Abstract
In the past century, gradual but sustained advances in our understanding of the molecular mechanisms involved in the growth and invasive properties of cancer cells have led to better management of tumors. However, many tumors still escape regulation and progress to advanced disease. Until recently, there has not been an organized and sustained focus on the “normal” cells in the vicinity of tumors. Interactions between the tumor and these host cells, as well as autonomous qualities of the host cells themselves, might explain why tumors in people with histologically similar cancers often behave and respond differently to treatment. Cells of the tumor microenvironment, variously referred to as cancer stroma, reactive stroma or carcinoma associated fibroblasts (CAF), exist in close proximity to the cancer epithelium. Both stromal and epithelial phenotypes co-evolve during tumorigenesis and it is now becoming clear that these stromal cells may not be the innocent bystanders they had been widely thought to be, but rather may be active contributors to carcinogenesis. Our group and others have shown the important role that CAF play in the progression of cancer. In this article we will address current trends in the study of the interactions between cancer stroma and tumor cells in different organs. We will also highlight perceived knowledge gaps and suggest research areas that need to be further explored to provide new targets for anti-cancer therapies.
Keywords: cancer associated fibroblast, stromal-epithelial interaction, tumor heterogeneity, tumor stroma, activated fibroblasts, myofibroblasts
Introduction
For many years malignant tumors were viewed as being composed of transformed cells that acquired cell-autonomous hyperproliferative and invasive survival capacities. However, tumors are complex organ facsimiles composed not only of the founder neoplastic cells, but also of tumor-associated stromal cells and an extracellular milieu that includes matrix components, chemokines and cytokines, all of which increase the complexity of the tumor microenvironment. Fibroblasts are an important component of the tumor stroma. The term fibroblast encompasses a number of stromal cells with a broadly similar phenotype. Most tumors incorporate an obvious biologically active, fibroblastic cell type known variously as reactive fibroblasts, myofibroblasts, or simply carcinoma-associated fibroblasts. These cells have received increased attention because of their participation in tumor development, including invasion and metastasis and their ability to act as markers of patient prognosis [1]. Thus, elucidation of the critical cellular and/or molecular events that orchestrate tumor evolution is important as these represent potentially attractive targets for therapeutic intervention. The purpose of this review is to summarize our current understanding of these cells, highlighting recent advances and analyzing the therapeutic potential of targeting CAF.
Stromal influence on adult tissue homeostasis
Maintenance of the anatomical status quo, or tissue homeostasis, is crucial for the preservation of normal tissue morphology and the proper function of organs. In adult tissue, this task is tightly controlled by the stromal compartment through interactions between the different cell types and the production of extracellular cell matrix (ECM) components that provide the structure for proper tissue architecture and function.
The identification of multipotent mesenchymal stem cells (MSC) derived from adult human tissues, including bone marrow stroma and a number of connective tissues, has provided a new understanding of tissue homeostasis. These cells have the potential to differentiate into chondrocytes, osteoblasts, adipocytes, fibroblasts, marrow stroma, and other tissues of mesenchymal origin. Interestingly, these MSC reside in a diverse host of tissues throughout the adult organism and possess the ability to ‘regenerate’ cell types specific for these tissues, reviewed in [2]. Given the wide distribution of the sources of MSC, the bone marrow stroma may be considered to be the source of a common pool of multipotent cells that gain access to various tissues via the circulation, subsequently adopting characteristics that meet the requirements of maintenance and repair of a specific tissue type. In fact, the presence of MSC in tissues other than the marrow stroma strongly suggests the existence of cell populations with more limited capacity for differentiation; specifically, monopotent or bipotent cells may have differentiation potentials developmentally adapted to (and perhaps restricted to) the tissues in which they reside and help to the maintain tissue architecture.
It is well known that during the wound healing process, fibroblast-to-myofibroblast differentiation is a key event in the physiological reconstruction of the connective tissue. Myofibroblasts remodel the ECM and induce angiogenesis [3]. Smooth muscle α-actin (αSMA) is the most common marker used to identify myofibroblasts, but other markers such as vimentin, desmin, or smooth muscle myosin can be used in combination with αSMA to distinguish between different subpopulations of myofibroblasts and normal fibroblasts or smooth muscle cells [4, 5]. Myofibroblast differentiation is induced by paracrine signals generated by repairing or injured tissues. Among those signals, transforming growth factor beta (TGF-β) is among the most potent. TGF-β activates myofibroblast differentiation of prostate fibroblasts [6]. The identification of myofibroblasts in prostate cancer patient tissue predicts tumor relapse [7] Under normal circumstances once the tissue is repaired, myofibroblasts and other resident cells undergo apoptosis, and the remaining collagen-rich tissue is slowly remodeled to a normal phenotype [8]. In cancers, sometimes described as resembling chronic wounds, the origin of myofibroblasts remains controversial; however, their presence may be essential for much invasive growth and is translated into poor clinical prognosis [9]. Soluble factors secreted by myofibroblasts have been shown to mediate the invasive growth of breast and colon cancer cells in vitro and in vivo [10]. Thus, management of stromal reaction through prevention of the formation of myofibroblasts is a potentially attractive opportunity for therapy.
