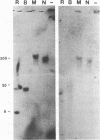
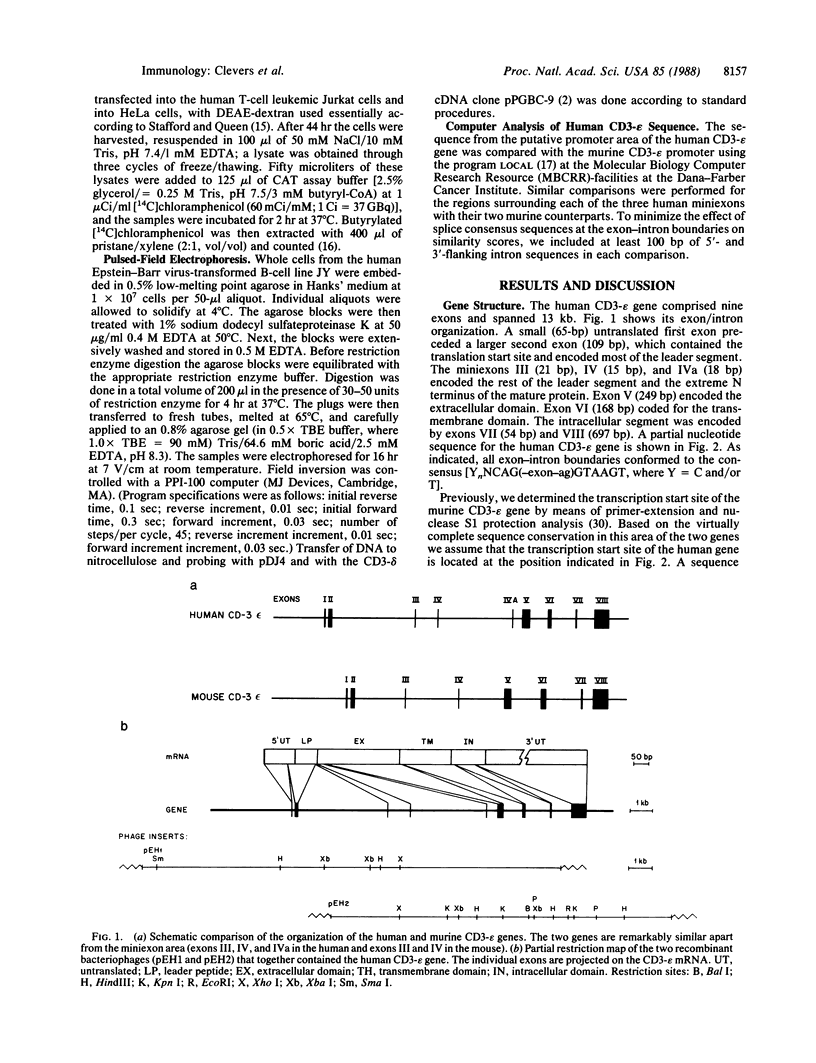
Abstract
The antigen receptor of the T lymphocyte consists of two variable T-cell receptor chains (either TCR-alpha, TCR-beta or TCR-gamma, TCR-delta) noncovalently linked to four different invariant membrane proteins (CD3-gamma, CD3-delta, CD3-epsilon, and the CD3-zeta homodimer). The CD3 genes are expressed early in thymocyte development, preceding the rearrangement and expression of the T-cell receptor genes. Here we report the isolation and structural analysis of the human CD3-epsilon gene. The gene consisted of nine exons. Three exons, encoding the junction of leader peptide and mature protein, were extremely small (21, 15, and 18 base pairs, respectively). The murine gene contained only two such miniexons, the sequences of which were not homologous to those of the three human miniexons. But from comparisons of intron sequences the regions surrounding the human miniexons III and IV appeared to be closely related to those surrounding the murine miniexons III and IV. The most-3' miniexon in the human gene (IVa) had no murine counterpart and appeared not to duplicate any of the other miniexons. Sequence analysis of CD3-epsilon cDNA clones isolated from four independent libraries gave no evidence for alternative use of these miniexons. Like CD3-delta, the CD3-epsilon gene was transcribed from a weak, nontissue-specific, TATA-less promoter. Pulsed-field electrophoresis showed that the human CD3-epsilon gene was separated from the CD3-gamma, CD3-delta gene pair by at least 30 kilobases, but by no more than 300 kilobases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aho S., Tate V., Boedtker H. Location of the 11 bp exon in the chicken pro alpha 2(I) collagen gene. Nucleic Acids Res. 1984 Aug 10;12(15):6117–6125. doi: 10.1093/nar/12.15.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart R. E., Nguyen H. T., Medford R. M., Destree A. T., Mahdavi V., Nadal-Ginard B. Intricate combinatorial patterns of exon splicing generate multiple regulated troponin T isoforms from a single gene. Cell. 1985 May;41(1):67–82. doi: 10.1016/0092-8674(85)90062-5. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Frank M., Olson M. V. Electrophoretic separations of large DNA molecules by periodic inversion of the electric field. Science. 1986 Apr 4;232(4746):65–68. doi: 10.1126/science.3952500. [DOI] [PubMed] [Google Scholar]
- Clevers H., Alarcon B., Wileman T., Terhorst C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol. 1988;6:629–662. doi: 10.1146/annurev.iy.06.040188.003213. [DOI] [PubMed] [Google Scholar]
- Clevers H., Dunlap S., Terhorst C. The transmembrane orientation of the epsilon chain of the TcR/CD3 complex. Eur J Immunol. 1988 May;18(5):705–710. doi: 10.1002/eji.1830180508. [DOI] [PubMed] [Google Scholar]
