Abstract
Extracellular matrix (ECM) components play essential roles in development, remodeling, and signaling in the cardiovascular system. They are also important in determining the mechanics of blood vessels, valves, pericardium, and myocardium. The goal of this brief review is to summarize available information regarding the mechanical contributions of ECM in the myocardium.
Fibrillar collagen, elastin, and proteoglycans all play crucial mechanical roles in many tissues in the body generally and in the cardiovascular system specifically. The myocardium contains all three components, but their mechanical contributions are relatively poorly understood. Most studies of ECM contributions to myocardial mechanics have focused on collagen, but quantitative prediction of mechanical properties of the myocardium, or changes in those properties with disease, from measured tissue structure is not yet possible. Circumstantial evidence suggests that the mechanics of cardiac elastin and proteoglycans merit further study. Work in other tissues used a combination of correlation, modification or digestion, and mathematical modeling to establish mechanical roles for specific ECM components; this work can provide guidance for new experiments and modeling studies in myocardium.
Keywords: collagen, constitutive model, elastin, proteoglycans, residual stress
1. Introduction
Extracellular matrix (ECM) components play an essential role in development, remodeling, and signaling in the cardiovascular system. They are also important in determining the mechanics of blood vessels, valves, pericardium, and myocardium. The goal of this brief review is to summarize available information regarding the mechanical contributions of ECM in the myocardium. This information is derived from three types of studies. The first clue to the mechanical importance of an ECM component is often a correlative study of a particular pathology; for example, myocardial collagen content and ventricular stiffness both increase during pressure-overload hypertrophy, suggesting that myocardial collagen might be an important determinant of chamber stiffness. Manipulating a specific matrix component by enzymatic digestion, altering synthesis, or genetic manipulation can provide a more definitive test of that component’s mechanical contribution. Finally, mathematical models are often important in separating the mechanical roles of multiple components within a tissue.
We set out to review the evidence from each type of study (correlation, manipulation, modeling) for the three ECM components known to be most important to the mechanics of other soft tissues: collagen, elastin, and proteoglycans. We found two things that may be somewhat surprising. First, despite hints that elastin and proteoglycans may be important in determining the mechanics of the myocardium, their contribution has received little attention. Second, the mechanical functions of all three components are much better understood in other soft tissues than in the heart. We decided, therefore, to organize this review into sections for each of these mechanically active ECM components, and to begin each section with an example from another tissue before proceeding to evidence in the heart. We have selected examples that illustrate both the level of understanding that should be set as a goal for future work in the myocardium and approaches that might be useful in achieving that understanding.
2. Collagen
2.1. Collagen in the Pericardium
Pericardium is a thin sheet of tissue composed largely of collagen that surrounds the heart. Unlike myocardium, pericardium can be excised and tested easily, without complications from ischemia or contracture. Furthermore, it is so thin that its mechanics can be treated as two-dimensional, simplifying testing and analysis. Despite this experimental accessibility, it has taken 25 years of work to develop a quantitative understanding of how collagen determines the mechanical properties of pericardium. Two recent reports by Sacks and colleagues [1, 2] reflect the current state of the art, providing inspiration and guidance for future studies of collagen and myocardial mechanics.
A complete understanding of the structural basis for a tissue’s mechanical properties should allow quantitative prediction of those mechanical properties from measured structure. Sacks achieved this goal for pericardium in 2003 [1]. Using a light-scattering technique, he measured the distribution of collagen fiber orientations in samples of bovine pericardium. Then, he performed a single equibiaxial stretch test on each sample while measuring force in two perpendicular directions. Because equibiaxial stretch subjects every fiber in the plane of the tissue to exactly the same strain, regardless of orientation, these test data provided the stress-strain curve for the individual collagen fibers, assuming collagen fibers dominate the mechanics and their density is known. Finally, Sacks used measured orientation and fiber stress-strain properties to predict the mechanical response of each sample to a wide range of stretch combinations, and showed that the predictions matched test data extremely well.
This model falls slightly short of the goal of predicting mechanics entirely from structure because it still required a single mechanical test per sample to determine the effective stress-strain curve for collagen fibers in that sample. There is ample evidence that the exponential stress-strain relationship of collagenous tissues reflects the combined effects of two microstructural events. Initially, collagen fibers uncoil or uncrimp, offering relatively little resistance to stretch; once collagen fibers are straight, stretching them requires much greater force. Measuring the degree of coiling or crimping of collagen and knowing the mechanical properties of straightened fibers should therefore allow prediction of the stress-strain response from structural information alone. Sacks and colleagues recently used x-ray diffraction to track the periodicity of the collagen molecules within each fiber during mechanical testing and separate uncoiling from axial stretch [2].
2.2. Collagen in the Myocardium
In contrast to the pericardium, collagen constitutes a relatively small fraction of myocardial mass. Nevertheless, the myocardial collagen network is essential to the mechanics of the heart [3]. In systole, myocytes bear most of the wall stress but the surrounding collagen weave and struts transmit force and help maintain myocyte alignment. In diastole, perimysial collagen fibers uncoil as the ventricle fills; once these fibers straighten, they resist further expansion, accounting for the steep portion of the end-diastolic pressure-volume curve and protecting myocytes from overstretch (Figure 1). This picture of the mechanical role of fibrillar collagen in the heart arose largely through correlative studies [4-7], complemented by studies that directly altered collagen content [8-13]. Accurate quantitative models relating collagen content and structure to myocardial mechanics are still lacking, and more work is needed to fully understand the mechanical impact of changes in collagen subtype ratios and collagen cross-linking.
Figure 1.