Tumor-promoting ability of carcinoma associated fibroblasts in different organs
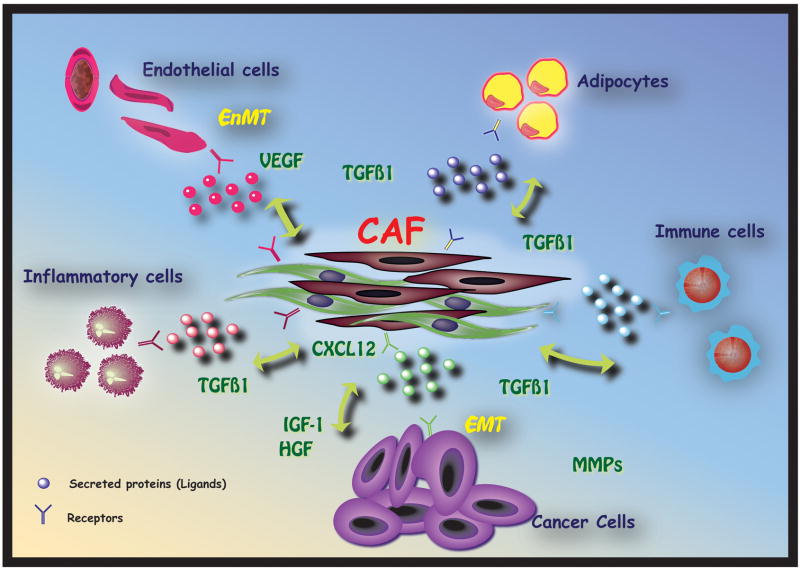
The role of cancer-associated fibroblasts in tumor progression is now well accepted. Many “in situ” cancers never progress to an invasive type, most likely due to host factors that prevent this development, a phenomenon termed “cancer without disease”. This highlights the fact that a transformed cell per se is not enough to cause lethal cancer, but that carcinogenesis requires the recruitment of a tumor microenvironment permissive of further tumor growth and metastasis [11]. The stromal microenvironment is complex and contains many cell types including fibroblasts, smooth muscle cells, immune and inflammatory cells, adipocytes and endothelial cells Figure 1. Of utmost significance for tumor progression, as the primary stromal cell type that produces extracellular matrix (ECM), fibroblasts can bi-directionally ‘sense’ signals between ECM and cells by means of their integrin-dependent cell–matrix adhesions, eliciting changes in stromal dynamics and composition.
Fig 1. Carcinoma Associated Fibroblasts play a central role in the modulation of cancer growth.
CAF are composed of a mixture of fibroblasts which might have different origins. When activated, these cells interact with cancer cells and start expressing several mitogenic and pro-invasive factors that create a favorable milieu for tumor cells to proliferate and invade into the surrounding tissue. Chemokines and cytokines can act in a paracrine manner binding to their cognate receptors resulting in bidirectional or multi-directional communication.
Changes in stromal characteristics can be perceived as an initial attempt to ‘repair the damage’ induced by transformed epithelium. There is a complex interplay of reciprocal stromal–epithelial interactions that essentially fools repair mechanisms resulting in changes in the ECM and the tumor stroma, a phenomena called “stromogenesis” [5]. Carcinogenesis then results from the loss or breakdown of biological organization induced by perturbed stromal–epithelial interactions, rather than from epithelial or stromal mutations alone [12]. There is substantial evidence for the contention that cancer-associated fibroblasts produce a tumor supportive ECM, which promotes the growth, expansion and dissemination of the pre-neoplastic epithelial cell population, creating a permissive ‘pasture’ for the emerging malignant cell [4]. Stromal cells are though to be critical drivers of tumor progression in a number of organs.
1. Prostate
Prostate cancer is the most common non-skin malignancy in men in the USA and the second leading cause of male cancer death [13]. The stromal component of prostate cancer co-evolves with tumor cells and several lines of experimental and clinical evidence show that a dynamic interaction between stroma and epithelium plays a critical role in this progression. Using a xenograft model of human cells, we and others have shown that recombination of CAF with non-tumorigenic epithelial cells results in permanent malignant transformation of non-tumorigenic epithelial cells [14, 15]. More recently, it has been shown that inhibition of the retinoblastoma gene function in epithelial cells can induce a selective expansion of a subpopulation of highly proliferative p53-null stromal cells through a paracrine mechanism [16].
Genetic stability and hormones
Although the stroma is usually genetically stable, lesions on chromosome 8p have been detected, and a high incidence (33%) of loss of heterogeneity (LOH) has been reported in the tumor stroma of prostate cancer patients [17] although this mechanism is not universally accepted. Several studies support the occurrence of a field effect for gene silencing in the stroma by cancer cells through hypermetylation. Among the genes implicated are GSTP1, APC, RASSF1A, H1N-1 and RARβ2 [18, 19]. More research is needed to clarify the contribution of any of these candidates in prostate cancer.
