1. Summary
Recent advances in understanding the physiological role of mast cells (MCs) points to an important regulatory role for these cells in adaptive immunity. MCs express a diverse array of molecules that can promote their interaction with T cells as well as with other immune cells. New evidence demonstrates that mast cells can directly and indirectly communicate with T cells. They can control both effector and regulatory T cell responses and their activity can in turn be modulated by these interactions. Here we briefly summarize these advances and discuss some of the major challenges in understanding the communication of MCs and T cells.
Keywords: communication, mast cells, molecular interaction, T cells, T effectors, Tregs
2. Introduction
Since the first description of the mast cell (MC) by Paul Ehrlich in 1878, the function of this tissue resident cell has been enigmatic (reviewed in [1]). These cells were named “Mastzellen” by Paul Ehrlich because he thought of them as providers of nutrition to tissues because of their notable granules. It was not until at least forty years later, with the discovery of a reaginic agent by Carl Prausnitz that elicited allergic reactions [2], and the later finding by J.F. Riley and G.B. West that MCs were the source for the release of allergic mediators such as histamine [3], that a role (albeit pathological) for MCs was delineated. The modern era in the study of MC function was ushered in by the identification of IgE by Kimishige and Teruko Ishizaka in the late 1960’s as the reaginic agent that caused MC activation [4] and by the cloning of the high affinity IgE receptor (Fcε RI) [5,6], which allowed structural and functional studies on mast cell function in vitro and in vivo. These seminal discoveries promoted the view that the MC was principally a villain that caused allergic disease [7], nonetheless, its protective role in some parasitic infections was also recognized [8].
Today, it has become widely accepted that the role of the MC is not restricted to allergic processes. This evolution was partly a consequence of the discovery that MCs produce cytokines [9,10], to varied stimuli, and that this can occur in the absence of MC degranulation. This was further cemented by the finding that MCs were essential to survival in a mouse model of sepsis [11] suggesting an important role for this cell type in innate immunity, which was further reinforced by the recognition that MCs express multiple pathogen-associated molecular pattern receptors, like members of the Toll-like receptor family (TLRs) [12–15]. Given their localization to tissues which can be in contact with the environment, and the accumulated evidence of a role for these cells in host defense, MCs are now recognized as innate immune cells [16]. The discovery of additional novel roles for MCs in host defense, particularly to snake and bee venoms, provide evidence of the MC as a first line of defense in specific circumstances [17,18].
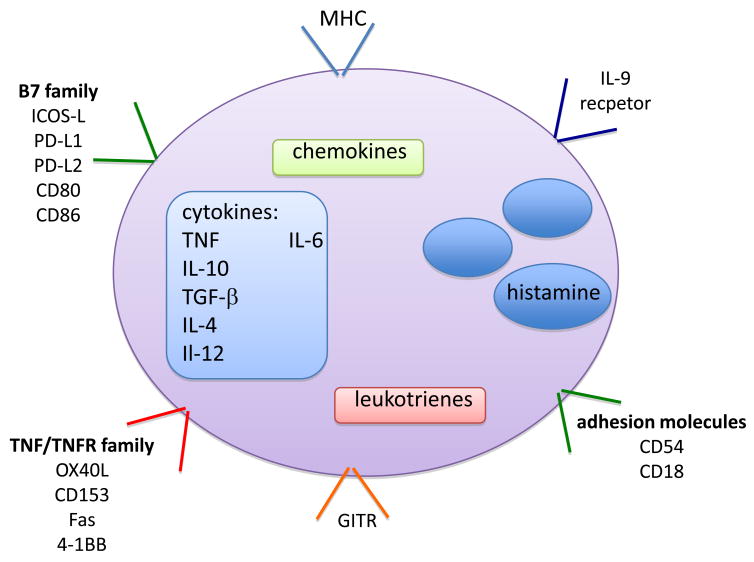
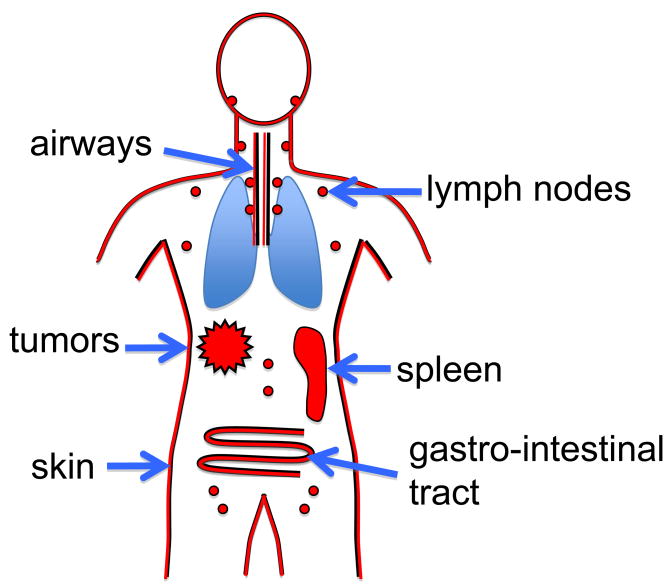
MCs can also function as amplifiers of the adaptive immune responses [19]. There is increasing evidence of MCs as modulators of T cell responses [20]. The concept that MCs communicate with T cells is hardly surprising, due to the fact that MCs express a wide array of molecules [21] that can potentially mediate this cross-talk (Fig. 1). These include major histocompatibility molecules, stimulatory and inhibitory proteins as well as a large number of cytokines and inflammatory mediators (reviewed in [19,22]). Furthermore, MCs have been identified in the proximity of T cells not only in peripheral tissues but also in lymphatic organs (Fig. 2). These new findings provide a continuing evolution of the role of MCs, as the recent data suggests an important regulatory role for these cells [16,19]. In this review, we summarize the findings on the various modes of communication between MC and T cells and, where known, the mechanisms and functional outcome of such interactions are also discussed. As this is a young field of investigation, we also discuss the current hurdles and future challenges towards understanding how MCs and T cells communicate.
Figure 1.