- Cooper T. A., Ordahl C. P. A single cardiac troponin T gene generates embryonic and adult isoforms via developmentally regulated alternate splicing. J Biol Chem. 1985 Sep 15;260(20):11140–11148. [PubMed] [Google Scholar]
- Furley A. J., Mizutani S., Weilbaecher K., Dhaliwal H. S., Ford A. M., Chan L. C., Molgaard H. V., Toyonaga B., Mak T., van den Elsen P. Developmentally regulated rearrangement and expression of genes encoding the T cell receptor-T3 complex. Cell. 1986 Jul 4;46(1):75–87. doi: 10.1016/0092-8674(86)90861-5. [DOI] [PubMed] [Google Scholar]
- Georgopoulos K., van den Elsen P., Bier E., Maxam A., Terhorst C. A T cell-specific enhancer is located in a DNase I-hypersensitive area at the 3' end of the CD3-delta gene. EMBO J. 1988 Aug;7(8):2401–2407. doi: 10.1002/j.1460-2075.1988.tb03085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold D. P., Puck J. M., Pettey C. L., Cho M., Coligan J., Woody J. N., Terhorst C. Isolation of cDNA clones encoding the 20K non-glycosylated polypeptide chain of the human T-cell receptor/T3 complex. Nature. 1986 May 22;321(6068):431–434. doi: 10.1038/321431a0. [DOI] [PubMed] [Google Scholar]
- Gold D. P., Puck J. M., Pettey C. L., Cho M., Coligan J., Woody J. N., Terhorst C. Isolation of cDNA clones encoding the 20K non-glycosylated polypeptide chain of the human T-cell receptor/T3 complex. Nature. 1986 May 22;321(6068):431–434. doi: 10.1038/321431a0. [DOI] [PubMed] [Google Scholar]
- Gold D. P., van Dongen J. J., Morton C. C., Bruns G. A., van den Elsen P., Geurts van Kessel A. H., Terhorst C. The gene encoding the epsilon subunit of the T3/T-cell receptor complex maps to chromosome 11 in humans and to chromosome 9 in mice. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1664–1668. doi: 10.1073/pnas.84.6.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Krissansen G. W., Gorman P. A., Kozak C. A., Spurr N. K., Sheer D., Goodfellow P. N., Crumpton M. J. Chromosomal locations of the gene coding for the CD3 (T3) gamma subunit of the human and mouse CD3/T-cell antigen receptor complexes. Immunogenetics. 1987;26(4-5):258–266. doi: 10.1007/BF00346520. [DOI] [PubMed] [Google Scholar]
- Krissansen G. W., Owen M. J., Verbi W., Crumpton M. J. Primary structure of the T3 gamma subunit of the T3/T cell antigen receptor complex deduced from cDNA sequences: evolution of the T3 gamma and delta subunits. EMBO J. 1986 Aug;5(8):1799–1808. doi: 10.1002/j.1460-2075.1986.tb04429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R., Mathey-Prevot B., Bernards A., Baltimore D. Neuronal pp60c-src contains a six-amino acid insertion relative to its non-neuronal counterpart. Science. 1987 Jul 24;237(4813):411–415. doi: 10.1126/science.2440106. [DOI] [PubMed] [Google Scholar]
