Abstract
The concept that extracellular matrix (ECM) turnover occurs during cardiac remodeling is a well-accepted paradigm. To date, a multitude of studies document that remodeling is accompanied by increases in the synthesis and deposition of ECM components as well as increases in extracellular proteases, especially matrix metalloproteinases (MMPs), which break down ECM components. Further, soluble ECM fragments generated from enzymatic action serve to stimulate cell behavior and have been proposed as candidate plasma biomarkers of cardiac remodeling. This review briefly summarizes our current knowledge base on cardiac ECM turnover following myocardial infarction (MI), but more importantly extends discussion by defining avenues that remain to be explored to drive the ECM remodeling field forward. Specifically, this review will discuss cause and effect roles for the ECM changes observed following MI and the potential role of the ECM changes that may serve as trigger points to regulate remodeling. While the pattern of remodeling following MI is qualititatively similar but quantitively different from various types of injury, the basic theme in remodeling is repeated. Therefore, while we use the MI model as the prototype injury model, the themes discussed here are also relevant to cardiac remodeling due to other types of injury.
Introduction
A myocardial infarction (MI) occurs when a previously atherosclerotic coronary artery becomes totally occluded, resulting in ischemia of the downstream myocardium. Metabolic distress of sufficient duration to result in cardiomyocyte necrosis is termed infarction. Following MI, the left ventricle (LV) undergoes a continuum of molecular, cellular, and extracellular responses that manifest clinically as changes in LV size, shape, and function.[2] Coined by Jan and Marc Pfeffer,[3] the term LV remodeling encompasses LV wall thinning, LV dilation, and infarct expansion; inflammation and necrotic myocyte resorption; fibroblast accumulation and scar formation; and endothelial cell activation and neovascularization.[4] LV remodeling is influenced by variations in inflammatory response (neutrophil and macrophage influx), hemodynamic load, molecular changes (neurohormonal activation and cytokine production), and extracellular responses (fibrosis and activation extracellular proteases including matrix metalloproteinases (MMPs) and serine proteases).[5] In addition, pre-existing conditions such as diabetes or periodontal disease and the use of drugs such as angiotensin converting enzyme inhibitors and β adrenergic receptor inhibitors can influence remodeling outcomes. Remodeling occurs in response to MI, but is also a common end point for the LV responses to pressure overload, volume overload, cardiomyopathy, infection, or cardiotoxic agents (see the other reviews in this issue for more detailed discussions). Basically, changes in the ECM represent a dynamic interaction between the various cell types and the acellular components. In this review, we will focus on ECM turnover in response to MI.
ECM gene and protein synthesis increases post-MI
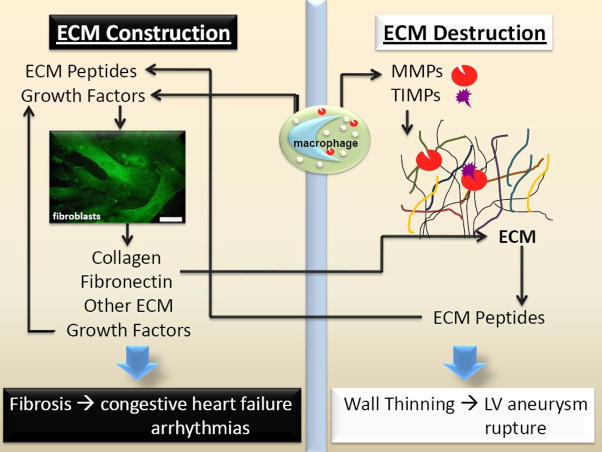
ECM is tissue specific, in that it varies in quality and quantity among different tissues. In the heart the ECM includes collagens (types I, III, IV, V, and VI); glycoproteins (fibronectin, laminins, periostin, fibromodulin, and vitronectin); proteoglycans (versican, lumican, and biglycan); glycosaminoglycans (hyaluronic acid and dermatan sulfate); and matricellular proteins (secreted protein acidic and rich in cysteine, thrombospondins, tenascins).[6] In addition, extracellular proteases include serine proteases and MMPs that are present either bound to the ECM, in various cell types, or circulating in blood. Several ECM proteins have been proposed as candidate markers of post-MI adverse LV remodeling (Table 1), and mechanistic roles have been elucidated using null mice.[7] In the baseline state, the cell source for most of the ECM proteins is primarily the cardiac fibroblast; post-MI, cell sources include the myofibroblast, neutrophils, mast cells, lymphocytes, and the macrophage. For example, post-MI the macrophage contributes increased levels of MMPs and osteopontin.[8] In addition, Schnoor and colleagues have recently demonstrated that macrophages contain mRNAs for a large number of collagens (particularly collagen VI) and fibronectin.[9] Fibroblasts in the post-MI LV are primarily myofibroblasts that have differentiated from resident fibroblasts or from infiltrating fibrocytes. Whether the source (resident or infiltrating) yields myofibroblasts with different characteristics has not been examined. Studies that isolate cardiac fibroblasts and stimulate in vitro assume that resident fibroblasts comprise the major source. The rapid changes to the ECM following MI are thought to be critical alterations that contribute to the propagation of structural and functional decline. As shown in Figure 1, the net balance between ECM construction and destruction determines the net structural and functional outcome and both sides of the scale stimulate the other.