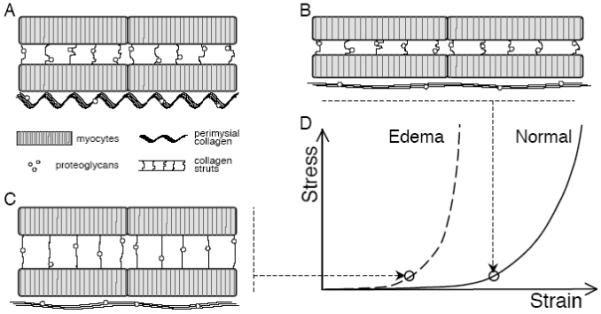
Contributions of ECM components to myocardial mechanics. A Schematic diagram shows myocytes connected by collagen struts, large perimysial collagen fibers aligned with the myocytes, and proteoglycans associated with the collagen. B During passive uniaxial stretch, titin in myocytes initially bears most of the force as perimysial collagen fibers uncoil; once straightened, collagen resists further extension, protecting myocytes from overstretch. C The location and mechanical role of cardiac proteoglycans are unknown; however, observed mechanical changes during edema could be explained by increased hydration of the proteoglycans pre-stressing the collagen network, as in cartilage. D The pre-stressed collagen network would resist deformation at much lower strains, shifting the uniaxial stress-strain curve to the left.
2.2.1. Correlative Approaches
Early studies of pressure-overload hypertrophy (PO) revealed that experimental pressure overload increases collagen content as well as myocardial mass [4]. Papillary muscles from PO hearts displayed elevated diastolic stiffness as well as contractile abnormalities (reduced maximal shortening velocity, prolonged time to peak tension) that could be due either to alterations in the contractile elements or to changes in the elastic elements of the muscle [4]. Continuing studies of cardiac hypertrophy produced data on myocyte hypertrophy, fibrosis, and mechanics across a broad array of diseases and experimental models; for example, Villari et al. demonstrated a nonlinear relationship between chamber stiffness and collagen content in patients with aortic valve disease [5]. In 1993, Weber and colleagues used these data from the literature to argue convincingly that increased diastolic stiffness correlates with ventricular fibrosis rather than with hypertrophy per se [6]. Comparisons between species provided additional support for the role of myocardial collagen in determining diastolic stiffness; for example, Borg et al. compared rat and hamster hearts and found that differences in diastolic stiffness correlated with the extent of the collagen network [7].
Cardiac pathologies affect not only collagen content but also other factors that may impact mechanics, including collagen cross-linking, fiber structure, and subtype ratios. Correlative studies typically provide the first indication of the potential importance of these factors. Diastolic stiffness correlates with the level of collagen cross-linking in some animal models of pressure overload [14] and in patients with heart failure [15], suggesting that collagen content and cross-linking are both important determinants of diastolic properties during hypertrophy and failure. In diabetic patients, high levels of advanced glycation end-products (AGEs) correlate with premature vascular and LV diastolic stiffening, suggesting that AGE-mediated collagen cross-linking can contribute to diastolic dysfunction; agents that prevent or reduce AGE-mediated cross-linking have shown promise in normalizing diastolic mechanics in experimental models of diabetes [16]. Following myocardial infarction, collagen content, fiber structure, and cross-linking all change dramatically as necrotic myocardium is replaced by scar tissue; correlation of these structural changes with changes in mechanical properties suggests that collagen fiber alignment and cross-linking can be as important as collagen content in determining mechanical properties of the healing scar [17]. Ratios of type I to type III collagen also change during hypertrophy [18], failure [19], and infarct healing [20], but the mechanical consequences of these changes are unclear. Correlative approaches provide limited insight in these situations, because total collagen content and type I/III ratios vary simultaneously.
2.2.2. Modification and Digestion
Once correlative studies suggested that collagen is a critical determinant of diastolic mechanics, researchers began degrading or altering collagen to test its role more directly. Some approached this problem by altering collagen turnover or deposition. Bing et al., who reported increased collagen content and increased diastolic stiffness in rat papillary muscles during pressure overload (PO), found that adding beta-amino proprionitrile (BAPN) to the rat chow prevented both the increase in collagen and the increase in stiffness [8]. Even in the absence of hemodynamic overload, 6 weeks of BAPN treatment decreased collagen content, collagen cross-linking, and diastolic chamber stiffness in pigs [9].
An alternate approach is to enzymatically degrade collagen in an acute experimental preparation and measure the resulting change in mechanical properties. Degrading collagen in an intact, perfused heart proved problematic. Collagenase treatment [10] and activation of endogenous proteases with 5,5′-dithio-2-nitrobenzoic acid (DTNB)[11] both altered diastolic mechanics but also induced prominent edema, complicating interpretation of the data. Experiments in isolated papillary muscles and trabeculae were more successful. Activating endogenous matrix metalloproteases (MMPs) with plasmin decreased collagen content, stiffness, and viscous damping in control and PO cat RV papillary muscles [12]. Interestingly, the reverse experiment - degrading everything else and measuring the properties of the remaining collagen - proved even more instructive. Granzier and Irving used KCl/KI to remove actin, myosin, and most of the associated proteins from skinned cardiac trabeculae and found that diastolic properties changed little at long muscle lengths [13]. Combining these studies with mechanical tests on individual myocytes, they concluded that intracellular titin explains much of the passive stiffness of cardiac muscle at short sarcomere lengths, while collagen explains most of the stiffness at longer lengths, in the steep portion of the passive stress-strain curve (Figure 1).
Studies on papillary muscles are experimentally convenient, but it is unclear how the results translate to the three-dimensional collagen network of the myocardium. The perimysial collagen network surrounds and connects sheets of myocardium several cells thick; this sheet structure has been described and extensively studied by LeGrice and colleagues [21]. While the same group has attempted to relate the three-dimensional perimysial collagen structure to behavior in multi-axial shear tests [22, 23], they have not yet performed multi-dimensional versions of the digestion studies described above.