A schematic representation of mast cell components and products which may mediate interactions with T-cells. The intermediary molecules are either secreted (e.g. cytokines, chemokines, leukotrienes, histamine) or membrane-bound (e.g. MHC, costimultory/coinhibitory molecules, adhesion molecules). It is anticipated that different inflammatory settings will result in varying expression of these components thus leading to the versatile role of MC in disease models.
Figure 2.
Likely sites for MC-T cell communication. Murine models suggest several sites in which these two cell types may possibly establish contact in health and disease. Physical interaction, however, may not be crucial in interactions that are driven by MC-derived secreted mediators.
3. Types of mast cell-T cell interactions
The potential interaction of MCs with T cells has long been suggested by early findings of cytotoxic T cell interactions with the target mastocytoma cell line P815 [23–25], and the demonstration that mast cells and lymphocytes can form rosettes in mixed cultures [26]. More recent studies have shown that MCs and activated T cells can interact via ICAM-1 (CD54) and LFA-1 (CD11a) expressed, respectively, on their cell surface [27] (Fig. 1). This interaction promoted histamine release from MCs and was found to enhance FcεRI-mediated MC degranulation as well [28]. Nonetheless, the mechanisms involved are unclear. That MCs should be able to interact with T cells might seem predictable since MCs express an array of surface molecules, including members of the B7 family (ICOS-L, PD-L1, PD-L2) the TNF/TNFR families (OX40L, CD153, Fas, 4-1BB) and the glucocorticoid-induced TNFR (GITR) [21] (Fig. 1). However, under what circumstances such interactions occur and what is the outcome of such interactions has only recently been a focus of active investigation. Recent work has begun to shed some light on the mechanisms and the physiological effects of MC-T cell interactions. In the next several sections we summarize some of these physiological outcomes and where possible we discuss potential mechanisms.
3.1 MCs can stimulate (and possibly suppress) T effector cells
The role of the MC as an effector cells in various types of inflammation, such as Th2-type diseases and autoimmunity, has long been recognized. Their possible role in experimental autoimmune encephalomyelitis (EAE) was suspected over several decades ago [29]. EAE is a mouse model of multiple sclerosis, a prototypical T cell-mediated autoimmune disease in the mouse, where MCs were shown to enhance the inflammatory process [30] and to promote CD4+ and CD8+ T-cell expansion in the central nervous system [31]. Several intriguing observations have been made with regards to MC and T cell communication in the context of EAE. First, reconstitution of bone marrow derived MCs in mast cell-deficient (Wv/Wv) mice exacerbated disease even in the absence of engraftment within the inflammatory site, implying that enhancement of T-effector activity occurred at a site remote to the inflammatory site (e.g. lymph nodes and spleen) [32]. Second, adoptive transfer experiments of autoreactive T-cells indicated that MCs have a role both in the onset and the severity of disease [31].
A possible mechanism for the enhancement of T-effector activity arises from the observation that MCs are able to act as antigen presenting cells [33]. Several recent studies provide mechanistic insight in support of such a model. Inducible expression of MHC class II by MCs was reported to occur when these cells were treated with LPS and IFN-γ [34] (Fig. 1). This study demonstrated that MHC class II-expressing MCs failed to stimulate naïve T cells, but could effectively support activated T effectors and cause the expansion of regulatory T cells (Tregs). Moreover, an LPS injection in mice caused an increased number of MCs in the lymph nodes and these MCs expressed MHC Class II molecules as well as the positive costimulatory B7 family members CD80 and CD86, demonstrating the potential to regulate T cell activity in the lymph nodes [34] (Fig. 1 and 2). In addition, another study [35] reported that treatment of peritoneal cell-derived mast cells (PCMCs) with IFN-γ and IL-4 induced expression of MHC class II molecules. These PCMCs were then able to present antigen to effector T cells causing their activation, proliferation, and the formation of an immunological synapse between the PCMC and the T cell [35]. It also appears that Notch signaling can induce MHC class II and OX40L expression on MCs. Consequently, these MCs could also cause proliferation of T cells and promoted a Th2 phenotype [36]. Thus, upregulation of MHC class II on MCs is a key component for stimulating T effector cells and Tregs in some settings. However, this role for MCs is not limited to MHC class II presentation, MHC class I-dependent cross presentation of MCs to CD8+ T cells was recently shown to increase CD8+ T cell proliferation and effector functions [37]. Moreover, in vivo studies demonstrated that MCs could regulate CD8+ T cell-specific priming in EAE. Thus, the findings argue that MCs are important in antigen-specific regulation of CD8+ T cells.
Various other mechanisms underlying the MC’s ability to activate T cells have been subject to investigation. Although some of the most recent work has focused on the engagement of OX40 on T cells by MC expressed OX40L [38–40] (this will be discussed in the following sections), the means by which MCs can potentially stimulate lymphocytes seems much more diverse. It has been shown that MCs can cause T cell proliferation and cytokine production in a TNF-dependent manner following FcεRI stimulation [41]. Moreover, in co-cultures of MCs with helper T cells, low numbers of MCs enhanced T cell proliferation whereas high numbers suppressed T cell proliferation [42]. Evidence for a role of histamine via the H1 receptor was suggested in this study, however, the underlying mechanism is unclear.
Much less is known about the role of MCs in suppression of T effectors. Nonetheless, some clues are being unveiled. In a mouse model of hepatocarcinoma, tumor cell-derived stem cell factor (SCF) promoted the recruitment of MCs to the tumor (Fig. 2) and the release of adenosine, which inhibits the production of IL-2 and IFN-γ by CD4+ T cells [43]. It was suggested that the high levels of SCF in the tumor microenvironment serve to promote the release of adenosine and cause the MCs to remain at the site of the tumor by inhibiting further migration. In addition, this immune suppression could be further enhanced by the increased presence of Tregs in the tumor site [43]. However, whether the recruitment of Tregs to the tumor environment is caused by MCs is unclear. Thus, in this model, MCs appear to be key in suppressing the immune response required for effective elimination of the tumor.
Obviously, the mechanism by which MCs may promote or suppress T effectors may differ depending on the inflammatory setting. Nonetheless, the findings underscore the potential capacity of MCs to facilitate T effector responses by diverse mechanisms.