Table 1.
Candidate Plasma ECM Biomarkers of Cardiac Remodeling
| Collagen I telopeptides (CITP) |
| Collagen III telopeptide (CIIITP) |
| Connective Tissue Growth Factor |
| Fibronectin |
| Granzyme B |
| Matrix Metalloproteinase-9 |
| Tenascin C |
| Thrombospondin-1 |
| Tissue Inhibitor of Metalloproteinase-1 |
| Transforming Growth Factor β |
Figure 1.
Post-MI, the net ECM response is determined by the balance between ECM construction and destruction. If ECM accumulation predominates, then fibrosis can predispose the LV to congestive heart failure and arrhythmias. If ECM degradation predominates, then wall thinning can lead to LV aneurysm and rupture. Both pathways interact and feed on each other.
Protease levels increase post-MI
Multiple MMPs and tissue inhibitors of metalloproteinases (TIMPs) have been shown to be altered post-MI in both human and animal studies. MMPs -1, -2, -3, -7, -8, -9, -12, -13, -14 and TIMPs -1 and -2 levels increase, while TIMP-3 and TIMP-4 levels decrease post-MI.[10–12] It is important to note that of the 25 MMPs identified to date, only the MMPs listed above have been evaluated post-MI. These studies are warranted, particularly for the other membrane type MMPs.
Of the MMPs that have been examined, MMP-9 has surfaced as a leading candidate for having direct effects on cardiac remodeling: a) MMP-9 levels increase early post-MI,[13] b) MMP inhibition improves post-MI outcomes by decreasing MMP-9 levels,[14, 15] and c) MMP-9 gene deletion reduces remodeling and stimulates post-MI angiogenesis.[16] Plasma MMP-9 levels predict cardiovascular mortality, as coronary artery disease patients with the highest MMP-9 levels at baseline showed the greatest cardiovascular mortality rates at the 4 year follow-up.[17] Horne and colleagues examined over 5000 patients for associations between MI and polymorphisms in 4 MMPs (MMP-1, -2, -3, and -9) and 3 TIMPs (TIMP-1, -2, and -3).[18] In this study, only composite MMP-9 genotypes, not any of the other MMP/TIMP polymorphisms, associated with MI. In another study of 404 post-MI patients, plasma MMP-9 (p=0.005) and TIMP-1 (p=0.036), but not NTproBNP, correlated with the change in LV EDV, indicating that MMP-9 may be a useful clinical biomarker of LV remodeling and adverse prognosis.[19] Following MI, the neutrophil is a rich source of MMP-9 and serine proteases including serine elastase, a potent MMP-9 activator.[13] The macrophage is also an abundant MMP-9 source.[20]
Another family of proteases include the a disintegrin and metalloproteinase (ADAM), a disintegrin and metalloproteinase with thrombospondins motifs (ADAMTS), and ADAM-TS-like (ADAMTSL) members. [21, 22] While the tumor necrosis factor α converting enzyme (TACE; ADAM-17) is the best known of these members, new roles for this family are currently under investigation. To date, however, fewer than a dozen papers are listed when ADAM and myocardial infarction are used as keywords in PubMed, indicating that this topic remains largely unexplored. Gilliam Murphy has written an excellent review of ADAM roles in cancer.[23] Based on this review, we can speculate that ADAMs -9, -12, -15, -17, and -19 may have important roles in the post-MI setting.
Soluble ECM fragments are generated post-MI
Matricryptins (also known as matrikines) are peptides generated from ECM proteins, including collagens (I, IV, XVIII, and XV), connective tissue glycoproteins (fibronectin, thrombospondin-1, laminin, and SPARC), elastin and potentially other ECM components.[24–27] Of these, collagen and fibronectin fragments have been the most studied in cardiac remodeling. In the late 1990’s, Diez and colleagues developed serum assays to measure the C-terminal propeptide of collagen I (PICP), the N-terminal propeptide of collagen III (PIIINP), and the C-terminal telopeptide of collagen I (ICTP). [28] Several groups have since measured serum markers of collagen I and III turnover post-MI. McGavigan and colleagues measured PICP and CITP, markers of collagen I synthesis and degradation, respectively, in 51 patients with acute MI.[29] When dichotomized to wall motion index, patients with abnormal wall motion index showed higher baseline levels of CITP and decreased levels of PICP, indicating a net increase in collagen I breakdown. Francisco Villarreal and colleagues assessed the time course of collagen I degradation post-MI in pigs.[30] ICTP levels peaked at 6 h post-MI and remained significantly elevated at day 2 post-MI. The increase in collagen I degradation was ameliorated by pre-treatment with doxycycline, implicating MMP activity. In patients with MI and percutaneous coronary intervention, day 4 PICP levels above 110 ug/l and PIIINP levels above 4 ug/l associated with increased LV dilation at 6 months follow up, indicating that increased collagen synthesis at day 4, above a threshold limit, was a predictor of adverse remodeling.[31] While these studies show that collagen peptides are generated post-MI, little has been done to examine the effect of these peptides on downstream cell signaling.