In a switch from its activity in the developing and normal adult organ, androgens promote prostate cancer growth through direct activation of the epithelial androgen receptor (AR) [20]. However, the role of AR in the tumor stroma has received little attention. Recent studies have shown that elevated AR expression in cancer cells concurrent with loss of AR in the surrounding stroma was correlated with higher clinical stage, higher PSA levels, and earlier and higher relapse rates after radical prostatectomy [21, 22]. It has been suggested that CAF could be derived from AR-negative fibroblasts that surround normal glands or from dedifferentiated smooth muscle cells [23]. More experimental studies are needed to further investigate the mechanism of AR loss within the stroma and its potential role in the progression of prostate cancer.
Soluble factors secreted by CAF
Stromal-epithelial interactions in prostate cancer involve a number of soluble factors and their receptors. These factors can act in a paracrine manner and work in coordination with other signaling molecules, such as ECM and integrins, which facilitate not only carcinogenesis, but cancer progression towards metastasis [24].
In cancer, Transforming growth factor beta 1 (TGF-β1) is expressed at elevated levels to a greater extent than TGF-β2 or TGF-β3. In normal prostate epithelial cells, TGF-β controls homeostasis by eliciting differentiation, inhibiting proliferation and inducing apoptosis. However, TGF-β signaling in fibroblasts modulates the growth and the oncogenic potential of adjacent epithelia. For example, inactivation of the TGFβR2 in mouse fibroblasts results in the appearance of prostatic intraepithelial neoplasia in prostate tissue in association with an increased number of stromal cells [25]. TGF-β often suppresses transformation and early tumorigenesis but, as cancer progresses, cancer cells become insensitive to the growth inhibitory effects of TGF-β [26]. Signaling mediated by TGF-β involves the activation of direct downstream effectors including PI3K/AKT, MAPK, TAK1, Ras, Rho. However, the only downstream targets that are thought to be TGF-β-specific are SMAD molecules. Loss of regulation of Wnt expression resulting from reduced TGF-β function has been implicated in prostate cancer progression [27]. TGF-β2 can promote cancer cell survival and resistance to apoptosis, effects that are attributable to the activation of nuclear factor-kappaB (NF-κB) [28]. These observations provide evidence supporting the clear association with cancer progression and makes TGF-β a potential target for therapeutic intervention.
Insulin-like growth factor-1 (IGF-1) is expressed primarily in prostatic stroma. Paracrine action of IGF includes proliferation of prostate epithelial cells through upregulation of MAPK, Akt and Cyclin D1 and downregulation of p27. Overexpression of IGF-1 has been shown to elicit neoplastic transformation of murine epithelium and significantly increased the invasive capacity of prostate cancer cell lines in vitro. Blockade of the IGF-1R or the use of specific inhibitors of the MAPK and PI3K pathways can decrease IGF-mediated tumor invasion [29]. Inhibition of IGF-1R signaling results in cytoplasmic AR retention accompanied by significant changes in androgen-regulated gene expression [30]. Another important interaction is that observed between IGF-1 and TGF-β1. Significantly, activation of the IGF-1R interferes with TGF-β1 activation of Smad3 thereby blocking TGF-β-mediated apoptosis in prostate epithelial cells [26]. Interactions between IGF-1, TGF-β1 and androgens highlight the importance of these factors during cancer progression and may account for the conversion to androgen-independence in the absence of androgen ligand.
HGF/scatter factor is expressed throughout the prostate stroma and acts in a paracrine fashion on epithelial cells where it binds to its receptor c-met. C-met is expressed in about 50% of localized prostate cancer and in almost all metastatic lesions [31]. Elevated expression of HGF/SF was shown to regulate invasion and growth of prostate cancer cells [32]. HGF can induce bone morphogenetic protein-7 (BMP-7), both in vitro and in vivo in prostate cancer models [33]. Androgen signaling downregulate c-met expression and androgen withdrawal can activate c-met indicating that c-met signaling may have a role in androgen independent progression of prostate cancer [34].
Recently several groups, including ours, have started to focus on the role of stromal-derived chemokines in prostate cancer progression. The pleiotropic actions of chemokines include the modulation of growth, angiogenesis, invasion, and metastasis [35]. While cancer cells can express all of the ligands, stromally derived chemokines include CXCL2, CXCL5, CXCL6 and CXCL12 [35].
In a recent study, we demonstrated that elevated TGF-β expression in the tumor stroma can activate CXCR4 expression in adjacent epithelial cells allowing the cognate ligand for this receptor, SDF1/CXCL12 (which is also expressed at elevated levels in the tumor stroma) to activate the receptor and thus stimulate phosphorylation of Akt in the epithelial cells [27, 36]. In addition, phosphorylation of Akt in human prostatic epithelium inhibits the nuclear translocation of the activated Smad2,3,4 complex thus allowing cells to ignore the growth inhibitory effects of TGF-β [37]. CXCR4 is elevated in localized and metastatic cancer and is a marker of poor prognosis [38].
2. Breast
Breast cancer is the most common form of cancer and is the second most common cause of cancer death in women [13]. The stroma of the breast is composed of fibroblasts, adipocytes, endothelial cells, pericytes, immune components and nerves. Stromal-epithelial interactions are crucial for proper breast development and breast tumor stroma can alter the proliferation, survival, differentiation, invasion and metastatic ability of cancer cells.