3.2 MCs suppress Tregs
The concept that MCs may act to promote inflammation through inhibition of Treg activity has been put forward by multiple recent reports. This possibility was first suggested several years ago by the finding that IFN-α treatment of FcεRI-activated cord blood derived human MCs caused a shift from TNF production to an enhanced production of IL-10 and TGF-β with a concomitant decrease in OX40L expression (Fig. 1), which resulted in the decreased ability of these cells to promote CD4+ T cell expansion [44]. Further mechanistic insight was provided by a more recent study where Tregs and T effectors were mixed with MCs [40]. This study showed that MCs counteracted the Treg suppression of effector T cells. Abrogation of the Treg suppressive activity depended on OX40/OX40L interaction (the latter being expressed on MCs) and T-cell derived IL-6. The result of the MC-mediated counteraction of Treg suppression of effector T cells was an increase in IL-17 producing T-cells [40]. In vivo analysis of inflammatory conditions where the localization of MCs with Tregs and Th-17 cells might occur, revealed that some MCs could co-localize with Tregs and Th17 cells in EAE. Likewise, these three cell types were identified in the BAL fluid of OTII transgenic mice challenged with ovalbumin intranasally, suggesting that this complex tripartite regulatory circuit might exist in such inflammatory conditions [40]. Similarly, a change in the suppressive phenotype of Tregs suppressive has been suggested in a mouse model of hereditary colon cancer (polyposis) [45], a conditions where MCs have been shown to be essential [46]. Progressive disease was shown to cause a shift of expanding Tregs from an IL-10 secreting to an IL-17 secreting phenotype with a concurrent development of a mastocytosis. Thus, it appears that in various inflammatory settings the presence of MCs with Tregs and T effectors may well promote inflammation via suppression of the IL-10 secreting Treg.
Another mechanism for counteraction of Treg suppressive activity has also been suggested by the binding of MC-derived histamine to H1 receptors on Tregs [47]. Exposure of Tregs to histamine caused a lowering of CD25 and Foxp3 expression but increased expression could be restored by treatment of the cells with the H1 antagonist loratadine. In this particular work, Tregs were exposed to histamine for 48 hours thus the physiological relevance of these findings are unclear given the short-half life of circulating histamine in vivo [48]. Nonetheless, there are some pathological conditions where histamine elevation is prolonged, for example, histamine intolerance can result from the ingestion of spoiled fish or other amine-rich foods, endogenous histamine overproduction, or an imbalance between the accumulation of histamine and its elimination [49–51]. This can cause severe allergic reactions or recurrent anaphylaxis, which may have suppression of Tregs as one component [52]. It should be noted, that histamine has also been suggested to act on T cells through its binding to H2 receptors. In an in vivo mouse model, administration of cimetidine, an H2 blocker, increased the immune response to a melanoma and inhibited its growth [53]. In a model of hepatitis B virus DNA vaccination, the enhancement of the immune response by cimetidine was demonstrated to result from elevated levels of IL-4 and IFN-γ producing CD4+ T effectors and downregulation of IL-10 and TGF-β secretion [54], which may also lead to reduced Treg activity. Collectively, these findings highlight the versatility of MCs as regulators of Treg activity in the immune system.
3.3 Tregs can suppress (and possibly enhance) MC function
Dampening of a large variety of immune responses to both self-antigens and to foreign antigens is a pivotal role for Tregs [55]. This suppression is the result of interactions between Tregs and other cellular targets, such as dendritic cells and T effectors. It was therefore hypothesized that MCs, which take part in allergic responses, may also submit to Treg-mediated inhibition. This was based largely on the findings that IL-10 and TGF-β, cytokines secreted by Tregs, can inhibit mast cell function [56,57]. An initial analysis of the effect of Tregs on MC function demonstrated that activated Tregs caused a reduction in the expression of Fcε RI on MCs [58]. While MCs were shown to recruit both Tregs and conventional CD4+ CD25− cells, only Tregs caused a reduction in Fcε RI expression whereas both T-cell subsets suppressed Fcε RI-mediated leukotriene C4 production. The inhibition of Fcε RI expression was shown to be, in part, dependent on IL-10 and TGF-β and was contact-dependent [58]. The picture, however, is quite complex, since inhibition of mast cell degranulation was not observed and cross-talk from both Tregs and conventional T cells was shown to enhance Fcε RI-induced Stat5 phosphorylation along with a concomitant increase in cytokine production. Thus, while the findings show that activated Tregs can suppress Fcε RI expression and leukotriene production, they in turn enhance cytokine production. Thus, the physiological significance of this work remains to be elucidated.
A considerable advance in understanding Treg and MC interactions was made with the discovery that these cells can interact via OX40 and OX40L [21,38,39,59–61] (Fig. 1). Consequently, it was reported that this interaction results in the suppression of MC degranulation [38]. In vitro experiments demonstrated the requirement for OX40 on the Treg and OX40L on the MC in order to suppress MC degranulation. Mechanistically, it was shown that Treg-MC contact impaired the influx of extracellular Ca2+ after Fcε RI stimulation. This was mediated through increased cAMP generation in the MC as a result of OX40L engagement. Inhibition of cAMP generation in MCs caused normal Ca2+ influx and normal MC degranulation even in the presence of Tregs, demonstrating that quenching of cAMP caused reversion to a normal phenotype. cAMP was previously demonstrated to inhibit MC calcium responses and degranulation [59–61], thus the findings suggest that OX40L engagement on MCs leads to the activation of a heterotrimeric GTP-binding protein that is linked to the activation of adenylyl cyclase, which generates cAMP. This is of considerable interest since OX40L engagement on dendritic cells enhances their maturation and antigen presenting ability [62], suggesting the possibility that Tregs may promote MC differentiation from an effector to antigen-presenting phenotype. It should be noted that this study [38] also provided in vivo data showing that suppression of MC degranulation in a model of anaphylaxis depended on Treg-derived OX40. However, the reciprocal experiment on the role of OX40L expressed by MCs could not be conducted as engraftment of these cells was impaired in MC-deficient mice.
