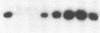Abstract
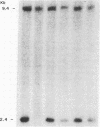
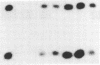
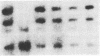
The cloning of a cDNA for the human androgen receptor gene has resulted in the availability of cDNA probes that span various parts of the gene, including the entire steroid-binding domain and part of the DNA-binding domain, as well as part of the 5' region of the gene. The radiolabeled probes were used to screen for androgen receptor mutations on Southern blots prepared by restriction endonuclease digestion of genomic DNA from human subjects with complete androgen insensitivity syndrome (AIS). In this investigation, we considered only patients presenting complete AIS and with the androgen receptor (-) form as the most probable subjects to show a gene deletion. One subject from each of six unrelated families with the receptor (-) form of complete AIS and 10 normal subjects (6 females and 4 males) were studied. In the 10 normal subjects and in 5 of the 6 patients, identical DNA restriction fragment patterns were observed with EcoRI and BamHI. In one affected individual, a partial deletion of the androgen receptor gene involving the steroid-binding domain was detected. Analysis of other members of this family confirmed the apparent gene deletion. Our data provide direct proof that complete AIS in some families can result from a deletion of the androgen receptor structural gene. However, other families do not demonstrate such a deletion, suggesting that point mutations (or small, undetectable deletions) may also result in the receptor (-) form of complete AIS, adding further to the genetic heterogeneity of this syndrome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiman J., Griffin J. E., Gazak J. M., Wilson J. D., MacDonald P. C. Androgen insensitivity as a cause of infertility in otherwise normal men. N Engl J Med. 1979 Feb 1;300(5):223–227. doi: 10.1056/NEJM197902013000503. [DOI] [PubMed] [Google Scholar]
- Amrhein J. A., Meyer W. J., 3rd, Jones H. W., Jr, Migeon C. J. Androgen insensitivity in man: evidence for genetic heterogeneity. Proc Natl Acad Sci U S A. 1976 Mar;73(3):891–894. doi: 10.1073/pnas.73.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis S. E., Waber P. G., Kittur S. D., Patel A. S., Kazazian H. H., Jr, Mellis M. A., Counts R. B., Stamatoyannopoulos G., Bowie E. J., Fass D. N. Hemophilia A. Detection of molecular defects and of carriers by DNA analysis. N Engl J Med. 1985 Oct 3;313(14):842–848. doi: 10.1056/NEJM198510033131402. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Karam J. H., Rutter W. J. Polymorphic DNA region adjacent to the 5' end of the human insulin gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5759–5763. doi: 10.1073/pnas.78.9.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. R., Maes M., Rothwell S. W., Migeon C. J. Human complete androgen insensitivity with normal dihydrotestosterone receptor binding capacity in cultured genital skin fibroblasts: evidence for a qualitative abnormality of the receptor. J Clin Endocrinol Metab. 1982 Jul;55(1):61–69. doi: 10.1210/jcem-55-1-61. [DOI] [PubMed] [Google Scholar]
- Brown T. R., Migeon C. J. Cultured human skin fibroblasts: a model for the study of androgen action. Mol Cell Biochem. 1981 Apr 13;36(1):3–22. doi: 10.1007/BF02354827. [DOI] [PubMed] [Google Scholar]
- Chang C. S., Kokontis J., Liao S. T. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988 Apr 15;240(4850):324–326. doi: 10.1126/science.3353726. [DOI] [PubMed] [Google Scholar]
- Donohoue P. A., van Dop C., McLean R. H., White P. C., Jospe N., Migeon C. J. Gene conversion in salt-losing congenital adrenal hyperplasia with absent complement C4B protein. J Clin Endocrinol Metab. 1986 May;62(5):995–1002. doi: 10.1210/jcem-62-5-995. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fichman K. R., Migeon B. R., Migeon C. J. Genetic disorders of male sexual differentiation. Adv Hum Genet. 1980;10:333-77, 387. doi: 10.1007/978-1-4615-8288-5_5. [DOI] [PubMed] [Google Scholar]
- Giannelli F., Choo K. H., Rees D. J., Boyd Y., Rizza C. R., Brownlee G. G. Gene deletions in patients with haemophilia B and anti-factor IX antibodies. Nature. 1983 May 12;303(5913):181–182. doi: 10.1038/303181a0. [DOI] [PubMed] [Google Scholar]