In breast tumors, the normal stromal microenvironment is drastically changed. These changes include increased numbers of fibroblasts, including myofibroblasts, infiltration of inflammatory cells, angiogenesis and ECM remodeling. This is coupled with changes in epithelial cell activities such as proliferation, differentiation, invasion and survival. Many studies have examined the ability of the altered stromal cell types to induce the observed changes in epithelial cell activity. In one study, mammary fibroblasts that are unable to respond to TGF-β were grafted with breast carcinoma cells and implanted into mice. Tumor growth, invasion and metastasis were increased [39, 40]. This shows that molecular alterations of tumor stromal cells alone can significantly alter the behavior and progression of tumors. Lethally irradiated fibroblasts or inclusion of fibroblast-conditioned medium in breast cancer cell grafts increased tumorigenicity and tumor growth, showing that soluble secreted products from fibroblasts promote tumors [41]. Of these soluble, secreted factors, CXCL12 and CXCL14 are secreted by myofibroblasts in breast tumors and increase proliferation, invasion and metastasis of breast cancer cells [42, 43]. It has been suggested that myofibroblasts in tumors may arise from infiltration of bone marrow-derived mesenchymal stem cells (MSC). Subcutaneous grafts of MSC mixed with breast cancer cells enhanced lung metastasis. Breast cancer cells from these grafts were re-derived from the grafts and subcutaneously grafted again. These grafts did not have the same increased metastatic rate, showing that there are no irreversible changes in the cancer cells and that enhanced metastasis is dependent on the paracrine interaction with MSC. This study went on to show that this activity is dependent on CCL5-CCR5 interactions [44]. The Hedgehog pathway, which is essential for normal mammary gland development, is also essential for maintenance of the CD44+/CD24low subpopulation of breast cancer cells that resemble mammary stem cells [45]. Expression of the Hedgehog target gene Gli1 correlates with poor prognosis in breast cancer [46]. In this study, Gli1 expression was primarily noted in cancer cells and correlated with another Hedgehog ligand, Sonic Hedgehog. This suggests that maintenance of a stem cell-like population of breast cancer cells operates on a pathway that is independent of the Ihh-Gli2 pathway that controls epithelial and fibroblast proliferation in ductal development. These studies demonstrate the links between normal developmental pathways and those active in cancer. Future studies should focus on the pathways, such as TGF-β, Hedgehog and chemokines, in breast cancer development and progression.
Examination of the molecular changes in stromal cells that control cancer development and progression in the breast have shown that these include alterations in expression of many genes. This includes genes that remodel ECM, alter growth factor and chemokine signaling, stimulate inflammation and increase migration and invasion [47]. Since many of these changes in gene expression are maintained for long periods, including in cell culture, it is possible that these changes are genetically heritable. These may include gene amplification and deletion or microsatellite instability and point mutations [48–51] as well as promoter methylation. However, this concept of heritable changes in CAF is not widely accepted since some studies have shown the absence of such aberrations in breast cancer stroma [52]. Future studies will clarify this and should delineate possible differences in breast cancer sub-types.
3. Pancreas
Pancreatic cancer is the most lethal abdominal malignancy. Mortality reaches an alarming 95% of patients within 5 years and on average, patients live only 6 months after diagnosis [53]. One of the defining characteristics of pancreatic ductal adenocarcinoma (PDAC) is the presence of extensive stromal response called desmoplasia. However, the precise role of the stroma in pancreatic cancer is poorly understood. The desmoplastic stroma consists of proliferating fibroblasts and pancreatic stellate cells, inflammatory cells, nerve fibers, and marrow-derived stem cells. The predominant mesenchymal cell within the stroma is the stellate cell, which resembles myofibroblasts commonly found in the stroma of other types of cancer. Stellate cells are rarely found in the normal pancreas but are prevalent during inflammation and in malignant disease. These cells when quiescent, express the intermediate filament protein desmin, and when activated, start expressing smooth muscle actin, produce large amounts of ECM proteins (mostly collagen I and III) and release other inflammatory mediators, including TGF-β [54]. Autocrine production of PDGF, TGF-β, cytokines (IL-1, IL-6, and tumor necrosis factor-related apoptosis-inducing ligand) and COX-2 potentiates the stromal phenotype. Despite the lack of effective in vivo systems to evaluate the role of the stromal reaction in pancreatic cancer, some clues have been obtained from xenograft models. Overexpression of TGF-β in Panc-1 tumor cells induced a dramatic response within the stroma in orthotopically transplanted nude mice, similar to that observed in human prostatic ductal adenocarcinoma [55].
KRAS proto-oncogene mutations are present in more than 90% of human pancreatic tumors. However, attempts to model in KRAS mutations in mice gave mixed results. Endogenous mutant expression of KRAS (G12D) alone or in combination with deletion of the tumor suppressor allele CDKN2/Ink-4a/Arf can induce the entire progression of pancreatic cancer, from preinvasive (PanIN-1 to PanIN-3) to invasive and metastatic disease. These tumors appear to bear a strong resemblance to human pancreatic cancer with a proliferative stromal component. Finally, one of the most compelling studies that supports a critical role for stroma in pancreatic cancer is the finding that mice with Smad7 deletion in stromal cells develop PanIN accompanied with increased fibrosis around the ductal regions [56]. Based on these observations and the aggressive behavior of this type of cancer more exhaustive research is needed to develop strategies for targeting the stroma of pancreatic cancer.