While less mechanistically defined, it has been shown that adoptive transfer of in vitro-stimulated Tregs can increase bacterial clearance and improve survival in a mouse model of sepsis [63]. This cecal ligation and puncture model has been shown to depend on MCs for survival and protection is at least in part mediated by TNF secretion [11], however, it is also likely that mast cell granule proteases play an important role through recruitment of neutrophils [64–66]. Transfer of IL-2, anti-CD3, and anti-CD28 in vitro stimulated Tregs was shown to enhance survival and was host T cell dependent and resulted in a marked increase in the numbers of MCs in the peritoneal cavity [63]. The mechanism for this enhancement is not clear, however, the result of increased MC numbers was translated to increased TNF in the peritoneal cavity. Thus, the findings suggest that activated Tregs can promote and increase MC response in this particular model of sepsis. Collectively, while the preponderance of evidence might suggest that MCs can be subject to Treg-mediated suppression there appears to be some cases where Tregs may promote MC function.
4. MCs as the effector arm of Treg-mediated suppression
The role of MC as immune suppressors or as the effectors of Treg activity is a novel concept that has been proposed in recent years by several reports. This role for the MC was not predicted based on the conventional view of MCs as proinflammatory cells. Nonetheless, evidence in support of this view has accumulated from various model systems. MCs have been implicated in the progression of tumor development, by inducing immunological tolerance to the neoplastic tumor ([43,67] and reviewed in [68]) (Fig. 2). One proposed mechanism for the anti-inflammatory or suppressive effect is MC-derived IL-10 [69]. This mechanism was shown to underlie the dampening of the immunological response to a hapten in combination with UVB [70], chronic UVB irradiation [69] and mosquito bites [71]. Other mechanisms for a protective or suppressive effect of MCs on the immune response may be mediated by the ability of mast cell granule proteases and other contents to cleave and inactivate various inflammatory or toxic agents as has been shown for sarafotoxins [17,18], a toxin in the venom of some snakes which is degraded by MC granule proteases leading to increased survival.
A detailed model of collaboration between Tregs and MC has been described in a skin allograft system [72]. In this work, it was noted that tolerant allografts acquire an MC-gene product signature and that tolerance was impaired in MC-deficient mice. The authors found that activated Tregs express high levels of IL-9 and that this cytokine recruited and activated MCs in the skin graft to achieve inhibition of rejection. The conclusion from this work is that MCs were essentials intermediaries in regulatory T-cell tolerance [72]. This, however, is not likely to be universal, since MC-deficient- and IL-9-null- mice do not exhibit the exaggerated inflammatory phenotype that is seen in the Treg-deficient Foxp3-null mice. Moreover, whether Tregs secrete IL-9 or induce, via TGF-β secretion, the differentiation of Th2 cells to the newly described Th9 cells [73] (as the source of IL-9 in this allograft model) remains unclear. Another level of complexity has been added to this model by a follow-up study that showed that, upon localized or systemic MC degranulation, peripheral tolerance was broken and thus allograft rejection is facilitated [74]. The proposed mechanism involved the transient loss of Tregs and MCs from the allograft and a decreased expression of suppressor molecules by the Treg. However, in contrast to the aforementioned work demonstrating that MCs can cause the shift of Tregs to Th-17 cells [45], this study found that Tregs lost the ability to secrete TGF-β, IL-10, and express other suppressive molecules without a change in Foxp3 expression (thus retaining a Treg phenotype) [74]. Thus, the conclusion from this study is that the same MCs that appear to be crucial for the establishment of tolerant skin allografts, also harbor within their granules the potential to initiate rejection. Nonetheless, given that the loss of MC’s or IL-9 expression does not lead to spontaneous inflammatory disease or to the type of inflammatory disease seen in the absence of Foxp3 (in unchallenged or challenged mice), the ability of MCs to act as intermediaries of Treg-mediated suppression is most likely circumstance-dependent and is not a generalized function of MCs.
5. What have we learned and where do we go from here?
The evidence briefly summarized herein provides multiple scenarios where MCs can be intimately linked to T cell biology and function. A common theme emerges whereby MC and T cell communication is regulatory in nature but not indispensable to carry out many of the effector functions of either cell type. There is evidence that:
MCs can present antigen to T cells. However, there are many types of antigen presenting cells and MCs do not appear to be professional antigen presenting cells like dendritic cells.
MCs can stimulate the proliferation of T effector cells. MCs appear to counteract the suppressive role of Tregs leading to increased T effector proliferation and function and this requires the OX40-OX40L axis and IL-6 production.
MCs can interact with Tregs via the OX40L-OX40 interactions, respectively. OX40 engagement suppresses Treg function (appears to shift some Tregs to IL-17 production).
Tregs can suppress some MC effector functions (particularly degranulation). OX40-OX40L interactions promotes cAMP in mast cells dampening calcium mobilization and thus degranulation.
Under certain circumstances, MCs may serve as the effector arm of Treg-mediated suppression and MCs may also serve to break tolerance upon their activation.
Having been discovered more than a century ago, it seems rather remarkable that the regulatory role for MCs remained elusive for so long. However, until recently, there has been a lack of information on the physiological role of MCs beyond allergy. Naturally, the need for an understanding of the in vivo role of MCs compromises the understanding of MC and T cell interactions in health and disease and, importantly, the ability to devise therapeutic strategies by modulation of such interactions.