To date, most studies examining matricryptins effects have evaluated angiogenic/anti-angiogenic and tumor promoting/inhibiting properties.[24, 32–35] In particular, how MMP-9 regulates ECM turnover by generating matricryptins post-MI remains unclear, and the effects of ECM peptides on inflammatory cell, endothelial, and fibroblast cell function needs further investigation.
MMP-9 degrades denatured collagen I and III, and these collagen peptides influence MMP production and stimulate collagen synthesis.[36, 37] Of note, MMP-9 processes collagen into more fragments than MMP-2 does, suggesting different downstream signaling when collagen is processed by different MMPs.[38] MMP-9 processes fibronectin to multiple peptides, including 70 and 45 kD peptides. Fibronectin and laminin peptides stimulate macrophage migration and MMP-9 secretion.[39, 40] TNFα-stimulated macrophages release fibronectin peptides, suggesting a role for macrophage-derived MMP-9 in fibronectin processing.[40] The laminin γ1 peptide KAFDITYVRLKF promotes migration and MMP-9 secretion in mouse melanoma cells, but effects on cardiac cells have not been examined.[41] When all overlapping peptides of the γ1 chain of laminin were systematically examined for cell adhesiveness, only 12 peptides had activity. SPARC is a matricellular protein that binds collagen and is upregulated by transforming growth factor β. For a detailed review of SPARC roles in extracellular matrix remodeling, please see Amy Bradshaw’s review in this issue. Produced by endothelial cells, fibroblasts, and macrophages, only M2 macrophages take up SPARC via the stabilin-1 receptor.[42] In blood monocytes, SPARC stimulates MMP-9, and SPARC peptides generated by MMP-9 induced MMP production.[43] MMP-9 also increases TGFβ activity.[39] Therefore, a positive feedback loop among MMP-9, SPARC, and TGFβ makes it difficult to isolate out individual roles.
Assigning cause and effect functions to ECM changes that occur post-MI
There is strong evidence that excess ECM turnover associates with LV functional capacity and outcome. In the Research into Etanercept Cytokine Antagonism in Ventricular Dysfunction (RECOVER) trial, baseline levels of the amino-terminal propeptide of collagen III (PIIINP), MMP-1, TIMP-1, high sensitivity C reactive protein, interleukin-10, and interleukin-18 were measured in 1009 patients at baseline.[44] In an adjusted multivariate model that included all 6 markers, only PIIINP and MMP-1 independently predicted 6 min walk test results, and only PIIINP associated with death and CHF hospitalization numbers. In addition, the fact that ECM genetic mutations can result in cardiomyopathy lends support to the concept that altered ECM can initiate LV dysfunction.[45] Using null and transgenic mice for several ECM and MMP genes has also allowed us to isolate out individual and overlapping functions, although many gene deletion and transgenic models remain to be examined (Table 2).[10, 16, 46–52] Interestingly, different interventions (e.g., deletion of MMP-9 or osteopontin) that similarly reduce collagen accumulation post-MI reduce or increase LV remodeling, indicating that measuring total collagen levels may not predict outcomes. This topic is also discussed in Marielle Scherrer-Crosbie’s review in this issue. ECM turnover lies between cardiomyocyte loss and LV functional decline, but the complete mechanistic pathway remains to be elucidated. Potentially, as more matrikines are identified, these peptides may serve as critical biomarkers and help to distinguish signaling pathways involved in adaptive versus maladaptive remodeling.
Table 2.
Summary of ECM and MMP Null and Transgenic Mice Post-MI Studies
| Factor | Post-MI Effects |
|---|---|
| Collagenase Resistant Collagen Transgenic [46] | No effect seen on early post-MI remodeling |
| Osteopontin Null [47] | Increased LV dilation and reduced collagen deposition in remote and infarct regions |
| Secreted Protein Acidic and Rich in Cysteine Null [48] | Increased cardiac rupture and LV dysfunction |
| Thrombospondin-1 Null reviewed in [49] | Sustained upregulation of chemokines and cytokines; increased macrophage and myofibroblast density; more extensive remodeling |
| MMP-2 Null [50] | Decreased early rupture rate and improved late survival and decreased LV dilation |
| MMP-7 Null [10] | Decreased connexin 43 processing, decreased arrhythmias, and improved survival |
| MMP-9 Null [16] | Decreased remodeling and collagen deposition, decreased macrophage infiltration, increased angiogenesis |
| TIMP-1 Null [51] | Increased LV end diastolic volume and pressure; more pronounced hypertrophic response |
| TIMP-3 Null [52] | Increased mortality, LV dilation, apoptosis, blood vessel numbers; decreased collagen |
Determining which ECM changes are trigger points for remodeling
Several issues need to be resolved before we can know how different ECM components change during remodeling to effect post-MI outcomes. For one, a complete catalogue of ECM changes that occur in the infarct, remote, and border zones over time need to be made. Since the relationship between the cellular and acellular components is dynamic, spatiotemporal considerations are especially important. The post-MI response is a continuum, and the same therapeutic perturbation given at different times will likely have different consequences. For example, measuring total collagen levels by picrosirius red staining at one time point and in one region will not provide as much information as individually measuring collagen I, III, IV, V, and VI over time and location.