Heterogeneity of stromal cells
Fibroblasts are highly heterogeneous [57]. In vivo the fibroblastic populations surrounding tumors have been shown to contain a number of distinct phenotypes [58]. Clonal modulation of fibroblast subsets is a potential mechanism underlying the pathogenesis of cancer and may play a role in modulating the proliferation of tumor cells. The stroma contains fibroblasts (normal and activated), inflammatory cells, immunocytes, macrophages, lymph and endothelial cells and nerves. Fibroblasts isolated from human cancer patients have been referred as simply carcinoma associated fibroblasts. However, CAF remains a poorly defined group of cells commonly identified using biological assays. CAF have classically been defined by the absence of epithelial cell marker cytokeratins, and the expression of vimentin and α-SMA. However, a recent study that used the vimentin, αSMA, type I collagen, FSP1 (S100A4), PDGFRβ and NG2 markers to examine fibroblasts heterogeneity within breast and pancreatic carcinomas indicated that distinct fibroblasts sub-populations could be identified and quantified within each tumor microenvironment [58]. Of these markers, co-expression of αSMA and FAP were characteristic of myofibroblasts. FSP1 could identify fibroblasts, and in combination with the other markers form a picture of what constitutes the CAF populations. It still remains to be determined whether the presence of any particular population or combination of populations within the stroma is essential for tumor progression. Some clues have come from a recent work in which mammary carcinoma cells in combination with FSP1+/+ mouse embryonic fibroblasts were able to promote progression to metastasis in FSP−/− mice [59]. Currently it is not known how many fibroblast sub-populations are present within the tumor stroma, and whether the presence of an individual population or the presence of all of these cell types are equally important for the initial steps during tumor development.
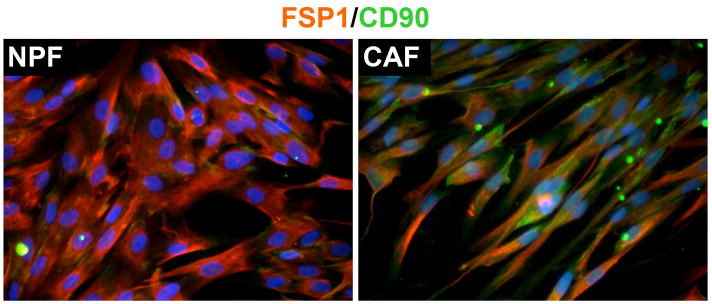
Another potentially controversial area of CAF research is focused on the origin of CAF. While local fibroblasts were originally considered to be the most likely major source of CAF, recent studies have shown that there are other additional sources depending on the type of cancer. For example bone marrow-derived MSC can contribute up to 25% of the total myofibroblast population in the tumor stroma in a mouse xenograft model of pancreatic carcinoma [60]. Once recruited, MSC become activated and start secreting matrix proteins helping cancer cells to metastasize. In a similar study, MSC had the ability to increase the metastatic potential of weakly metastatic breast cancer cells by upregulation of the chemokine CCL5 [44]. It has been shown recently that fibroblasts isolated from human prostate cancer samples that express high levels of the MSC marker CD90 (CD90hi) may have tumor-promoting potential compared to a subpopulation of low CD90 expressors (CD90lo) [61] Figure 2. On the other hand MSC have been shown to inhibit tumor growth in a model of Kaposi’s sarcoma [62]. Due to their ability to localize in tumor sites, genetically modified MSC carrying the human interferonβ gene were injected in tumor bearing mice. These cells were able to decrease not only the size of the primary tumor, but also the number of metastasis compared to control animals [63]. A better understanding of the interplay between MSC and tumor cells in relation to the tumor microenvironment will be important in developing strategies for therapy. It has also been suggested that malignant epithelial cells undergoing EMT can integrate within the tumor stroma and contribute to the CAF population. While this is a plausible hypothesis definitive in vivo experiments and clinical correlates are not presently available. Studies on genetic alterations in tumor stroma described above have the potential to shed some light in this area.
Fig 2. Immunofluorescence analysis of fibroblast-specific protein 1 (FSP1) and the mesenchymal stem marker CD90.
Human normal prostate fibroblast (NPF) and carcinoma associated fibroblast (CAF) were isolated, double-labeled with antibodies against FSP1 and CD90 and visualized using fluorochrome-conjugated (FITC or TRITC) secondary antibodies. NPF show homogeneous expression of FSP1 and low expression of CD90. However CAF presented not only a heterogeneous expression of FSP1 and CD90 but also high intensity pattern of expression for CD90 positive cells.
Recently, studies have demonstrated a phenomena called endothelial-to-mesenchymal transition occurs in areas of fibrosis and can account for up to 40% of the tumor associated fibroblasts [64]. The molecular mechanism of EnMT in tumors has not yet been specifically studied, but it has been suggested to be mediated by TGF-β and inhibited by BMP-7 [65]. EnMT may play a role in the genesis of vascular networks composed of “tip cells” (vessels without a lumen) an important mechanism for stabilizing the neovasculature. Clearly, more research is warranted in this area to: a) identify and characterize the biological relevant subpopulation(s), b) identify the factors responsible for the activation/maintenance/recruitment of CAF and c) investigate how to modulate them to possibly normalize the stroma by generation of therapeutic agents.