2.2.3. Modeling
Mathematical and computational models typically represent the mechanical properties of myocardium using phenomenologic constitutive equations - equations that describe stress-strain behavior of the tissue without attempting to relate it to the underlying structure [24]. Such models provide little insight about the importance of individual structural components, and are not easily modified to reflect what is known about structural changes in disease. Models based on measured structure, like the model described for pericardium earlier in this article, are conceptually attractive. Their utility for modeling myocardium has been limited primarily by the fact that such models typically have a large number of parameters that are difficult to measure directly, complicating the search for unique parameter values when fitting data [23]. One interesting exception is the simple structural model proposed by MacKenna and colleagues [25]. They modeled perimysial collagen fibers as helical springs and found that relatively small differences in fiber diameter, tortuosity, and density predicted large enough changes in the mechanics of uncoiling to explain reported differences in passive stiffness between rat and dog myocardium.
One of the difficulties in building a mathematical model based on tissue structure, and one of the reasons that structural models often end up including so many parameters, is that multiple components can each bear load directly and through interactions. Using existing tissue engineering techniques, it is now possible to consider assembling a physical model of a tissue, building it up one component at a time, using a stepwise approach to understand the role of each component and its interactions. We used fibroblast-populated collagen gels as a simple physical model of myocardial scar tissue to help us understand the importance of collagen fiber alignment in determining scar mechanical properties [26, 27]. A combination of modeling and experiments revealed that fiber alignment could not fully explain the anisotropy observed in these simple gels, supporting the idea that fiber prestress and/or rotation must be taken into account to properly describe mechanical behavior [27]. Others used cross-linking by lysyl oxidase [28] or glycation [29] to stiffen collagen gels for use as tissue engineering scaffolds, simultaneously providing insight into the mechanical consequences of enzymatic and non-enzymatic collagen cross-linking.
3. Elastin
3.1. Elastin in Arteries
Arteries are composed of layers of elastin, collagen, and smooth muscle and are lined with a layer of endothelial cells. Like pericardium, large arteries are an experimentally tractable system - they can be excised for mechanical testing and even cultured to study structural remodeling in response to altered mechanical loading. Arteries display a nonlinear stress-strain relationship, which arises from interaction of parallel elastin and collagen fibers in the arterial wall [30]. At low pressures, elastin bears most of the load and the artery is fairly compliant. At normal blood pressures, elastin allows the aorta to stretch and store blood as it is ejected by the heart during systole, then recoil during diastole to provide a relatively constant perfusion pressure and flow to the body. At higher pressures, load is transferred to collagen fibers, which gradually straighten and limit arterial expansion. This interplay between a rubber-like, distensible component (elastin or similar proteins) and stiff collagen fibers in the arteries is apparently common to all species with closed circulatory systems; Shadwick [30] provides an excellent review of relevant studies with an emphasis on comparisons across species.
The studies that established how elastin and collagen determine the mechanical properties of arteries employed a mix of correlation and digestion. In large arteries, elastin content decreases and collagen content increases with distance from the heart [31]; the drop in elastin:collagen ratio correlates with reported increases in stiffness along the arterial tree [32]. Similarly, arterial stiffening with age correlates with a decreased elastin:collagen ratio due to fragmentation of elastin fibers and deposition of collagen [33]. Roach and Burton tested arteries before and after digestion of elastin (with trypsin) or collagen (with formic acid) and confirmed that elastin was responsible for the mechanical properties at low stresses, while collagen determined properties at high stresses [34].
Despite a clear understanding of the mechanical roles of elastin and collagen in arteries, quantitatively predicting arterial properties from measured structure has proved surprisingly difficult. This may foreshadow the difficulty of developing structural constitutive models for the heart, which is geometrically and structurally more complex than arteries. Nevertheless, new mathematical models of arterial growth and remodeling should inspire important new work in the heart. Humphrey and colleagues formulated mathematical models of the turnover of elastin, collagen, and smooth muscle during growth and remodeling of blood vessels [35]. Their models explain many of the observed features of arterial growth and remodeling. For example, unloaded arterial rings are under residual stress - tension in the outer wall and compression in the inner wall - and therefore spring open into an arc when this stress is relieved by transecting the wall. This residual stress is important to arterial function, because it helps reduce the stress concentration that would normally be present in the inner wall of an inflated tube. Humphrey and colleagues showed that the changes in residual stress observed during arterial growth and remodeling could arise through degradation of components deposited at previous stress levels and replacement with components deposited at a new stress. Similar models may prove very useful in understanding how turnover of myocardial collagen, muscle proteins, and other components impact cardiac mechanics during hypertrophy and remodeling.
3.2. Elastin in the Heart
The myocardium contains elastin, both in the walls of coronary blood vessels and in the interstitium, but it is unclear whether elastin contributes significantly to myocardial mechanics. Correlative approaches are limited because most pathologies known to alter elastin also alter collagen. We found no reports of definitive digestion experiments analogous to those reviewed above for arteries. Mathematical models of myocardium and the heart have not yet incorporated elastin.
3.2.1. Correlative Approaches
Interstitial elastin fibers are disrupted by acute ischemia [36] and during pressure overload [37] and subsequent heart failure [38]; cathepsin S may account for the elevated elastolytic activity in extracts from failing myocardium [38]. A decade ago, Tyagi et al. speculated that changes in the elastin:collagen ratio, rather than in collagen content alone, underlie the increased diastolic stiffness in pressure overload [39]. This would certainly be consistent with studies in aging arteries, but has not been explored fully in the heart. Correlative studies can be difficult to interpret if pathologies that alter myocardial elastin also alter collagen content, as is the case for ischemia, heart failure, and pressure overload. A definitive test will require selective alteration of myocardial elastin through digestion or genetic manipulation.