There are several reasons, both conceptual and technical, for the relative paucity of in vivo studies. Perhaps the most critical problem is the lack of an appropriate mouse model, in which the sole problem is the absence of MC. Currently, the gold standard for demonstration of in vivo MC function are lines arising from a spontaneous mutation (W/Wv) [75] or chromosomal inversion (Wsh/Wsh) [76] of the Kit gene. These MC-deficient mice have multiple defects including immunological and hematological abnormalities. For example, Wsh/Wsh mice may develop splenomegaly with expanded myeloid and megakaryocyte populations and aberrant bone marrow and when repopulated with MCs not all tissues repopulate like wild type mice [76–78]. These abnormalities can potentially exert effects on MC and T cell communication and/or function. In order to draw conclusions about the role of MCs, one is required to compare wild type mice with both MC reconstituted and non-MC reconstituted mice. Ideally, since W/Wv and Wsh/Wsh differ in their abnormalities, studies should include both strains of mice to ascertain that the reconstituted effect is indeed mediated by MCs. Apart from being technically lengthy, cumbersome and expensive, this type of protocol possesses several inherent biological drawbacks. First, reconstitution is invariably done with bone marrow derived cultured MC. These cells are differentiated in vitro, they are immature and they do not accurately recapitulate the different MC phenotypes found in vivo [22]. Heterogeneity of MCs in tissues is a hallmark of their in vivo localization and while two major types of MCs have been described, connective tissue and mucosal MCs, its is likely that MC heterogeneity is widely varied and depends on the specific microenvironment in which they reside [16,79]. A second major disadvantage of MC reconstitution models is the clear difference in the numbers and tissue distribution of MCs when comparing MC-reconstituted Kit-deficient mice to wild type mice. For reasons that are not entirely clear, intravenous injection of MCs results in a massive engraftment in the spleen but not in the skin and other sites [78]. To some extent this can be overcome by intradermal reconstitution of MCs, however, it is not known whether the local skin reconstitution would alter MC interactions and/or trafficking that may be needed to manifest their regulatory role, such as their presence in lymphoid secondary tissues. We anticipate that future breakthroughs in the understanding of MC function in health and disease will require better mouse models that enable their specific depletion and genetic manipulation.
As alluded to above, the functional hierarchy between MCs and T cells differs and this needs to be considered in the investigation of their interactions. T cells, both regulatory and effector subsets are critical for immunological homeostasis [80–82]. Global or specific alterations may result in immunodeficiencies and even fatal phenotypes. The same is not true for MCs, whose deficiency is not associated with considerable spontaneous manifestations of disease in mice. Moreover, to the best of our knowledge, there is no described human disease of MC deficiency. This can be viewed as the MC being vital and thus MC-deficiencies are not viable, however, multiple lines of evidence argue against this notion. Both c-Kit and STAT5 [83] -deficient mice, which are MC-deficient, are viable. Suppression of MC function with pharmacological agents in humans or in mice does not result in high susceptibility to infectious disease nor affect the viability of the MC [84,85]. Taken together, we conclude that the MC fine tunes the immunological responses. Long term, chronic, immunological models in mice where MCs are specifically depleted will probably yield considerably more insight on the role of the MC in vivo and its impact on the T cell compartment.
In summary, the body of data on MC signaling and function, which has accumulated thus far, offers a number of mechanisms for potential MC communication with T-cell. Several of these cooperations have been demonstrated both in vitro and in vivo. Nevertheless, some fundamental questions have yet to be answered. For example, the site (or sites) of MC and T cell cross-talk have not been convincingly demonstrated (e.g. site of inflammation, the lymphatic tissues, etc.) (Fig. 2). The advent and further development of novel technologies, such as intra-vital imaging, has the potential to shed light on such critical questions. MCs express a wide variety of molecules that act as positive and negative ligands for T cell activation and responses [21] (Fig. 1). When are these molecules used in health and disease? Is there regulation of their expression on MCs in specific tissues? While it is clear that MCs possess the ability to both augment and to suppress inflammation, what is the relevance of this property to the role of the T cell in inflammation and to the development of potential therapies? It is hoped that the continued characterization of MC behavior in vivo, in the setting of inflammation, will effectively address these fundamental issues and will shed new light on the role of the MC in immune regulation and its partnership with the T cell.
Acknowledgments
The research of the authors included herein was supported in part by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beaven MA. Our perception of the mast cell from Paul Ehrlich to now. Eur J Immunol. 2009;39:11–25. doi: 10.1002/eji.200838899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prausnitz C. The passive transfer of allergy. Int Arch Allergy Appl Immunol. 1955;6:260–269. doi: 10.1159/000228183. [DOI] [PubMed] [Google Scholar]
- 3.Riley JF, West GB. Tissue mast cells: studies with a histamine-liberator of low toxicity (compound 48/80) J Pathol Bacteriol. 1955;69:269–282. doi: 10.1002/path.1700690135. [DOI] [PubMed] [Google Scholar]
- 4.Ishizaka K, Ishizaka T. Identification of -E-antibodies as a carrier of reaginic activity. J Immunol. 1967;99:1187–1198. [PubMed] [Google Scholar]