- Miyazaki H., Fukamizu A., Hirose S., Hayashi T., Hori H., Ohkubo H., Nakanishi S., Murakami K. Structure of the human renin gene. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5999–6003. doi: 10.1073/pnas.81.19.5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Koyama T., Georgopoulos K., Clevers H., Haser W. G., LeBien T., Tonegawa S., Terhorst C. Close linkage of the mouse and human CD3 gamma- and delta-chain genes suggests that their transcription is controlled by common regulatory elements. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9131–9134. doi: 10.1073/pnas.84.24.9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. F., Waterman M. S. Identification of common molecular subsequences. J Mol Biol. 1981 Mar 25;147(1):195–197. doi: 10.1016/0022-2836(81)90087-5. [DOI] [PubMed] [Google Scholar]
- Tunnacliffe A., Buluwela L., Rabbitts T. H. Physical linkage of three CD3 genes on human chromosome 11. EMBO J. 1987 Oct;6(10):2953–2957. doi: 10.1002/j.1460-2075.1987.tb02600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunnacliffe A., Sims J. E., Rabbitts T. H. T3 delta pre-mRNA is transcribed from a non-TATA promoter and is alternatively spliced in human T cells. EMBO J. 1986 Jun;5(6):1245–1252. doi: 10.1002/j.1460-2075.1986.tb04353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman A. M., Baniyash M., Hou D., Samelson L. E., Burgess W. H., Klausner R. D. Molecular cloning of the zeta chain of the T cell antigen receptor. Science. 1988 Feb 26;239(4843):1018–1021. doi: 10.1126/science.3278377. [DOI] [PubMed] [Google Scholar]
- Wingender E. Compilation of transcription regulating proteins. Nucleic Acids Res. 1988 Mar 25;16(5):1879–1902. doi: 10.1093/nar/16.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen J. J., Quertermous T., Bartram C. R., Gold D. P., Wolvers-Tettero I. L., Comans-Bitter W. M., Hooijkaas H., Adriaansen H. J., de Klein A., Raghavachar A. T cell receptor-CD3 complex during early T cell differentiation. Analysis of immature T cell acute lymphoblastic leukemias (T-ALL) at DNA, RNA, and cell membrane level. J Immunol. 1987 Feb 15;138(4):1260–1269. [PubMed] [Google Scholar]
- van den Elsen P., Bruns G., Gerhard D. S., Pravtcheva D., Jones C., Housman D., Ruddle F. A., Orkin S., Terhorst C. Assignment of the gene coding for the T3-delta subunit of the T3-T-cell receptor complex to the long arm of human chromosome 11 and to mouse chromosome 9. Proc Natl Acad Sci U S A. 1985 May;82(9):2920–2924. doi: 10.1073/pnas.82.9.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Elsen P., Georgopoulos K., Shepley B. A., Orkin S., Terhorst C. Exon/intron organization of the genes coding for the delta chains of the human and murine T-cell receptor/T3 complex. Proc Natl Acad Sci U S A. 1986 May;83(9):2944–2948. doi: 10.1073/pnas.83.9.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Elsen P., Shepley B. A., Borst J., Coligan J. E., Markham A. F., Orkin S., Terhorst C. Isolation of cDNA clones encoding the 20K T3 glycoprotein of human T-cell receptor complex. 1984 Nov 29-Dec 5Nature. 312(5993):413–418. doi: 10.1038/312413a0. [DOI] [PubMed] [Google Scholar]