It is important to note that morphometric techniques for measuring ECM components are frequently less accurate than biochemical analyses. With improved genomic and proteomic techniques, assigning key roles to individual ECM components is only now beginning. Specific protein and RNA arrays are now available that give a broader profile of the dynamic interaction among the various components. For example, measuring total levels of ECM proteins or integrins that connect cells to the ECM do not tell us the quality of these proteins, in terms of whether full-length or soluble forms are present. MMPs release ECM to cell connections by direct processing of the ECM and by shedding integrins from the cell surface, which also prevents the development of mechanical tension. As we begin to amass this information, more sophisticated statistical approaches such as cluster analysis and principle component analysis and mathematical modeling using differential equations can be used to systematically evaluate ECM roles in cardiac remodeling.
Another issue that we need to deal with is individual biology versus population biology. In human, animal, and cell studies there is individuality in responses, meaning that the average applies for the majority but not necessarily the entirety of the group. This is often seen clinically, in the variation between drug responders and non-responders. We also see this with our post-MI mice, where a group of mice with identical strain and age and with equal infarct sizes will show a wide range of LV dilation (Figure 2, unpublished data). Paying attention to and understanding unequal responses will provide insight into how disparities occur, which will be important for developing optimal therapeutic strategies.
Figure 2.
Infarct size predicts the average, but not the individual, LV remodeling response. For 65 C57/BL6J mice examined at day 7 post-MI, infarct size positively associated with the change in end diastolic dimension. However, for mice within a similar infarct size range (40–50% infarct size is highlighted by the dashed box), the response was not predictable.
Pathoplasticity refers to the way in which a disease process is molded by the context in which it occurs, and this concept is relevant to the post-MI LV. For example, strain, gender, and age-related differences in mice responses are well documented. C57/BL6J male mice are highly prone to rupture post-MI and will develop heart failure symptoms,[46] while FVB mice are resistant to developing heart failure[53] and aging mice show poorer survival rates post-MI.[54] Using computational simulation approaches that are soundly based on real experimental data, we can develop flexible applications of therapeutic models that will meet the needs of diverse individuals and allow us to identify the high need for therapy patient group at an earlier intervention time.
MMP inhibition as a practical therapeutic option
In the Prevention of Myocardial Infarction Early Remodelling (PREMIER) trial, 253 patients with ST segment elevation MI and ejection fractions <40% were randomized to placebo or oral administration of the MMP inhibitor PG-116800.[55] At 90 days follow-up, there were no significant effects on LV remodeling indices or clinical outcomes. Since the failure of this and several other MMP inhibitor trials, we must ask ourselves if it is wise to continue thinking about MMP inhibition as a clinically viable therapeutic option.
One issue with MMP inhibition is that to date the inhibitors used have very broad specificity and selectivity profiles.[56] The fact that most inhibitors were developed when MMPs 1–3 and TIMP-1 were the only known players and the fact that MMPs share high sequence homology in the active site region means that developing drugs that target only one MMP will be challenging. In addition, pro-MMPs have function that is activation-independent. For example, pro-MMP-9 has catalytic activity when it is bound to substrate,[57] and pro-MMP-9 can stimulate cell migration that does not depend on MMP activation or ECM substrate cleavage.[58] The hemopexin domain of MMP-9 can bind several membrane receptors, including lipoprotein receptor-related proteins (LRP-1 and -2), CD44, reversion-inducing-cysteine-rich protein with kazal motifs (RECK), and the DNA repair protein KU. Dendritic cells secrete MMP-9, which can bind αMβ2 integrin and to CD44 to modulate CCL5 and activate the jun N-terminal kinase (JNK) signaling pathway. Pro-MMP-9 effects on cell migration, therefore, may reflect intracellular signaling.[58] Thus, coming up with a strategy that targets MMP-9 will be challenging. Interestingly, Yamamoto and his group have shown that several angiotensin converting enzyme (ACE) inhibitors, including imidapril and lisinopril, can bind to the active site of MMP-9.[59] Whether ACE inhibitors can bind to pro-MMP-9 or other MMPs has not been investigated. Nonetheless, designing ACE inhibitors with improved MMP-9 inhibition may be a useful strategy.