- 5.Blank U, Ra C, Miller L, White K, Metzger H, Kinet JP. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature. 1989;337:187–189. doi: 10.1038/337187a0. [DOI] [PubMed] [Google Scholar]
- 6.Metzger H, Blank U, Kinet J-P, Kochan J, Ra C, Rivera J. Emerging picture of the receptor with high affinity for IgE. Int Arch Allergy Appl Immunol. 1989;88:14–17. [PubMed] [Google Scholar]
- 7.Ishizaka T, White JR, Saito H. Activation of basophils and mast cells for mediator release. Int Arch AllergyAppl Immunol. 1987;82:327–332. doi: 10.1159/000234218. [DOI] [PubMed] [Google Scholar]
- 8.Askenase PW. Immune inflammatory responses to parasites: the role of basophils, mast cells and vasoactive amines. Am J Trop Med Hyg. 1977;26:96–103. doi: 10.4269/ajtmh.1977.26.96. [DOI] [PubMed] [Google Scholar]
- 9.Burd PR, Rogers HW, Sundararajan J, Wilson SD, Dvorak AM, Galli SJ, et al. Interleukin 3-dependent and -independent mast cells stimulated with IgE and antigen express multiple cytokines. J Exp Med. 1989;170:245–257. doi: 10.1084/jem.170.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plaut M, Pierce JH, Watson CJ, Hanley-Hyde J, Nordan RP, Paul WE. Mast cell lines produce lymphokines in response to cross-linkage of Fcε RI or to calcium ionophores. Nature. 1989;339:64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- 11.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 12.Leal-Berumen I, Conlon P, Marshall JS. IL-6 production by rat peritoneal mast cells is not necessarily preceded by histamine release and can be induced by bacterial lipopolysaccharide. J Immunol. 1994;152:5468–5476. [PubMed] [Google Scholar]
- 13.Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787–799. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- 14.Mekori YA, Metcalfe DD. Mast cells in innate immunity. Immunol Rev. 2000;173:131–140. doi: 10.1034/j.1600-065x.2000.917305.x. [DOI] [PubMed] [Google Scholar]
- 15.Okumura S, Kashiwakura J, Tomita H, Matsumoto K, Nakajima T, Saito H, et al. Identification of specific gene expression profiles in human mast cells mediated by Toll-like receptor 4 and Fcε RI. Blood. 2003;102:2547–2554. doi: 10.1182/blood-2002-12-3929. [DOI] [PubMed] [Google Scholar]
- 16.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 17.Rivera J. Snake bites and bee stings: the mast cell strikes back. Nat Med. 2006;12:999–1000. doi: 10.1038/nm0906-999. [DOI] [PubMed] [Google Scholar]
- 18.Metz M, Piliponsky AM, Chen CC, Lammel V, Abrink M, Pejler G, et al. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313:526–530. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- 19.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 20.Sayed BA, Brown MA. Mast cells as modulators of T-cell responses. Immunol Rev. 2007;217:53–64. doi: 10.1111/j.1600-065X.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- 21.Nakae S, Suto H, Iikura M, Kakurai M, Sedgwick JD, Tsai M, et al. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol. 2006;176:2238–2248. doi: 10.4049/jimmunol.176.4.2238. [DOI] [PubMed] [Google Scholar]
- 22.Rao KN, Brown MA. Mast cells: multifaceted immune cells with diverse roles in health and disease. Ann N Y Acad Sci. 2008;1143:83–104. doi: 10.1196/annals.1443.023. [DOI] [PubMed] [Google Scholar]
- 23.Kolsch E. The genetic control of T cell-mediated immunity against the DBA/2 mastocytoma P-815. II. Low responsiveness in T cell-mediated cytotoxicity accompanied by the inability to produce antibodies in a secondary response. Eur J Immunol. 1975;5:527–532. doi: 10.1002/eji.1830050804. [DOI] [PubMed] [Google Scholar]
- 24.Balk SP, Mescher MF. Specific reversal of cytolytic T lymphocyte--target cell interaction. J Supramol Struct Cell Biochem. 1981;16:43–52. doi: 10.1002/jsscb.1981.380160105. [DOI] [PubMed] [Google Scholar]
- 25.Balk SP, Walker J, Mescher MF. Kinetics of cytolytic T lymphocyte binding to target cells in suspension. J Immunol. 1981;126:2177–2183. [PubMed] [Google Scholar]
- 26.Giulling EV, Nikol’skii IS, Diugovskaia LA, Chernenko OD, Ovsienko VV. Contact interaction of mast cells and lymphocytes during ontogenetic antigen-induced differentiation and malignant transformation of lymphoid cells. Biull Eksp Biol Med. 1980;89:584–585. [PubMed] [Google Scholar]
- 27.Fox CC, Jewell SD, Whitacre CC. Rat peritoneal mast cells present antigen to a PPD-specific T cell line. Cell Immunol. 1994;158:253–264. doi: 10.1006/cimm.1994.1272. [DOI] [PubMed] [Google Scholar]
- 28.Inamura N, Mekori YA, Bhattacharyya SP, Bianchine PJ, Metcalfe DD. Induction and enhancement of Fcε RI-dependent mast cell degranulation following coculture with activated T cells: dependency on ICAM-1- and leukocyte function-associated antigen (LFA)-1-mediated heterotypic aggregation. J Immunol. 1998;160:4026–4033. [PubMed] [Google Scholar]
- 29.Orr EL. Presence and distribution of nervous system-associated mast cells that may modulate experimental autoimmune encephalomyelitis. Ann N Y Acad Sci. 1988;540:723–726. doi: 10.1111/j.1749-6632.1988.tb27226.x. [DOI] [PubMed] [Google Scholar]
- 30.Secor VH, Secor WE, Gutekunst CA, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med. 2000;191:813–822. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gregory GD, Robbie-Ryan M, Secor VH, Sabatino JJ, Jr, Brown MA. Mast cells are required for optimal autoreactive T cell responses in a murine model of multiple sclerosis. Eur J Immunol. 2005;35:3478–3486. doi: 10.1002/eji.200535271. [DOI] [PubMed] [Google Scholar]
- 32.Tanzola MB, Robbie-Ryan M, Gutekunst CA, Brown MA. Mast cells exert effects outside the central nervous system to influence experimental allergic encephalomyelitis disease course. J Immunol. 2003;171:4385–4391. doi: 10.4049/jimmunol.171.8.4385. [DOI] [PubMed] [Google Scholar]
- 33.Frandji P, Tkaczyk C, Oskeritzian C, David B, Desaymard C, Mecheri S. Exogenous and endogenous antigens are differentially presented by mast cells to CD4+ T lymphocytes. Eur J Immunol. 1996;26:2517–2528. doi: 10.1002/eji.1830261036. [DOI] [PubMed] [Google Scholar]