3.2.2. Modification and Digestion
We found no reports of digestion experiments designed to isolate the mechanical contributions of myocardial elastin. Jobsis et al. recently proposed that the elastic fibers in the visceral pericardium may play an important role in elastic recoil and untwisting during early diastole [40]. They showed that disrupting the visceral pericardium affected passive mechanics and residual stress, but did not separate the contributions of epimysial elastin and collagen.
Globally perturbing elastin expression also provides limited information about myocardial elastin, because effects on arteries dominate the phenotype. Elastin knockout mice are born with stiff, tortuous arteries and marked cardiac hypertrophy; they die within 72 hours due to smooth muscle cell proliferation that occludes the aorta [41]. Mice with one elastin allele deleted have stiffer arteries, elevated blood pressure, mild cardiac hypertrophy, and a normal life span [42]. Cardiac function appears normal in these animals at birth [41], and subsequent changes are believed to be secondary to decreased arterial compliance and hypertension [42]. The mild cardiac phenotype in elastin hemizygous mice suggests a limited role for elastin in myocardial mechanics; experiments in animals with cardiac-restricted deletion of one or both elastin alleles could definitively address this question.
The ability to manipulate elastin expression provides opportunities to test its role in normal and diseased myocardium, but also opportunities to envision new therapeutic roles. For example, Mizuno et al. proposed that elastin might be beneficial in reinforcing healing myocardial infarcts [43]. They employed transplanted vascular endothelial cells to express elastin in healing rat infarcts and found reduced infarct expansion, reduced left ventricular remodeling, and improved heart function.
4. Proteoglycans
4.1. Proteoglycans in Articular Cartilage
Proteoglycans are equally important to collagen for determining the mechanical properties of articular cartilage; a combination of correlation, digestion, and modeling revealed how these two components work together to absorb large compressive loads. During rapid loading, the force applied to articular cartilage is initially borne entirely by the fluid within it [44]. The proteoglycans provide charge and osmotic forces that limit fluid flow out of the cartilage, while the collagen fibers share the compressive load and prevent expansion of the tissue perpendicular to the applied load.
Correlative studies provided the first clues to this dual role, revealing that collagen orientation and content correlate strongly with tensile properties while proteoglycan content correlates strongly with compressive viscoelastic properties. Tensile testing of articular cartilage from human cadaver knee joints demonstrated a correlation between collagen organization and tensile modulus [45]. Aligned collagen in surface zone cartilage had a significantly higher modulus than disorganized collagen in deep zone cartilage. A significant positive correlation between the tensile modulus and the ratio of collagen to proteoglycan content demonstrated the importance of collagen for the tensile properties of cartilage. A strong positive correlation between proteoglycan content and the equilibrium compressive modulus of cartilage suggested that the compressive properties of cartilage, on the other hand, are determined by the proteoglycan content [46, 47].
These correlative studies are supported by studies where cartilage proteoglycan content was reduced either experimentally or pathologically. Osteoarthritis, a degenerative condition which results in a loss of proteoglycans, dramatically lowers the compressive modulus of human cartilage [48]. Animal models of osteoarthritis show similar changes [48]. As early as 1972, enzymatic degradation was shown to significantly increase the compressibility of cartilage [49]. A more recent study showed that degradation of proteoglycans by either chondroitinase or hyaluronidase greatly reduced the shear modulus of cartilage [50]. The proteoglycan network typically swells the tissue and prestresses the collagen network by drawing in and retaining fluid; degrading the proteoglycans lowered the prestress and loosened the collagen network, thus significantly reducing the shear stiffness of the solid matrix.
Mathematical models provided a quantitative understanding of the contribution of proteoglycans to the mechanical properties of cartilage. The biphasic theory described cartilage in terms of interacting solid (collagens and proteoglycans) and fluid phases [44]. This theory was later expanded to include a third phase and to incorporate the effects of fixed charges [44]. In these multiphase models, proteoglycans control the flow of water in and out of cartilage, largely determining the ability of cartilage to resist compression. Dense negative charges on the proteoglycan side chains have two major effects: 1) attraction of counterions which lead to a Donnan osmotic pressure and 2) repulsion of side chains from each other leading to increased swelling pressure and resistance to compression. At the onset of compressive loading, all load is carried by the fluid phase of the tissue. With time, fluid flows out of the tissue and at equilibrium all load is carried by the solid matrix. As proteoglycans are lost, permeability and fluid flow increase, and the modulus of the tissue decreases. These mathematical models have allowed investigators to develop quantitative structure-function relationships for articular cartilage. The composition and structural organization of the solid and fluid phases can be used to accurately predict the mechanical behavior of the tissue under normal and pathologic conditions.
4.2. Proteoglycans in the Heart
Proteoglycans regulate cartilage mechanics by controlling the flow of water in and out of the tissue during loading. There is no evidence that proteoglycans play a similar role in myocardium, but it is intriguing to speculate (Figure 1). Fluid movement is likely more important to myocardial mechanics than generally acknowledged, and is poorly understood. Proteoglycans are present in the myocardium but are frequently ignored when examining the cardiac ECM. Azeloglu et al. recently demonstrated that proteoglycans are an important determinant of residual stress in arteries, where, as in myocardium, their presence had been largely ignored [51]. Analogous studies in the heart are certainly overdue.