What about ECM peptides as therapeutic options?
One way to bypass the non-selective nature of MMP inhibitors is to focus on the downstream substrates processed by MMPs. Because MMPs process substrates that are both detrimental and beneficial to the MI response, selective MMP inhibition may not be clinically desirable. Instead of focusing on inhibiting MMP-2 or MMP-3 or MMP-9, perhaps a more practical strategy will be to focus on the substrates processed by MMPs. For example, MMP-9 processes fibronectin to release a peptide that contains the RGD sequence, and RGD has been extensively evaluated in cell culture and in vivo experiments. Randall Lee’s group recently showed that rats injected with RGD modified alginate at 5 weeks following ischemia/reperfusion had improved function compared to uninjected hearts, and this benefit was due to stimulation of the angiogenic response. [60] These data suggest that over-expressing RGD may serve a net beneficial function. Given the many ECM and non-ECM substrates available for MMP processing, this line of questioning remains fruitful.
Conclusion
In summary, the ECM is a dynamic component involved in every stage of remodeling in the infarcted myocardium. While many studies have documented the qualitative and quantitative changes in individual components, few have approached the problem of remodeling by dealing with the complexity of the interactions between the cellular and acellular components. From these studies, it is anticipated that new insights into the potential regulatory nature of the ECM will provide new therapeutic insights.
Table 3.
Questions that remain to be answered
|
Acknowledgments
We acknowledge the many insightful comments from Dr. Thomas K. Borg and thank him for critically reviewing this article. We also acknowledge funding to RZ from NIH (T32 HL07446); and funding to MLL from NIH (R01 HL075360), the American Heart Association (GIA 0855119F), and the Morrison Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coats WD, Whittaker P, Cheung DT, Currier JW, Han B, Faxon DP. Collagen Content Is Significantly Lower in Restenotic Versus Nonrestenotic Vessels After Balloon Angioplasty in the Atherosclerotic Rabbit Model. Circulation. 1997 March 4;95(5):1293–300. doi: 10.1161/01.cir.95.5.1293. [DOI] [PubMed] [Google Scholar]
- 2.Cohn JN, Ferrari R, Sharpe N. Cardiac Remodeling- Concepts and Clinical Implications: A Consensus Paper From an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35(3):569–82. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer MA, Braunwald E. Ventricular Remodeling After Myocardial Infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–72. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 4.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Journal of the American College of Cardiology. 2000;35(3):569–82. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 5.Lindsey ML. MMP induction and inhibition in myocardial infarction. Heart Fail Rev. 2004 Jan;9(1):7–19. doi: 10.1023/B:HREV.0000011390.44039.b7. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee I, Yekkala K, Borg TK, Baudino TA. Dynamic interactions between myocytes, fibroblasts, and extracellular matrix. Annals of the New York Academy of Sciences. 2006 Oct;1080:76–84. doi: 10.1196/annals.1380.007. [DOI] [PubMed] [Google Scholar]
- 7.Sundstrom J, Vasan RS. Circulating biomarkers of extracellular matrix remodeling and risk of atherosclerotic events. Curr Opin Lipidol. 2006 Feb;17(1):45–53. doi: 10.1097/01.mol.0000203891.34890.b5. [DOI] [PubMed] [Google Scholar]
- 8.Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol. 2008 Nov 12;130(2):147–58. doi: 10.1016/j.ijcard.2008.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnoor M, Cullen P, Lorkowski J, Stolle K, Robenek H, Troyer D, et al. Production of Type VI Collagen by Human Macrophages: A New Dimension in Macrophage Functional Heterogeneity. J Immunol. 2008 April 15;180(8):5707–19. doi: 10.4049/jimmunol.180.8.5707. [DOI] [PubMed] [Google Scholar]
- 10.Lindsey ML, Escobar GP, Mukherjee R, Goshorn DK, Sheats NJ, Bruce JA, et al. Matrix Metalloproteinase-7 Affects Connexin-43 Levels, Electrical Conduction, and Survival After Myocardial Infarction. Circulation. 2006 June 27;113(25):2919–28. doi: 10.1161/CIRCULATIONAHA.106.612960. [DOI] [PubMed] [Google Scholar]
- 11.Peterson JT, Li H, Dillon L, Bryant JW. Evolution of matrix metalloprotease and tissue inhibitor expression during heart failure progression in the infarcted rat. Cardiovascular Research. 2000;46:307–15. doi: 10.1016/s0008-6363(00)00029-8. [DOI] [PubMed] [Google Scholar]