Summary and Future Opportunities
The current trend in cancer research is the inclusion of the microenvironment as a major contributor of cancer progression. The tumor-inducing actions of CAF start during the conversion of stressed normal epithelial cells to cancer cells and continue through progression to metastasis.
The demonstrated tumor-promoting capacity of CAF has increased interest in exploiting them as therapeutic targets for anticancer therapy. Inhibiting the secretion of tumor-promoting factors, or neutralization of these factors seems to be the first line approach. Several molecular candidates have been proposed in this article. However a better understanding of the effects of targeting these pathways is needed. Another potential therapeutic approach is the interference with the recruitment or activation of CAF. Promising results in experimental models have been obtained when targeting growth factors such as PDGF, TGF-β, chemokines like CXCL12 and inhibitors to the Hedgehog signaling. Similar results have been documented in immunotherapy approaches when using FAP antibodies in animal models. Together, these studies emphasize the importance of cross talk between stromal and malignant cells of the tumor. It is likely that the continued characterization of these interactions, and the molecular identification of key mediators, will provide insights into tumor biology and suggest further novel therapeutic options.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ayala G, Tuxhorn JA, Wheeler TM, Frolov A, Scardino PT, Ohori M, et al. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin Cancer Res. 2003;9(13):4792–801. [PubMed] [Google Scholar]
- 2.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9(1):204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuxhorn JA, McAlhany SJ, Dang TD, Ayala GE, Rowley DR. Stromal cells promote angiogenesis and growth of human prostate tumors in a differential reactive stroma (DRS) xenograft model. Cancer Res. 2002;62(11):3298–307. [PubMed] [Google Scholar]
- 4.Kunz-Schughart LA, Knuechel R. Tumor-associated fibroblasts (part I): Active stromal participants in tumor development and progression? Histol Histopathol. 2002;17(2):599–621. doi: 10.14670/HH-17.599. [DOI] [PubMed] [Google Scholar]
- 5.Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol. 2001;166(6):2472–83. [PubMed] [Google Scholar]
- 6.Tuxhorn JA, McAlhany SJ, Yang F, Dang TD, Rowley DR. Inhibition of transforming growth factor-beta activity decreases angiogenesis in a human prostate cancer-reactive stroma xenograft model. Cancer Res. 2002;62(21):6021–5. [PubMed] [Google Scholar]
- 7.Yanagisawa N, Li R, Rowley D, Liu H, Kadmon D, Miles BJ, et al. Stromogenic prostatic carcinoma pattern (carcinomas with reactive stromal grade 3) in needle biopsies predicts biochemical recurrence-free survival in patients after radical prostatectomy. Hum Pathol. 2007;38(11):1611–20. doi: 10.1016/j.humpath.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol. 2004;36(6):1031–7. doi: 10.1016/j.biocel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315(26):1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 10.De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. International Journal of Cancer. 2008;123(10):2229–2238. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 11.Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427(6977):787. doi: 10.1038/427787a. [DOI] [PubMed] [Google Scholar]
- 12.Weaver VM, Gilbert P. Watch thy neighbor: cancer is a communal affair. J Cell Sci. 2004;117(Pt 8):1287–90. doi: 10.1242/jcs.01137. [DOI] [PubMed] [Google Scholar]
- 13.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 14.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59(19):5002–11. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, Grossfeld GD, et al. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res. 2001;61(22):8135–42. [PubMed] [Google Scholar]
- 16.Hill R, Song Y, Cardiff RD, Van Dyke T. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell. 2005;123(6):1001–11. doi: 10.1016/j.cell.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 17.Macintosh CA, Stower M, Reid N, Maitland NJ. Precise microdissection of human prostate cancers reveals genotypic heterogeneity. Cancer Res. 1998;58(1):23–8. [PubMed] [Google Scholar]
- 18.Grover AC, Tangrea MA, Woodson KG, Wallis BS, Hanson JC, Chuaqui RF, et al. Tumor-associated endothelial cells display GSTP1 and RARbeta2 promoter methylation in human prostate cancer. J Transl Med. 2006;4:13. doi: 10.1186/1479-5876-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krop I, Player A, Tablante A, Taylor-Parker M, Lahti-Domenici J, Fukuoka J, et al. Frequent HIN-1 promoter methylation and lack of expression in multiple human tumor types. Mol Cancer Res. 2004;2(9):489–94. [PubMed] [Google Scholar]