4.2.1. Correlative Approaches
While examining the collagen network of the heart, Borg et al. reported the presence of significant quantities of material presumed to be glycosaminoglycans in the myocardium [7]. This material leached from the tissue during normal histologic processing but could be retained by adding 1% cetylpyridinium chloride during fixation. The fact that proteoglycans are lost during traditional fixation may explain why few classic studies of ECM changes in disease commented on proteoglycan content. More recently, proteoglycans are receiving attention for their potential signaling roles, a topic that is discussed elsewhere in this special issue and in recent reviews (for example, see Bereczki and Santha [52] for a discussion of biglycan).
Myocardial tissue volume changes are important to myocardial mechanics, but it is not yet clear what role proteoglycans play. The coronary vasculature interacts with the surrounding tissue, with perfusion increasing both myocardial volume and myocardial stiffness during mechanical testing [53]; it is difficult to separate intravascular and extravascular volume changes during such experiments. Perfusion with solutions of varying osmolarities alters opening angle in left ventricular slices, indicating changes in residual stresses and strains that could significantly alter myocardial mechanics [54]. Although Lanir and colleagues attributed these changes in the opening angle to myocyte swelling and shrinkage that altered tension on the surrounding collagen network, Azeloglu showed that in arteries the inhomogeneous transmural distribution of proteoglycans, rather than cellular swelling, explained changes of opening angle with solution osmolarity [51]. Perhaps the best example of water content influencing cardiac mechanics occurs following myocardial infarction. The infarct becomes edematous within hours, and drugs that decrease inflammation and edema greatly increase the extent of infarct expansion and risk of rupture [17]; the specific role of proteoglycans in post-infarction mechanics merits further study.
4.2.2. Modification and Digestion
Transgenic models revealed a critical role for proteoglycans in development and collagen fibrillogenesis. Knockouts for versican [55], aggrecan, [56] and perlecan [57] are lethal; animals die prenatally or immediately postnatally and typically show cardiac as well as skeletal malformations. Knockouts for decorin [58] and byglycan [59] are viable but show abnormal collagen morphology in skin, tendon, and elsewhere in the musculoskeletal system. These animals do not display obvious cardiac abnormalities at baseline, but infarct healing is altered [60-62], presumably because decorin and byglycan are required to form normal collagen fibrils in the healing scar. In humans, the mucopolysaccharidoses, a group of genetic disorders characterized by accumulation of proteoglycans and skeletal defects, also cause cardiac defects and cardiomyopathy. After enzyme replacement therapy, valve abnormalities persist but ventricular mechanics improve, suggesting that direct mechanical effects of accumulated GAGs may be important in the etiology of this cardiomyopathy [63].
Selective digestion of proteoglycans would be the best way to examine their mechanical role in the heart. As with the collagen digestion experiments reviewed earlier in this article, it will be challenging to dissolve proteoglycans selectively in the intact heart without inducing other changes that confound mechanical measurements. Isolated papillary muscles or trabeculae may prove the most useful experimental system for these studies. This experimental system also avoids another potential complication of residual stress and opening angle studies - because models predict that the transmural distribution of proteoglycans, rather than proteoglycan content, determines the opening angle, incomplete or nonuniform digestion could lead easily to erroneous conclusions [51].
5. Conclusions
Fibrillar collagen, elastin, and proteoglycans all play crucial mechanical roles in many tissues in the body generally and in the cardiovascular system specifically. The myocardium contains all three components, but their mechanical contributions are relatively poorly understood. Most studies of ECM contributions to myocardial mechanics have focused on collagen, but quantitative prediction of mechanical properties of the myocardium, or changes in those properties with disease, from measured tissue structure is not yet possible. Circumstantial evidence suggests that the mechanics of cardiac elastin and proteoglycans merit further study.
Table 1 lists our suggestions for experiments needed to clarify the role of ECM components in myocardial mechanics. These experiments are easy to propose but admittedly difficult to perform, because they require cardiac-specific manipulation of individual matrix components. The first priority is simply to definitively test the role of collagen types I and III, elastin, and proteoglycans by selectively manipulating each component. These experiments should be done in normal hearts as well in multiple disease models (hemodynamic overload, infarct healing, etc.), since the relative importance of different components likely varies with remodeling. Once these experiments are performed, we suggest that the next priority should be developing quantitative models of cardiac ECM changes with remodeling, with attention to both shifts in composition and shifts in pre-stress on individual components.
Table 1. Proposed Studies on the Mechanical Role of Cardiac ECM Components.