- 12.Krishnamurthy P, Peterson J, Subramanian V, Singh M, Singh K. Inhibition of matrix metalloproteinases improves left ventricular function in mice lacking osteopontin after myocardial infarction. Molecular and Cellular Biochemistry. 2009;322(1):53–62. doi: 10.1007/s11010-008-9939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindsey M, Wedin K, Brown MD, Keller C, Evans AJ, Smolen J, et al. Matrix-Dependent Mechanism of Neutrophil-Mediated Release and Activation of Matrix Metalloproteinase 9 in Myocardial Ischemia/Reperfusion. Circulation. 2001;103:2181–7. doi: 10.1161/01.cir.103.17.2181. [DOI] [PubMed] [Google Scholar]
- 14.Rohde LE, Ducharme A, Arroyo LH, Aikawa M, Sukhova GH, Lopez-Anaya A, et al. Matrix metalloproteinase inhibition attenuates early left ventricular enlargement after experimental myocardial infarction in mice. Circulation. 1999;15(23):3063–70. doi: 10.1161/01.cir.99.23.3063. [DOI] [PubMed] [Google Scholar]
- 15.Villarreal FJ, Griffin M, Omens J, Dillmann W, Nguyen J, Covell J. Early Short-Term Treatment With Doxycycline Modulates Postinfarction Left Ventricular Remodeling. Circulation. 2003 September 23;108(12):1487–92. doi: 10.1161/01.CIR.0000089090.05757.34. [DOI] [PubMed] [Google Scholar]
- 16.Lindsey ML, Escobar GP, Dobrucki LW, Goshorn DK, Bouges S, Mingoia JT, et al. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006 January 1;290(1):H232–9. doi: 10.1152/ajpheart.00457.2005. [DOI] [PubMed] [Google Scholar]
- 17.Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, et al. Plasma Concentrations and Genetic Variation of Matrix Metalloproteinase 9 and Prognosis of Patients With Cardiovascular Disease. Circulation. 2003 April 1;107(12):1579–85. doi: 10.1161/01.CIR.0000058700.41738.12. [DOI] [PubMed] [Google Scholar]
- 18.Horne BD, Camp NJ, Carlquist JF, Muhlestein JB, Kolek MJ, Nicholas ZP, et al. Multiple-polymorphism associations of 7 matrix metalloproteinase and tissue inhibitor metalloproteinase genes with myocardial infarction and angiographic coronary artery disease. American heart journal. 2007 Oct;154(4):751–8. doi: 10.1016/j.ahj.2007.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly D, Khan SQ, Thompson M, Cockerill G, Ng LL, Samani N, et al. Plasma tissue inhibitor of metalloproteinase-1 and matrix metalloproteinase-9: novel indicators of left ventricular remodelling and prognosis after acute myocardial infarction. Eur Heart J. 2008 September 1;29(17):2116–24. doi: 10.1093/eurheartj/ehn315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolaczkowska E, Chadzinska M, Scislowska-Czarnecka A, Plytycz B, Opdenakker G, Arnold B. Gelatinase B/matrix metalloproteinase-9 contributes to cellular infiltration in a murine model of zymosan peritonitis. Immunobiology. 2006;211(3):137–48. doi: 10.1016/j.imbio.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. The Biochemical journal. 2005 Feb 15;386(Pt 1):15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manso AM, Elsherif L, Kang S-M, Ross RS. Integrins, membrane-type matrix metalloproteinases and ADAMs: Potential implications for cardiac remodeling. Cardiovascular Research. 2006;69(3):574–84. doi: 10.1016/j.cardiores.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Murphy G. The ADAMs: signalling scissors in the tumour microenvironment. Nat Rev Cancer. 2008;8(12):932–41. doi: 10.1038/nrc2459. [DOI] [PubMed] [Google Scholar]
- 24.Maquart F-X, Pasco S, Ramont L, Hornebeck W, Monboisse J-C. An introduction to matrikines: extracellular matrix-derived peptides which regulate cell activity: Implication in tumor invasion. Critical Reviews in Oncology/Hematology. 2004/3;49(3):199–202. doi: 10.1016/j.critrevonc.2003.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Schellings MWM, Pinto YM, Heymans S. Matricellular proteins in the heart: possible role during stress and remodeling. Cardiovascular Research. 2004/10/1;64(1):24–31. doi: 10.1016/j.cardiores.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Sternlicht M, Werb Z. How Matrix Metalloproteinases Regulate Cell Behavior. Annu Rev Cell Dev Biol. 2001;17(1):463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasaki T, Gohring W, Mann K, Maurer P, Hohenester E, Knauper V, et al. Limited cleavage of extracellular matrix protein BM-40 by matrix metalloproteinases increases its affinity for collagens. The Journal of biological chemistry. 1997 Apr 4;272(14):9237–43. doi: 10.1074/jbc.272.14.9237. [DOI] [PubMed] [Google Scholar]
