1 and Fgfr2 have distinct differentiation- and proliferation-related roles in the developing mouse skull vault. Development. 1999;126:5611–20. doi: 10.1242/dev.126.24.5611. [DOI] [PubMed] [Google Scholar]
- 11.Lewandoski M, Sun X, Martin GR. Fgf8 signalling from the AER is essential for normal limb development. Nat Genet. 2000;26:460–3. doi: 10.1038/82609. [DOI] [PubMed] [Google Scholar]
- 12.Kosaki R, Naito Y, Torii C, Takahashi T, Nakajima T, Kosaki K. Split hand foot malformation with whorl-like pigmentary pattern: phenotypic expression of somatic mosaicism for the p63 mutation. Am J Med Genet A. 2008;146A:2574–7. doi: 10.1002/ajmg.a.32415. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki K, Haraguchi R, Ogata T, Barbieri O, Alegria O, Vieux-Rochas M, Nakagata N, Ito M, Mills AA, Kurita T, Levi G, Yamada G. Abnormal urethra formation in mouse models of split-hand/split-foot malformation type 1 and type 4. Eur J Hum Genet. 2008;16:36–44. doi: 10.1038/sj.ejhg.5201925. [DOI] [PubMed] [Google Scholar]
- 14.Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. Embo J. 1999;18:5931–42. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn K, Mishina Y, Hanks MC, Behringer RR, Crenshaw EB., 3rd BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal-ventral patterning of the limb. Development. 2001;128:4449–61. doi: 10.1242/dev.128.22.4449. [DOI] [PubMed] [Google Scholar]
- 16.Tarutani M, Itami S, Okabe M, Ikawa M, Tezuka T, Yoshikawa K, Kinoshita T, Takeda J. Tissue-specific knockout of the mouse Pig-a gene reveals important roles for GPI-anchored proteins in skin development. Proc Natl Acad Sci U S A. 1997;94:7400–5. doi: 10.1073/pnas.94.14.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 18.Nakaya MA, Biris K, Tsukiyama T, Jaime S, Rawls JA, Yamaguchi TP. Wnt3a links left-right determination with segmentation and anteroposterior axis elongation. Development. 2005;132:5425–36. doi: 10.1242/dev.02149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haraguchi R, Motoyama J, Sasaki H, Satoh Y, Miyagawa S, Nakagata N, Moon A, Yamada G. Molecular analysis of coordinated bladder and urogenital organ formation by Hedgehog signaling. Development. 2007;134:525–33. doi: 10.1242/dev.02736. [DOI] [PubMed] [Google Scholar]
- 20.Haraguchi R, Suzuki K, Murakami R, Sakai M, Kamikawa M, Kengaku M, Sekine K, Kawano H, Kato S, Ueno N, Yamada G. Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development. 2000;127:2471–9. doi: 10.1242/dev.127.11.2471. [DOI] [PubMed] [Google Scholar]
- 21.Jones CM, Lyons KM, Hogan BL. Involvement of Bone Morphogenetic Protein-4 (BMP-4) and Vgr-1 in morphogenesis and neurogenesis in the mouse. Development. 1991;111:531–42. doi: 10.1242/dev.111.2.531. [DOI] [PubMed] [Google Scholar]
- 22.Soshnikova N, Zechner D, Huelsken J, Mishina Y, Behringer RR, Taketo MM, Crenshaw EB, 3rd, Birchmeier W. Genetic interaction between Wnt/beta-catenin and BMP receptor signaling during formation of the AER and the dorsal-ventral axis in the limb. Genes Dev. 2003;17:1963–8. doi: 10.1101/gad.263003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrow JR, Thomas KR, Boussadia-Zahui O, Moore R, Kemler R, Capecchi MR, McMahon AP. Ectodermal Wnt3/beta-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev. 2003;17:394–409. doi: 10.1101/gad.1044903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macias D, Ganan Y, Ros MA, Hurle JM. In vivo inhibition of programmed cell death by local administration of FGF-2 and FGF-4 in the interdigital areas of the embryonic chick leg bud. Anat Embryol (Berl) 1996;193:533–41. doi: 10.1007/BF00187925. [DOI] [PubMed] [Google Scholar]
- 25.He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–21. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 26.Butterfield NC, Metzis V, McGlinn E, Bruce SJ, Wainwright BJ, Wicking C. Patched 1 is a crucial determinant of asymmetry and digit number in the vertebrate limb. Development. 2009;136:3515–24. doi: 10.1242/dev.037507. [DOI] [PubMed] [Google Scholar]
- 27.Verheyden JM, Lewandoski M, Deng C, Harfe BD, Sun X. Conditional inactivation of Fgfr1 in mouse defines its role in limb bud establishment, outgrowth and digit patterning. Development. 2005;132:4235–45. doi: 10.1242/dev.02001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez-Martinez R, Castro-Obregon S, Covarrubias L. Progressive interdigital cell death: regulation by the antagonistic interaction between fibroblast growth factor 8 and retinoic acid. Development. 2009;136:3669–78. doi: 10.1242/dev.041954. [DOI] [PubMed] [Google Scholar]
- 29.Ovchinnikov DA, Selever J, Wang Y, Chen YT, Mishina Y, Martin JF, Behringer RR. BMP receptor type IA in limb bud mesenchyme regulates distal outgrowth and patterning. Dev Biol. 2006;295:103–15. doi: 10.1016/j.ydbio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki T, Hasso SM, Fallon JF. Unique SMAD1/5/8 activity at the phalanx-forming region determines digit identity. Proc Natl Acad Sci U S A. 2008;105:4185–90. doi: 10.1073/pnas.0707899105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beermann F, Kaloulis K, Hofmann D, Murisier F, Bucher P, Trumpp A. Identification of evolutionarily conserved regulatory elements in the mouse Fgf8 locus. Genesis. 2006;44:1–6. doi: 10.1002/gene.20177. [DOI] [PubMed] [Google Scholar]
- 32.Izpisua-Belmonte JC, Dolle P, Renucci A, Zappavigna V, Falkenstein H, Duboule D. Primary structure and embryonic expression pattern of the mouse Hox-4.3 homeobox gene. Development. 1990;110:733–45. [PubMed] [Google Scholar]
- 33.Kosaki K, Kosaki R, Suzuki T, Yoshihashi H, Takahashi T, Sasaki K, Tomita M, McGinnis W, Matsuo N. Complete mutation analysis panel of the 39 human HOX genes. Teratology. 2002;65:50–62. doi: 10.1002/tera.10009. [DOI] [PubMed] [Google Scholar]
- 34.Narita T, Sasaoka S, Udagawa K, Ohyama T, Wada N, Nishimatsu S, Takada S, Nohno T. Wnt10a is involved in AER formation during chick limb development. Dev Dyn. 2005;233:282–7. doi: 10.1002/dvdy.20321. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Knezevic V, Ervin V, Hutson R, Ward Y, Mackem S. Direct interaction with Hoxd proteins reverses Gli3-repressor function to promote digit formation downstream of Shh. Development. 2004;131:2339–47. doi: 10.1242/dev.01115. [DOI] [PubMed] [Google Scholar]