| Question | Proposed Study | Guiding Examples |
|---|---|---|
| Is collagen type I/III ratio an important determinant of LV mechanics? |
|
|
| Is elastin an important determinant of LV mechanics? |
|
Arteries [34] |
| Do proteoglycans play an important role in LV mechanics? |
|
Cartilage [50] |
| How does turnover of myocytes and ECM components govern changes in mechanics with disease? |
|
Arteries [35] |
Acknowledgments
Portions of this work were supported by R01 HL075639 (Holmes) and K01 EB004347 (Thomopoulos) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Sacks MS. Incorporation of experimentally-derived fiber orientation into a structural constitutive model for planar collagenous tissues. J Biomech Eng. 2003 Apr;125(2):280–7. doi: 10.1115/1.1544508. [DOI] [PubMed] [Google Scholar]
- [2].Liao J, Yang L, Grashow J, Sacks MS. Molecular orientation of collagen in intact planar connective tissues under biaxial stretch. Acta Biomater. 2005 Jan;1(1):45–54. doi: 10.1016/j.actbio.2004.09.007. [DOI] [PubMed] [Google Scholar]
- [3].Weber KT. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol. 1989 Jun;13(7):1637–52. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- [4].Bing OH, Matsushita S, Fanburg BL, Levine HJ. Mechanical properties of rat cardiac muscle during experimental hypertrophy. Circ Res. 1971 Feb;28(2):234–45. doi: 10.1161/01.res.28.2.234. [DOI] [PubMed] [Google Scholar]
- [5].Villari B, Campbell SE, Hess OM, Mall G, Vassalli G, Weber KT, et al. Influence of collagen network on left ventricular systolic and diastolic function in aortic valve disease. J Am Coll Cardiol. 1993 Nov 1;22(5):1477–84. doi: 10.1016/0735-1097(93)90560-n. [DOI] [PubMed] [Google Scholar]
- [6].Weber KT, Brilla CG, Janicki JS. Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc Res. 1993 Mar;27(3):341–8. doi: 10.1093/cvr/27.3.341. [DOI] [PubMed] [Google Scholar]
- [7].Borg TK, Ranson WF, Moslehy FA, Caulfield JB. Structural basis of ventricular stiffness. Lab Invest. 1981 Jan;44(1):49–54. [PubMed] [Google Scholar]
- [8].Bing OH, Fanburg BL, Brooks WW, Matsushita S. The effect of lathyrogen beta-amino proprionitrile (BAPN) on the mechanical properties of experimentally hypertrophied rat cardiac muscle. Circ Res. 1978 Oct;43(4):632–7. doi: 10.1161/01.res.43.4.632. [DOI] [PubMed] [Google Scholar]
- [9].Kato S, Spinale FG, Tanaka R, Johnson W, Cooper Gt, Zile MR. Inhibition of collagen cross-linking: effects on fibrillar collagen and ventricular diastolic function. Am J Physiol. 1995 Sep;269(3 Pt 2):H863–8. doi: 10.1152/ajpheart.1995.269.3.H863. [DOI] [PubMed] [Google Scholar]
- [10].MacKenna DA, Omens JH, McCulloch AD, Covell JW. Contribution of collagen matrix to passive left ventricular mechanics in isolated rat hearts. Am J Physiol. 1994 Mar;266(3 Pt 2):H1007–18. doi: 10.1152/ajpheart.1994.266.3.H1007. [DOI] [PubMed] [Google Scholar]
- [11].Todaka K, Jiang T, Chapman JT, Gu A, Zhu SM, Herzog E, et al. Functional consequences of acute collagen degradation studied in crystalloid perfused rat hearts. Basic Res Cardiol. 1997 Jun;92(3):147–58. doi: 10.1007/BF00788632. [DOI] [PubMed] [Google Scholar]
- [12].Stroud JD, Baicu CF, Barnes MA, Spinale FG, Zile MR. Viscoelastic properties of pressure overload hypertrophied myocardium: effect of serine protease treatment. Am J Physiol Heart Circ Physiol. 2002 Jun;282(6):H2324–35. doi: 10.1152/ajpheart.00711.2001. [DOI] [PubMed] [Google Scholar]
- [13].Granzier HL, Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J. 1995 Mar;68(3):1027–44. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Badenhorst D, Maseko M, Tsotetsi OJ, Naidoo A, Brooksbank R, Norton GR, et al. Cross-linking influences the impact of quantitative changes in myocardial collagen on cardiac stiffness and remodelling in hypertension in rats. Cardiovasc Res. 2003 Mar;57(3):632–41. doi: 10.1016/s0008-6363(02)00733-2. [DOI] [PubMed] [Google Scholar]
- [15].Lopez B, Querejeta R, Gonzalez A, Beaumont J, Larman M, Diez J. Impact of treatment on myocardial lysyl oxidase expression and collagen cross-linking in patients with heart failure. Hypertension. 2009 Feb;53(2):236–42. doi: 10.1161/HYPERTENSIONAHA.108.125278. [DOI] [PubMed] [Google Scholar]
- [16].Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens. 2003 Jan;21(1):3–12. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]
- [17].Holmes JW, Borg TK, Covell JW. Structure and mechanics of healing myocardial infarcts. Annu Rev Biomed Eng. 2005;7:223–53. doi: 10.1146/annurev.bioeng.7.060804.100453. [DOI] [PubMed] [Google Scholar]
- [18].Chapman D, Weber KT, Eghbali M. Regulation of fibrillar collagen types I and III and basement membrane type IV collagen gene expression in pressure overloaded rat myocardium. Circ Res. 1990 Oct;67(4):787–94. doi: 10.1161/01.res.67.4.787. [DOI] [PubMed] [Google Scholar]
- [19].Pauschinger M, Knopf D, Petschauer S, Doerner A, Poller W, Schwimmbeck PL, et al. Dilated cardiomyopathy is associated with significant changes in collagen type I/III ratio. Circulation. 1999 Jun 1;99(21):2750–6. doi: 10.1161/01.cir.99.21.2750. [DOI] [PubMed] [Google Scholar]