- 28.Diez J, Panizo A, Gil MJ, Monreal I, Hernandex M, Mindan JP. Serum Markers of Collagen Type I Metabolism in Spontaneously Hypertensive Rats. Circulation. 1996;93:1026–32. doi: 10.1161/01.cir.93.5.1026. [DOI] [PubMed] [Google Scholar]
- 29.McGavigan AD, Maxwell PR, Dunn FG. Serological evidence of altered collagen homeostasis reflects early ventricular remodeling following acute myocardial infarction. International Journal of Cardiology. 2006;111(2):267–74. doi: 10.1016/j.ijcard.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 30.Villarreal F, Omens J, Dillmann W, Risteli J, Nguyen J, Covell J. Early degradation and serum appearance of type I collagen fragments after myocardial infarction. Journal of Molecular and Cellular Cardiology. 2004;36(4):597–601. doi: 10.1016/j.yjmcc.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Radovan J, Vaclav P, Petr W, Jan C, Michal A, Richard P, et al. Changes of collagen metabolism predict the left ventricular remodeling after myocardial infarction. Molecular and Cellular Biochemistry. 2006;293(1):71–8. doi: 10.1007/s11010-006-2955-5. [DOI] [PubMed] [Google Scholar]
- 32.Bellon G, Martiny L, Robinet A. Matrix metalloproteinases and matrikines in angiogenesis. Critical Reviews in Oncology/Hematology. 2004/3;49(3):203–20. doi: 10.1016/j.critrevonc.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Pasco S, Ramont L, Maquart F-X, Monboisse JC. Control of melanoma progression by various matrikines from basement membrane macromolecules. Critical Reviews in Oncology/Hematology. 2004/3;49(3):221–3. doi: 10.1016/j.critrevonc.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Schor SL, Schor AM. Phenotypic and genetic alterations in mammary stroma: implications for tumour progression. Breast Cancer Res. 2001;3(6):373–9. doi: 10.1186/bcr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tran KT, Griffith L, Wells A. Extracellular matrix signaling through growth factor receptors during wound healing. Wound Repair Regen. 2004 June 01;12(3):262–8. doi: 10.1111/j.1067-1927.2004.012302.x. [DOI] [PubMed] [Google Scholar]
- 36.Gardi C, Calzoni P, Marcolongo P, Cavarra E, Vanni L, Lungarella G. Collagen breakdown products and lung collagen metabolism: an in vitro study on fibroblast cultures. Thorax. 1994 Apr;49(4):312–8. doi: 10.1136/thx.49.4.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. The International Journal of Biochemistry & Cell Biology. 2008;40(6–7):1101–10. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia T, Akers K, Eisen AZ, Seltzer JL. Comparison of cleavage site specificity of gelatinase A and B using collagenous peptides. Biochim Biophys Acta. 1996;1293(2):259–66. doi: 10.1016/0167-4838(95)00259-6. [DOI] [PubMed] [Google Scholar]
- 39.Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005 Apr-Jun;9(2):267–85. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marom B, Rahat MA, Lahat N, Weiss-Cerem L, Kinarty A, Bitterman H. Native and fragmented fibronectin oppositely modulate monocyte secretion of MMP-9. J Leukoc Biol. 2007 June 1;81(6):1466–76. doi: 10.1189/jlb.0506328. [DOI] [PubMed] [Google Scholar]
- 41.Kuratomi Y, Nomizu M, Tanaka K, Ponce ML, Komiyama S, Kleinman HK, et al. Laminin gamma 1 chain peptide, C-16 (KAFDITYVRLKF), promotes migration, MMP-9 secretion, and pulmonary metastasis of B16-F10 mouse melanoma cells. Br J Cancer. 2002 Apr 8;86(7):1169–73. doi: 10.1038/sj.bjc.6600187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kzhyshkowska J, Workman G, Cardo-Vila M, Arap W, Pasqualini R, Gratchev A, et al. Novel Function of Alternatively Activated Macrophages: Stabilin-1-Mediated Clearance of SPARC. J Immunol. 2006 May 15;176(10):5825–32. doi: 10.4049/jimmunol.176.10.5825. [DOI] [PubMed] [Google Scholar]
- 43.Shankavaram UT, DeWitt DL, Funk SE, Sage EH, Wahl LM. Regulation of Human Monocyte Matrix Metalloproteinases by SPARC. J of Cellular Physiology. 1997;173:327–34. doi: 10.1002/(SICI)1097-4652(199712)173:3<327::AID-JCP4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 44.Radauceanu A, Ducki C, Virion JM, Rossignol P, Mallat Z, McMurray J, et al. Extracellular matrix turnover and inflammatory markers independently predict functional status and outcome in chronic heart failure. J Card Fail. 2008 Aug;14(6):467–74. doi: 10.1016/j.cardfail.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 45.Miller AD, Tyagi SC. Mutation in collagen gene induces cardiomyopathy in transgenic mice. J Cell Biochem. 2002;85(2):259–67. doi: 10.1002/jcb.10130. [DOI] [PubMed] [Google Scholar]