- 20.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25(2):276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 21.Ricciardelli C, Choong CS, Buchanan G, Vivekanandan S, Neufing P, Stahl J, et al. Androgen receptor levels in prostate cancer epithelial and peritumoral stromal cells identify non-organ confined disease. Prostate. 2005;63(1):19–28. doi: 10.1002/pros.20154. [DOI] [PubMed] [Google Scholar]
- 22.Henshall SM, Quinn DI, Lee CS, Head DR, Golovsky D, Brenner PC, et al. Altered expression of androgen receptor in the malignant epithelium and adjacent stroma is associated with early relapse in prostate cancer. Cancer Res. 2001;61(2):423–7. [PubMed] [Google Scholar]
- 23.Wikstrom P, Marusic J, Stattin P, Bergh A. Low stroma androgen receptor level in normal and tumor prostate tissue is related to poor outcome in prostate cancer patients. Prostate. 2009;69(8):799–809. doi: 10.1002/pros.20927. [DOI] [PubMed] [Google Scholar]
- 24.Chung LW, Baseman A, Assikis V, Zhau HE. Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J Urol. 2005;173(1):10–20. doi: 10.1097/01.ju.0000141582.15218.10. [DOI] [PubMed] [Google Scholar]
- 25.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303(5659):848–51. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 26.Danielpour D. Functions and regulation of transforming growth factor-beta (TGF-beta) in the prostate. Eur J Cancer. 2005;41(6):846–57. doi: 10.1016/j.ejca.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Placencio V, Iturregui JM, Uwamariya C, Sharif-Afshar AR, Koyama T, et al. Prostate tumor progression is mediated by a paracrine TGF-beta/Wnt3a signaling axis. Oncogene. 2008;27(56):7118–30. doi: 10.1038/onc.2008.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu T, Burdelya LG, Swiatkowski SM, Boiko AD, Howe PH, Stark GR, et al. Secreted transforming growth factor beta2 activates NF-kappaB, blocks apoptosis, and is essential for the survival of some tumor cells. Proc Natl Acad Sci U S A. 2004;101(18):7112–7. doi: 10.1073/pnas.0402048101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saikali Z, Setya H, Singh G, Persad S. Role of IGF-1/IGF-1R in regulation of invasion in DU145 prostate cancer cells. Cancer Cell Int. 2008;8:10. doi: 10.1186/1475-2867-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu JD, Haugk K, Woodke L, Nelson P, Coleman I, Plymate SR. Interaction of IGF signaling and the androgen receptor in prostate cancer progression. J Cell Biochem. 2006;99(2):392–401. doi: 10.1002/jcb.20929. [DOI] [PubMed] [Google Scholar]
- 31.Knudsen BS, Edlund M. Prostate cancer and the met hepatocyte growth factor receptor. Adv Cancer Res. 2004;91:31–67. doi: 10.1016/S0065-230X(04)91002-0. [DOI] [PubMed] [Google Scholar]
- 32.Verras M, Lee J, Xue H, Li TH, Wang Y, Sun Z. The androgen receptor negatively regulates the expression of c-Met: implications for a novel mechanism of prostate cancer progression. Cancer Res. 2007;67(3):967–75. doi: 10.1158/0008-5472.CAN-06-3552. [DOI] [PubMed] [Google Scholar]
- 33.Ye L, Lewis-Russell JM, Sanders AJ, Kynaston H, Jiang WG. HGF/SF up-regulates the expression of bone morphogenetic protein 7 in prostate cancer cells. Urol Oncol. 2008;26(2):190–7. doi: 10.1016/j.urolonc.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 34.Maeda A, Nakashiro K, Hara S, Sasaki T, Miwa Y, Tanji N, et al. Inactivation of AR activates HGF/c-Met system in human prostatic carcinoma cells. Biochem Biophys Res Commun. 2006;347(4):1158–65. doi: 10.1016/j.bbrc.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 35.Vindrieux D, Escobar P, Lazennec G. Emerging roles of chemokines in prostate cancer. Endocr Relat Cancer. 2009;16(3):663–73. doi: 10.1677/ERC-09-0109. [DOI] [PubMed] [Google Scholar]
- 36.Ao M, Franco OE, Park D, Raman D, Williams K, Hayward SW. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007;67(9):4244–53. doi: 10.1158/0008-5472.CAN-06-3946. [DOI] [PubMed] [Google Scholar]
- 37.Ao M, Williams K, Bhowmick NA, Hayward SW. Transforming growth factor-beta promotes invasion in tumorigenic but not in nontumorigenic human prostatic epithelial cells. Cancer Res. 2006;66(16):8007–16. doi: 10.1158/0008-5472.CAN-05-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akashi T, Koizumi K, Tsuneyama K, Saiki I, Takano Y, Fuse H. Chemokine receptor CXCR4 expression and prognosis in patients with metastatic prostate cancer. Cancer Sci. 2008;99(3):539–42. doi: 10.1111/j.1349-7006.2007.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng N, Bhowmick NA, Chytil A, Gorksa AE, Brown KA, Muraoka R, et al. Loss of TGF-beta type II receptor in fibroblasts promotes mammary carcinoma growth and invasion through upregulation of TGF-alpha-, MSP- and HGF-mediated signaling networks. Oncogene. 2005;24(32):5053–68. doi: 10.1038/sj.onc.1208685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng N, Chytil A, Shyr Y, Joly A, Moses HL. Enhanced hepatocyte growth factor signaling by type II transforming growth factor-beta receptor knockout fibroblasts promotes mammary tumorigenesis. Cancer Res. 2007;67(10):4869–77. doi: 10.1158/0008-5472.CAN-06-3381. [DOI] [PubMed] [Google Scholar]