- [20].Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995 Aug;147(2):325–38. [PMC free article] [PubMed] [Google Scholar]
- [21].Pope AJ, Sands GB, Smaill BH, LeGrice IJ. Three-dimensional transmural organization of perimysial collagen in the heart. Am J Physiol Heart Circ Physiol. 2008 Sep;295(3):H1243–H52. doi: 10.1152/ajpheart.00484.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dokos S, Smaill BH, Young AA, LeGrice IJ. Shear properties of passive ventricular myocardium. Am J Physiol Heart Circ Physiol. 2002 Dec;283(6):H2650–9. doi: 10.1152/ajpheart.00111.2002. [DOI] [PubMed] [Google Scholar]
- [23].Schmid H, O’Callaghan P, Nash MP, Lin W, LeGrice IJ, Smaill BH, et al. Myocardial material parameter estimation: a non-homogeneous finite element study from simple shear tests. Biomech Model Mechanobiol. 2008 Jun;7(3):161–73. doi: 10.1007/s10237-007-0083-0. [DOI] [PubMed] [Google Scholar]
- [24].Costa KD, Holmes JW, McCulloch AD. Modeling cardiac mechanical properties in three dimensions. Phil Trans R Soc Lond A. 2001;359:1233–50. [Google Scholar]
- [25].MacKenna DA, Vaplon SM, McCulloch AD. Microstructural model of perimysial collagen fibers for resting myocardial mechanics during ventricular filling. Am J Physiol. 1997 Sep;273(3 Pt 2):H1576–86. doi: 10.1152/ajpheart.1997.273.3.H1576. [DOI] [PubMed] [Google Scholar]
- [26].Thomopoulos S, Fomovsky GM, Holmes JW. The development of structural and mechanical anisotropy in fibroblast populated collagen gels. J Biomech Eng. 2005 Oct;127(5):742–50. doi: 10.1115/1.1992525. [DOI] [PubMed] [Google Scholar]
- [27].Thomopoulos S, Fomovsky GM, Chandran PL, Holmes JW. Collagen fiber alignment does not explain mechanical anisotropy in fibroblast populated collagen gels. J Biomech Eng. 2007 Oct;129(5):642–50. doi: 10.1115/1.2768104. [DOI] [PubMed] [Google Scholar]
- [28].Elbjeirami WM, Yonter EO, Starcher BC, West JL. Enhancing mechanical properties of tissue-engineered constructs via lysyl oxidase crosslinking activity. J Biomed Mater Res A. 2003 Sep 1;66(3):513–21. doi: 10.1002/jbm.a.10021. [DOI] [PubMed] [Google Scholar]
- [29].Girton TS, Oegema TR, Tranquillo RT. Exploiting glycation to stiffen and strengthen tissue equivalents for tissue engineering. J Biomed Mater Res. 1999 Jul;46(1):87–92. doi: 10.1002/(sici)1097-4636(199907)46:1<87::aid-jbm10>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- [30].Shadwick RE. Mechanical design in arteries. J Exp Biol. 1999 Dec;202(Pt 23):3305–13. doi: 10.1242/jeb.202.23.3305. [DOI] [PubMed] [Google Scholar]
- [31].Milnor WR. Hemodynamics. Williams & Wilkins; Baltimore: 1982. [Google Scholar]
- [32].Bergel DH. The dynamic elastic properties of the arterial wall. J Physiol. 1961 May;156(3):458–69. doi: 10.1113/jphysiol.1961.sp006687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Izzo JL, Jr., Mitchell GF. Aging and arterial structure-function relations. Adv Cardiol. 2007;44:19–34. doi: 10.1159/000096701. [DOI] [PubMed] [Google Scholar]
- [34].Roach MR, Burton AC. The reason for the shape of the distensibility curves of arteries. Can J Biochem Physiol. 1957 Aug;35(8):681–90. [PubMed] [Google Scholar]
- [35].Gleason RL, Jr., Humphrey JD. A 2D constrained mixture model for arterial adaptations to large changes in flow, pressure and axial stretch. Math Med Biol. 2005 Dec;22(4):347–69. doi: 10.1093/imammb/dqi014. [DOI] [PubMed] [Google Scholar]
- [36].Sato S, Ashraf M, Millard RW, Fujiwara H, Schwartz A. Connective tissue changes in early ischemia of porcine myocardium: an ultrastructural study. J Mol Cell Cardiol. 1983 Apr;15(4):261–75. doi: 10.1016/0022-2828(83)90281-x. [DOI] [PubMed] [Google Scholar]
- [37].Henderson BC, Sen U, Reynolds C, Moshal KS, Ovechkin A, Tyagi N, et al. Reversal of systemic hypertension-associated cardiac remodeling in chronic pressure overload myocardium by ciglitazone. Int J Biol Sci. 2007;3(6):385–92. doi: 10.7150/ijbs.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cheng XW, Obata K, Kuzuya M, Izawa H, Nakamura K, Asai E, et al. Elastolytic cathepsin induction/activation system exists in myocardium and is upregulated in hypertensive heart failure. Hypertension. 2006 Nov;48(5):979–87. doi: 10.1161/01.HYP.0000242331.99369.2f. [DOI] [PubMed] [Google Scholar]
- [39].Mujumdar VS, Tyagi SC. Temporal regulation of extracellular matrix components in transition from compensatory hypertrophy to decompensatory heart failure. J Hypertens. 1999 Feb;17(2):261–70. doi: 10.1097/00004872-199917020-00011. [DOI] [PubMed] [Google Scholar]
- [40].Jobsis PD, Ashikaga H, Wen H, Rothstein EC, Horvath KA, McVeigh ER, et al. The visceral pericardium: macromolecular structure and contribution to passive mechanical properties of the left ventricle. Am J Physiol Heart Circ Physiol. 2007 Dec;293(6):H3379–87. doi: 10.1152/ajpheart.00967.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wagenseil JE, Ciliberto CH, Knutsen RH, Levy MA, Kovacs A, Mecham RP. Reduced Vessel Elasticity Alters Cardiovascular Structure and Function in Newborn Mice. Circ Res. 2009 Apr 16; doi: 10.1161/CIRCRESAHA.108.192054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, et al. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Invest. 2003 Nov;112(9):1419–28. doi: 10.1172/JCI19028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mizuno T, Yau TM, Weisel RD, Kiani CG, Li RK. Elastin stabilizes an infarct and preserves ventricular function. Circulation. 2005 Aug 30;112(9 Suppl):I81–8. doi: 10.1161/01.CIRCULATIONAHA.105.523795. [DOI] [PubMed] [Google Scholar]