- 46.Lindsey ML, Yoshioka J, MacGillivray C, Muangman S, Gannon J, Verghese A, et al. Effect of a Cleavage-Resistant Collagen Mutation on Left Ventricular Remodeling. Circ Res. 2003 August 8;93(3):238–45. doi: 10.1161/01.RES.0000085580.45279.60. [DOI] [PubMed] [Google Scholar]
- 47.Trueblood NA, Xie Z, Communal C, Sam F, Ngoy S, Liaw L, et al. Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ Res. 2001 May 25;88(10):1080–7. doi: 10.1161/hh1001.090842. [DOI] [PubMed] [Google Scholar]
- 48.Schellings MW, Vanhoutte D, Swinnen M, Cleutjens JP, Debets J, van Leeuwen RE, et al. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. The Journal of experimental medicine. 2009 Jan 16;206(1):113–23. doi: 10.1084/jem.20081244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blankesteijn WM, Creemers E, Lutgens E, Cleutjens JP, Daemen MJ, Smits JF. Dynamics of cardiac wound healing following myocardial infarction: observations in genetically altered mice. Acta Physiol Scand. 2001 Sep;173(1):75–82. doi: 10.1046/j.1365-201X.2001.00887.x. [DOI] [PubMed] [Google Scholar]
- 50.Hayashidani S, Tsutsui H, Ikeuchi M, Shiomi T, Matsusaka H, Kubota T, et al. Targeted deletion of MMP-2 attenuates early LV rupture and late remodeling after experimental myocardial infarction. Am J Physiol Heart Circ Physiol. 2003 August 7;285(3):H1229–35. doi: 10.1152/ajpheart.00207.2003. [DOI] [PubMed] [Google Scholar]
- 51.Creemers EEJM, Davis JN, Parkhurst AM, Leenders P, Dowdy KB, Hapke E, et al. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2003 January 1;284(1):H364–71. doi: 10.1152/ajpheart.00511.2002. [DOI] [PubMed] [Google Scholar]
- 52.Tian H, Cimini M, Fedak PWM, Altamentova S, Fazel S, Huang M-L, et al. TIMP-3 deficiency accelerates cardiac remodeling after myocardial infarction. Journal of Molecular and Cellular Cardiology. 2007;43(6):733–43. doi: 10.1016/j.yjmcc.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Weinberg EO, Mirotsou M, Gannon J, Dzau VJ, Lee RT, Pratt RE. Sex dependence and temporal dependence of the left ventricular genomic response to pressure overload. Physiol Genomics. 2003 January 15;12(2):113–27. doi: 10.1152/physiolgenomics.00046.2002. [DOI] [PubMed] [Google Scholar]
- 54.Bujak M, Kweon HJ, Chatila K, Li N, Taffet G, Frangogiannis NG. Aging-Related Defects Are Associated With Adverse Cardiac Remodeling in a Mouse Model of Reperfused Myocardial Infarction. Journal of the American College of Cardiology. 2008;51(14):1384–92. doi: 10.1016/j.jacc.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hudson MP, Armstrong PW, Ruzyllo W, Brum J, Cusmano L, Krzeski P, et al. Effects of Selective Matrix Metalloproteinase Inhibitor (PG-116800) to Prevent Ventricular Remodeling After Myocardial Infarction: Results of the PREMIER (Prevention of Myocardial Infarction Early Remodeling) Trial. Journal of the American College of Cardiology. 2006;48(1):15–20. doi: 10.1016/j.jacc.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 56.Peterson JT. The importance of estimating the therapeutic index in the development of matrix metalloproteinase inhibitors. Cardiovasc Res. 2006 2006/2/15;69(3):677–87. doi: 10.1016/j.cardiores.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 57.Bannikov GA, Karelina TV, Collier IE, Marmer BL, Goldberg GI. Substrate binding of gelatinase B induces its enzymatic activity in the presence of intact propeptide. The Journal of biological chemistry. 2002 2002/2/11;:M110931200. doi: 10.1074/jbc.M110931200. [DOI] [PubMed] [Google Scholar]
- 58.Dufour A, Sampson NS, Zucker S, Cao J. Role of the hemopexin domain of matrix metalloproteinases in cell migration. J Cell Physiol. 2008 Dec;217(3):643–51. doi: 10.1002/jcp.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamamoto D, Takai S, Jin D, Inagaki S, Tanaka K, Miyazaki M. Molecular mechanism of imidapril for cardiovascular protection via inhibition of MMP-9. J Mol Cell Cardiol. 2007 Dec;43(6):670–6. doi: 10.1016/j.yjmcc.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 60.Yu J, Gu Y, Du KT, Mihardja S, Sievers RE, Lee RJ. The effect of injected RGD modified alginate on angiogenesis and left ventricular function in a chronic rat infarct model. Biomaterials. 2009 Feb;30(5):751–6. doi: 10.1016/j.biomaterials.2008.09.059. [DOI] [PubMed] [Google Scholar]