- 34.Kambayashi T, Allenspach EJ, Chang JT, Zou T, Shoag JE, Reiner SL, et al. Inducible MHC class II expression by mast cells supports effector and regulatory T cell activation. J Immunol. 2009;182:4686–4695. doi: 10.4049/jimmunol.0803180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaudenzio N, Espagnole N, Mars LT, Liblau R, Valitutti S, Espinosa E. Cell-cell cooperation at the T helper cell/mast cell immunological synapse. Blood. 2009 doi: 10.1182/blood-2009-02-202648. [DOI] [PubMed] [Google Scholar]
- 36.Nakano N, Nishiyama C, Yagita H, Koyanagi A, Akiba H, Chiba S, et al. Notch signaling confers antigen-presenting cell functions on mast cells. J Allergy Clin Immunol. 2009;123:74–81. e71. doi: 10.1016/j.jaci.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 37.Stelekati E, Bahri R, D’Orlando O, Orinska Z, Mittrucker HW, Langenhaun R, et al. Mast cell-mediated antigen presentation regulates CD8+ T cell effector functions. Immunity. 2009;31:665–676. doi: 10.1016/j.immuni.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 38.Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, et al. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kashiwakura J, Yokoi H, Saito H, Okayama Y. T cell proliferation by direct cross-talk between OX40 ligand on human mast cells and OX40 on human T cells: comparison of gene expression profiles between human tonsillar and lung-cultured mast cells. J Immunol. 2004;173:5247–5257. doi: 10.4049/jimmunol.173.8.5247. [DOI] [PubMed] [Google Scholar]
- 40.Piconese S, Gri G, Tripodo C, Musio S, Gorzanelli A, Frossi B, et al. Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation. Blood. 2009;114:2639–2648. doi: 10.1182/blood-2009-05-220004. [DOI] [PubMed] [Google Scholar]
- 41.Nakae S, Suto H, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: Importance of mast cell-derived TNF. Proc Natl Acad Sci U S A. 2005;102:6467–6472. doi: 10.1073/pnas.0501912102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan MM, Strober S, Melmon KL. Regulatory effects of mast cells on lymphoid cells: the role of histamine type 1 receptors in the interaction between mast cells, helper T cells and natural suppressor cells. Cell Immunol. 1986;103:41–53. doi: 10.1016/0008-8749(86)90066-3. [DOI] [PubMed] [Google Scholar]
- 43.Huang B, Lei Z, Zhang GM, Li D, Song C, Li B, et al. SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood. 2008;112:1269–1279. doi: 10.1182/blood-2008-03-147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujita T, Kambe N, Uchiyama T, Hori T. Type I interferons attenuate T cell activating functions of human mast cells by decreasing TNF-α production and OX40 ligand expression while increasing IL-10 production. J Clin Immunol. 2006;26:512–518. doi: 10.1007/s10875-006-9043-1. [DOI] [PubMed] [Google Scholar]
- 45.Gounaris E, Blatner NR, Dennis K, Magnusson F, Gurish MF, Strom TB, et al. T-regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Res. 2009;69:5490–5497. doi: 10.1158/0008-5472.CAN-09-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gounaris E, Erdman SE, Restaino C, Gurish MF, Friend DS, Gounari F, et al. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci U S A. 2007;104:19977–19982. doi: 10.1073/pnas.0704620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forward NA, Furlong SJ, Yang Y, Lin TJ, Hoskin DW. Mast cells down-regulate CD4+CD25+ T regulatory cell suppressor function via histamine H1 receptor interaction. J Immunol. 2009;183:3014–3022. doi: 10.4049/jimmunol.0802509. [DOI] [PubMed] [Google Scholar]
- 48.Sedor JR, Abboud HE. Actions and metabolism of histamine in glomeruli and tubules of the human kidney. Kidney Int. 1984;26:144–152. doi: 10.1038/ki.1984.148. [DOI] [PubMed] [Google Scholar]
- 49.Hershko AY, Dranitzki Z, Ulmanski R, Levi-Schaffer F, Naparstek Y. Constitutive hyperhistaminaemia: a possible mechanism for recurrent anaphylaxis. Scand J Clin Lab Invest. 2001;61:449–452. doi: 10.1080/00365510152567086. [DOI] [PubMed] [Google Scholar]
- 50.Maintz L, Novak N. Histamine and histamine intolerance. Am J Clin Nutr. 2007;85:1185–1196. doi: 10.1093/ajcn/85.5.1185. [DOI] [PubMed] [Google Scholar]
- 51.Morrow JD, Margolies GR, Rowland J, Roberts LJ., 2nd Evidence that histamine is the causative toxin of scombroid-fish poisoning. N Engl J Med. 1991;324:716–720. doi: 10.1056/NEJM199103143241102. [DOI] [PubMed] [Google Scholar]
- 52.Jin H, Kang Y, Zhao L, Xiao C, Hu Y, She R, et al. Induction of adaptive T regulatory cells that suppress the allergic response by coimmunization of DNA and protein vaccines. J Immunol. 2008;180:5360–5372. doi: 10.4049/jimmunol.180.8.5360. [DOI] [PubMed] [Google Scholar]
- 53.Nordlund JJ, Askenase PW. The effect of histamine, antihistamines, and a mast cell stabilizer on the growth of cloudman melanoma cells in DBA/2 mice. J Invest Dermatol. 1983;81:28–31. doi: 10.1111/1523-1747.ep12538356. [DOI] [PubMed] [Google Scholar]
- 54.Wang J, Su B, Ding Z, Du X, Wang B. Cimetidine enhances immune response of HBV DNA vaccination via impairment of the regulatory function of regulatory T cells. Biochem Biophys Res Commun. 2008;372:491–496. doi: 10.1016/j.bbrc.2008.04.191. [DOI] [PubMed] [Google Scholar]
- 55.Lohr J, Knoechel B, Abbas AK. Regulatory T cells in the periphery. Immunol Rev. 2006;212:149–162. doi: 10.1111/j.0105-2896.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- 56.Kennedy Norton S, Barnstein B, Brenzovich J, Bailey DP, Kashyap M, Speiran K, et al. IL-10 suppresses mast cell IgE receptor expression and signaling in vitro and in vivo. J Immunol. 2008;180:2848–2854. doi: 10.4049/jimmunol.180.5.2848. [DOI] [PubMed] [Google Scholar]
- 57.Norozian F, Kashyap M, Ramirez CD, Patel N, Kepley CL, Barnstein BO, et al. TGFβ1 induces mast cell apoptosis. Exp Hematol. 2006;34:579–587. doi: 10.1016/j.exphem.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 58.Kashyap M, Thornton AM, Norton SK, Barnstein B, Macey M, Brenzovich J, et al. Cutting edge: CD4 T cell-mast cell interactions alter IgE receptor expression and signaling. J Immunol. 2008;180:2039–2043. doi: 10.4049/jimmunol.180.4.2039. [DOI] [PubMed] [Google Scholar]