- [44].Mow VC, Ratcliffe A. Structure and Function of Articular Cartilage and Meniscus. In: Mow VC, Hayes WC, editors. Basic Orthopaedic Biomechanics. Lippincott-Raven; Philadelphia: 1997. pp. 113–77. [Google Scholar]
- [45].Akizuki S, Mow VC, Muller F, Pita JC, Howell DS, Manicourt DH. Tensile properties of human knee joint cartilage: I. Influence of ionic conditions, weight bearing, and fibrillation on the tensile modulus. J Orthop Res. 1986;4(4):379–92. doi: 10.1002/jor.1100040401. [DOI] [PubMed] [Google Scholar]
- [46].Armstrong CG, Mow VC. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J Bone Joint Surg Am. 1982 Jan;64(1):88–94. [PubMed] [Google Scholar]
- [47].Kempson GE, Muir H, Swanson SA, Freeman MA. Correlations between stiffness and the chemical constituents of cartilage on the human femoral head. Biochim Biophys Acta. 1970 Jul 21;215(1):70–7. doi: 10.1016/0304-4165(70)90388-0. [DOI] [PubMed] [Google Scholar]
- [48].Setton LA, Elliott DM, Mow VC. Altered mechanics of cartilage with osteoarthritis: human osteoarthritis and an experimental model of joint degeneration. Osteoarthritis Cartilage. 1999 Jan;7(1):2–14. doi: 10.1053/joca.1998.0170. [DOI] [PubMed] [Google Scholar]
- [49].Harris ED, Jr., Parker HG, Radin EL, Krane SM. Effects of proteolytic enzymes on structural and mechanical properties of cartilage. Arthritis Rheum. 1972 Sep-Oct;15(5):497–503. doi: 10.1002/art.1780150505. [DOI] [PubMed] [Google Scholar]
- [50].Zhu W, Mow VC, Koob TJ, Eyre DR. Viscoelastic shear properties of articular cartilage and the effects of glycosidase treatments. J Orthop Res. 1993 Nov;11(6):771–81. doi: 10.1002/jor.1100110602. [DOI] [PubMed] [Google Scholar]
- [51].Azeloglu EU, Albro MB, Thimmappa VA, Ateshian GA, Costa KD. Heterogeneous transmural proteoglycan distribution provides a mechanism for regulating residual stresses in the aorta. Am J Physiol Heart Circ Physiol. 2008 Mar;294(3):H1197–205. doi: 10.1152/ajpheart.01027.2007. [DOI] [PubMed] [Google Scholar]
- [52].Bereczki E, Santha M. The role of biglycan in the heart. Connect Tissue Res. 2008;49(3):129–32. doi: 10.1080/03008200802148504. [DOI] [PubMed] [Google Scholar]
- [53].May-Newman K, McCulloch AD. Homogenization modeling for the mechanics of perfused myocardium. Prog Biophys Mol Biol. 1998;69(23):463–81. doi: 10.1016/s0079-6107(98)00020-0. [DOI] [PubMed] [Google Scholar]
- [54].Lanir Y, Hayam G, Abovsky M, Zlotnick AY, Uretzky G, Nevo E, et al. Effect of myocardial swelling on residual strain in the left ventricle of the rat. Am J Physiol. 1996 May;270(5 Pt 2):H1736–43. doi: 10.1152/ajpheart.1996.270.5.H1736. [DOI] [PubMed] [Google Scholar]
- [55].Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR. The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev Biol. 1998 Oct 1;202(1):56–66. doi: 10.1006/dbio.1998.9001. [DOI] [PubMed] [Google Scholar]
- [56].Watanabe H, Kimata K, Line S, Strong D, Gao LY, Kozak CA, et al. Mouse cartilage matrix deficiency (cmd) caused by a 7 bp deletion in the aggrecan gene. Nat Genet. 1994 Jun;7(2):154–7. doi: 10.1038/ng0694-154. [DOI] [PubMed] [Google Scholar]
- [57].Costell M, Carmona R, Gustafsson E, Gonzalez-Iriarte M, Fassler R, Munoz-Chapuli R. Hyperplastic conotruncal endocardial cushions and transposition of great arteries in perlecan-null mice. Circ Res. 2002 Jul 26;91(2):158–64. doi: 10.1161/01.res.0000026056.81424.da. [DOI] [PubMed] [Google Scholar]
- [58].Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997 Feb 10;136(3):729–43. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Xu T, Bianco P, Fisher LW, Longenecker G, Smith E, Goldstein S, et al. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat Genet. 1998 Sep;20(1):78–82. doi: 10.1038/1746. [DOI] [PubMed] [Google Scholar]
- [60].Weis SM, Zimmerman SD, Shah M, Covell JW, Omens JH, Ross J, Jr., et al. A role for decorin in the remodeling of myocardial infarction. Matrix Biol. 2005 Jun;24(4):313–24. doi: 10.1016/j.matbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- [61].Campbell PH, Hunt DL, Jones Y, Harwood F, Amiel D, Omens JH, et al. Effects of biglycan deficiency on myocardial infarct structure and mechanics. Mol Cell Biomech. 2008 Mar;5(1):27–35. [PMC free article] [PubMed] [Google Scholar]
- [62].Westermann D, Mersmann J, Melchior A, Freudenberger T, Petrik C, Schaefer L, et al. Biglycan is required for adaptive remodeling after myocardial infarction. Circulation. 2008 Mar 11;117(10):1269–76. doi: 10.1161/CIRCULATIONAHA.107.714147. [DOI] [PubMed] [Google Scholar]
- [63].Braunlin EA, Berry JM, Whitley CB. Cardiac findings after enzyme replacement therapy for mucopolysaccharidosis type I. Am J Cardiol. 2006 Aug 1;98(3):416–8. doi: 10.1016/j.amjcard.2006.02.047. [DOI] [PubMed] [Google Scholar]