- 41.Noel A, De Pauw-Gillet MC, Purnell G, Nusgens B, Lapiere CM, Foidart JM. Enhancement of tumorigenicity of human breast adenocarcinoma cells in nude mice by matrigel and fibroblasts. Br J Cancer. 1993;68(5):909–15. doi: 10.1038/bjc.1993.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 43.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 44.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka H, Nakamura M, Kameda C, Kubo M, Sato N, Kuroki S, et al. The Hedgehog signaling pathway plays an essential role in maintaining the CD44+CD24-/low subpopulation and the side population of breast cancer cells. Anticancer Res. 2009;29(6):2147–57. [PubMed] [Google Scholar]
- 46.ten Haaf A, Bektas N, von Serenyi S, Losen I, Arweiler EC, Hartmann A, et al. Expression of the glioma-associated oncogene homolog (GLI) 1 in human breast cancer is associated with unfavourable overall survival. BMC Cancer. 2009;9:298. doi: 10.1186/1471-2407-9-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sadlonova A, Bowe DB, Novak Z, Mukherjee S, Duncan VE, Page GP, et al. Identification of Molecular Distinctions Between Normal Breast-Associated Fibroblasts and Breast Cancer-Associated Fibroblasts. Cancer Microenviron. 2009 doi: 10.1007/s12307-008-0017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6(1):17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 49.Kurose K, Gilley K, Matsumoto S, Watson PH, Zhou XP, Eng C. Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat Genet. 2002;32(3):355–7. doi: 10.1038/ng1013. [DOI] [PubMed] [Google Scholar]
- 50.Moinfar F, Man YG, Arnould L, Bratthauer GL, Ratschek M, Tavassoli FA. Concurrent and independent genetic alterations in the stromal and epithelial cells of mammary carcinoma: implications for tumorigenesis. Cancer Res. 2000;60(9):2562–6. [PubMed] [Google Scholar]
- 51.Fukino K, Shen L, Matsumoto S, Morrison CD, Mutter GL, Eng C. Combined total genome loss of heterozygosity scan of breast cancer stroma and epithelium reveals multiplicity of stromal targets. Cancer Res. 2004;64(20):7231–6. doi: 10.1158/0008-5472.CAN-04-2866. [DOI] [PubMed] [Google Scholar]
- 52.Qiu W, Hu M, Sridhar A, Opeskin K, Fox S, Shipitsin M, et al. No evidence of clonal somatic genetic alterations in cancer-associated fibroblasts from human breast and ovarian carcinomas. Nat Genet. 2008;40(5):650–5. doi: 10.1038/ng.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lillemoe KD, Yeo CJ, Cameron JL. Pancreatic cancer: state-of-the-art care. CA Cancer J Clin. 2000;50(4):241–68. doi: 10.3322/canjclin.50.4.241. [DOI] [PubMed] [Google Scholar]
- 54.Aoki H, Ohnishi H, Hama K, Shinozaki S, Kita H, Yamamoto H, et al. Existence of autocrine loop between interleukin-6 and transforming growth factor-beta1 in activated rat pancreatic stellate cells. J Cell Biochem. 2006;99(1):221–8. doi: 10.1002/jcb.20906. [DOI] [PubMed] [Google Scholar]
- 55.Lohr M, Schmidt C, Ringel J, Kluth M, Muller P, Nizze H, et al. Transforming growth factor-beta1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res. 2001;61(2):550–5. [PubMed] [Google Scholar]
- 56.Kuang C, Xiao Y, Liu X, Stringfield TM, Zhang S, Wang Z, et al. In vivo disruption of TGF-beta signaling by Smad7 leads to premalignant ductal lesions in the pancreas. Proc Natl Acad Sci U S A. 2006;103(6):1858–63. doi: 10.1073/pnas.0508977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schor SL, Schor AM. Clonal heterogeneity in fibroblast phenotype: implications for the control of epithelial-mesenchymal interactions. Bioessays. 1987;7(5):200–4. doi: 10.1002/bies.950070503. [DOI] [PubMed] [Google Scholar]
- 58.Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5(12):1640–6. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- 59.Grum-Schwensen B, Klingelhofer J, Berg CH, El-Naaman C, Grigorian M, Lukanidin E, et al. Suppression of tumor development and metastasis formation in mice lacking the S100A4(mts1) gene. Cancer Res. 2005;65(9):3772–80. doi: 10.1158/0008-5472.CAN-04-4510. [DOI] [PubMed] [Google Scholar]
- 60.Ishii G, Sangai T, Oda T, Aoyagi Y, Hasebe T, Kanomata N, et al. Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction. Biochem Biophys Res Commun. 2003;309(1):232–40. doi: 10.1016/s0006-291x(03)01544-4. [DOI] [PubMed] [Google Scholar]
- 61.Zhao HJ, Peehl DM. Tumor-Promoting Phenotype of CD90(hi) Prostate Cancer-Associated Fibroblasts. Prostate. 2009;69(9):991–1000. doi: 10.1002/pros.20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J Exp Med. 2006;203(5):1235–47. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96(21):1593–603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 64.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67(21):10123–8. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 65.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13(8):952–61. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]