- 59.Holgate ST, Lewis RA, Austen KF. Role of adenylate cyclase in immunologic release of mediators from rat mast cells: agonist and antagonist effects of purine- and ribose-modified adenosine analogs. Proc Natl Acad Sci USA. 1980;77:6800–6804. doi: 10.1073/pnas.77.11.6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Izushi K, Tasaka K. Histamine release from -escin-permeabilized rat peritoneal mast cells and its inhibition by intracellular Ca2+ blockers, calmodulin inhibitors and cAMP. Immunopharmacology. 1989;18:177–186. doi: 10.1016/0162-3109(89)90015-5. [DOI] [PubMed] [Google Scholar]
- 61.Winslow CM, Lewis RA, Austen KF. Mast cell mediator release as a function of cyclic AMP-dependent protein kinase activation. J Exp Med. 1981;154:1125–1133. doi: 10.1084/jem.154.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohshima Y, Tanaka Y, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J Immunol. 1997;159:3838–3848. [PubMed] [Google Scholar]
- 63.Heuer JG, Zhang T, Zhao J, Ding C, Cramer M, Justen KL, et al. Adoptive transfer of in vitro-stimulated CD4+CD25+ regulatory T cells increases bacterial clearance and improves survival in polymicrobial sepsis. J Immunol. 2005;174:7141–7146. doi: 10.4049/jimmunol.174.11.7141. [DOI] [PubMed] [Google Scholar]
- 64.Huang C, De Sanctis GT, O’Brien PJ, Mizgerd JP, Friend DS, Drazen JM, et al. Evaluation of the substrate specificity of human mast cell tryptase beta I and demonstration of its importance in bacterial infections of the lung. J Biol Chem. 2001;276:26276–26284. doi: 10.1074/jbc.M102356200. [DOI] [PubMed] [Google Scholar]
- 65.Huang C, Friend DS, Qiu WT, Wong GW, Morales G, Hunt J, et al. Induction of a selective and persistent extravasation of neutrophils into the peritoneal cavity by tryptase mouse mast cell protease 6. J Immunol. 1998;160:1910–1919. [PubMed] [Google Scholar]
- 66.Thakurdas SM, Melicoff E, Sansores-Garcia L, Moreira DC, Petrova Y, Stevens RL, et al. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem. 2007;282:20809–20815. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- 67.Ju MJ, Qiu SJ, Gao Q, Fan J, Cai MY, Li YW, et al. Combination of peritumoral mast cells and T-regulatory cells predicts prognosis of hepatocellular carcinoma. Cancer Sci. 2009;100:1267–1274. doi: 10.1111/j.1349-7006.2009.01182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wasiuk A, de Vries VC, Hartmann K, Roers A, Noelle RJ. Mast cells as regulators of adaptive immunity to tumours. Clin Exp Immunol. 2009;155:140–146. doi: 10.1111/j.1365-2249.2008.03840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 70.Hart PH, Grimbaldeston MA, Swift GJ, Jaksic A, Noonan FP, Finlay-Jones JJ. Dermal mast cells determine susceptibility to ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J Exp Med. 1998;187:2045–2053. doi: 10.1084/jem.187.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Depinay N, Hacini F, Beghdadi W, Peronet R, Mecheri S. Mast cell-dependent down-regulation of antigen-specific immune responses by mosquito bites. J Immunol. 2006;176:4141–4146. doi: 10.4049/jimmunol.176.7.4141. [DOI] [PubMed] [Google Scholar]
- 72.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 73.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, et al. Transforming growth factor- ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 74.de Vries VC, Wasiuk A, Bennett KA, Benson MJ, Elgueta R, Waldschmidt TJ, et al. Mast cell degranulation breaks peripheral tolerance. Am J Transplant. 2009;9:2270–2280. doi: 10.1111/j.1600-6143.2009.02755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kitamura Y, Go S, Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978;52:447–452. [PubMed] [Google Scholar]
- 76.Nigrovic PA, Gray DH, Jones T, Hallgren J, Kuo FC, Chaletzky B, et al. Genetic inversion in mast cell-deficient (Wsh) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am J Pathol. 2008;173:1693–1701. doi: 10.2353/ajpath.2008.080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wolters PJ, Mallen-St Clair J, Lewis CC, Villalta SA, Baluk P, Erle DJ, et al. Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient Kit(W−sh)/Kit(W−sh) sash mice. Clin Exp Allergy. 2005;35:82–88. doi: 10.1111/j.1365-2222.2005.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W−sh/W−sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pawankar R, Ra C. Heterogeneity of mast cells and T cells in the nasal mucosa. J Allergy Clin Immunol. 1996;98:S248–262. doi: 10.1016/s0091-6749(96)70073-8. [DOI] [PubMed] [Google Scholar]
- 80.Goyal R, Bulua AC, Nikolov NP, Schwartzberg PL, Siegel RM. Rheumatologic and autoimmune manifestations of primary immunodeficiency disorders. Curr Opin Rheumatol. 2009;21:78–84. doi: 10.1097/BOR.0b013e32831cb939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fietta P. Life-or-death fate in the adaptive immune system. Riv Biol. 2007;100:267–283. [PubMed] [Google Scholar]
- 82.Candotti F, Oakes SA, Johnston JA, Notarangelo LD, O’Shea JJ, Blaese RM. In vitro correction of JAK3-deficient severe combined immunodeficiency by retroviral-mediated gene transduction. J Exp Med. 1996;183:2687–2692. doi: 10.1084/jem.183.6.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shelburne CP, McCoy ME, Piekorz R, Sexl V, Roh KH, Jacobs-Helber SM, et al. Stat5 expression is critical for mast cell development and survival. Blood. 2003;102:1290–1297. doi: 10.1182/blood-2002-11-3490. [DOI] [PubMed] [Google Scholar]
- 84.Zhu Y, Herlaar E, Masuda ES, Burleson GR, Nelson AJ, Grossbard EB, et al. Immunotoxicity assessment for the novel Spleen tyrosine kinase inhibitor R406. Toxicol Appl Pharmacol. 2007;221:268–277. doi: 10.1016/j.taap.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 85.Braselmann S, Taylor V, Zhao H, Wang S, Sylvain C, Baluom M, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks Fc receptor signaling and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther. 2006;319:998–1008. doi: 10.1124/jpet.106.109058. [DOI] [PubMed] [Google Scholar]