- Griffin J. E., Punyashthiti K., Wilson J. D. Dihydrotestosterone binding by cultured human fibroblasts. Comparison of cells from control subjects and from patients with hereditary male pseudohermaphroditism due to androgen resistance. J Clin Invest. 1976 May;57(5):1342–1351. doi: 10.1172/JCI108402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs H. H., Brown M. S., Russell D. W., Davignon J., Goldstein J. L. Deletion in the gene for the low-density-lipoprotein receptor in a majority of French Canadians with familial hypercholesterolemia. N Engl J Med. 1987 Sep 17;317(12):734–737. doi: 10.1056/NEJM198709173171204. [DOI] [PubMed] [Google Scholar]
- Hughes I. A., Evans B. A. Complete androgen insensitivity syndrome characterized by increased concentration of a normal androgen receptor in genital skin fibroblasts. J Clin Endocrinol Metab. 1986 Aug;63(2):309–315. doi: 10.1210/jcem-63-2-309. [DOI] [PubMed] [Google Scholar]
- Kaufman M., Pinsky L., Baird P. A., McGillivray B. C. Complete androgen insensitivity with a normal amount of 5 alpha-dihydrotestosterone-binding activity in labium majus skin fibroblasts. Am J Med Genet. 1979;4(4):401–411. doi: 10.1002/ajmg.1320040410. [DOI] [PubMed] [Google Scholar]
- Kaufman M., Straisfeld C., Pinsky L. Male pseudohermaphroditism presumably due to target organ unresponsiveness to androgens. Deficient 5alpha-dihydrotestosterone binding in cultured skin fibroblasts. J Clin Invest. 1976 Aug;58(2):345–350. doi: 10.1172/JCI108478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan B. S., Meyer W. J., 3rd, Hadjian A. J., Jones H. W., Migeon C. J. Syndrome of androgen insensitivity in man: absence of 5 alpha-dihydrotestosterone binding protein in skin fibroblasts. J Clin Endocrinol Metab. 1974 Jun;38(6):1143–1146. doi: 10.1210/jcem-38-6-1143. [DOI] [PubMed] [Google Scholar]
- Lubahn D. B., Joseph D. R., Sullivan P. M., Willard H. F., French F. S., Wilson E. M. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science. 1988 Apr 15;240(4850):327–330. doi: 10.1126/science.3353727. [DOI] [PubMed] [Google Scholar]
- Lyon M. F., Hawkes S. G. X-linked gene for testicular feminization in the mouse. Nature. 1970 Sep 19;227(5264):1217–1219. doi: 10.1038/2271217a0. [DOI] [PubMed] [Google Scholar]
- Meyer W. J., 3rd, Migeon B. R., Migeon C. J. Locus on human X chromosome for dihydrotestosterone receptor and androgen insensitivity. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1469–1472. doi: 10.1073/pnas.72.4.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeon C. J., Brown T. R., Lanes R., Palacios A., Amrhein J. A., Schoen E. J. A clinical syndrome of mild androgen insensitivity. J Clin Endocrinol Metab. 1984 Oct;59(4):672–678. doi: 10.1210/jcem-59-4-672. [DOI] [PubMed] [Google Scholar]
- Monaco A. P., Bertelson C. J., Middlesworth W., Colletti C. A., Aldridge J., Fischbeck K. H., Bartlett R., Pericak-Vance M. A., Roses A. D., Kunkel L. M. Detection of deletions spanning the Duchenne muscular dystrophy locus using a tightly linked DNA segment. 1985 Aug 29-Sep 4Nature. 316(6031):842–845. doi: 10.1038/316842a0. [DOI] [PubMed] [Google Scholar]
- Pinsky L., Kaufman M., Gil-Esteban C., Sumbulian D. Regulation of the androgen receptor in human genital skin fibroblasts, with a review of sex steroid receptor regulation by homologous and heterologous steroids. Can J Biochem Cell Biol. 1983 Jul;61(7):770–778. doi: 10.1139/o83-097. [DOI] [PubMed] [Google Scholar]
- Rivarola M. A., Saez J. M., Meyer W. J., Kenny F. M., Migeon C. J. Studies of androgens in the syndrome of male pseudohermaphroditism with testicular feminization. J Clin Endocrinol Metab. 1967 Mar;27(3):371–378. doi: 10.1210/jcem-27-3-371. [DOI] [PubMed] [Google Scholar]
- Stanley A. J., Gumbreck L. G., Allison J. E., Easley R. B. Male pseudohermaphroditism in the laboratory Norway rat. Recent Prog Horm Res. 1973;29:43–64. doi: 10.1016/b978-0-12-571129-6.50005-1. [DOI] [PubMed] [Google Scholar]
- Tremblay R. R., Foley T. P., Jr, Corvol P., Park I. J., Kowarski A., Blizzard R. M., Jones H. W., Jr, Migeon C. J. Plasma concentration of testosterone, dihydrotestosterone, testosterone-oestradiol binding globulin, and pituitary gonadotrophins in the syndrome of male pseudo-hermaphroditism with testicular feminization. Acta Endocrinol (Copenh) 1972 Jun;70(2):331–341. doi: 10.1530/acta.0.0700331. [DOI] [PubMed] [Google Scholar]
- Wieacker P., Griffin J. E., Wienker T., Lopez J. M., Wilson J. D., Breckwoldt M. Linkage analysis with RFLPs in families with androgen resistance syndromes: evidence for close linkage between the androgen receptor locus and the DXS1 segment. Hum Genet. 1987 Jul;76(3):248–252. doi: 10.1007/BF00283617. [DOI] [PubMed] [Google Scholar]