Abstract
Proinflammatory cytokines, such as IL-1β, have been implicated in the cellular and behavioral effects of stress and in mood disorders, although the downstream signaling pathways underlying these effects have not been determined. In the present study, we demonstrate a critical role for NF-κB signaling in the actions of IL-1β and stress. Stress inhibition of neurogenesis in the adult hippocampus, which has been implicated in the prodepressive effects of stress, is blocked by administration of an inhibitor of NF-κB. Further analysis reveals that stress activates NF-κB signaling and decreases proliferation of neural stem-like cells but not early neural progenitor cells in the adult hippocampus. We also find that depressive-like behaviors caused by exposure to chronic stress are mediated by NF-κB signaling. Together, these data identify NF-κB signaling as a critical mediator of the antineurogenic and behavioral actions of stress and suggest previously undescribed therapeutical targets for depression.
Keywords: cytokine, IL-1, inflammation, neural progenitor cell, depression
Mood disorders represent a major health concern, affecting over 15% of the population in developed countries, resulting in enormous personal and economic costs and, in many cases, suicide (1, 2). Despite significant efforts, the neurobiological mechanisms underlying depression have not been characterized. Both genetic and environmental factors contribute to depression, and traumatic or repeated stress is known to precipitate or exacerbate mood disorders (3 –5). In addition, proinflammatory cytokines, including IL-1β, IL-6, and TNF-α, that are induced by injury and infection as well as by psychological stress have been implicated in depressive behavior in rodent models and depressed patients (6 –8).
Exposure to stress and depression can result in atrophy of limbic brain regions that control emotion and mood, including inhibition of neurogenesis in the adult hippocampus (5, 9, 10). Inhibition of neurogenesis is observed with many different types of physical and psychological stressors, but the types of cells, neural stem-like cells (NSCs) or intermediate transient amplifying neural progenitor cells (ANPs), that are influenced have not been characterized (9, 11). A role for proinflammatory cytokines is supported by a recent report that IL-1β signaling is necessary and sufficient for the antineurogenic and behavioral effects of stress (6). One possible signaling cascade that could mediate the effects of IL-1β is NF-κB, which is activated by IL-1β and other cytokines both in peripheral immune cells and in the brain (8, 12). Chronic stress enhances the activation of NF-κB in response to inflammatory stimuli (13, 14), and social stress increases NF-κB signaling in healthy subjects and produces an exaggerated response in depressed patients (15, 16).
In the present study, we investigate the role of NF-κB in the cellular and behavioral responses to acute and chronic stress. The results demonstrate that the inhibition of neurogenesis by stress occurs via activation of NF-κB in NSCs and that stress-induced anhedonia, a core symptom of depression, is dependent on NF-κB.
Results
NF-κB Signaling Mediates the Suppression of Hippocampal Neurogenesis Caused by Acute and Chronic Stress.
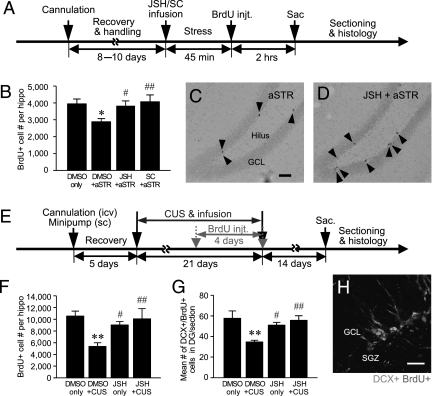
To examine the role of NF-κB in the effects of acute stress, rats were administered an inhibitor of NF-κB before acute immobilization stress (45 min) (Fig. 1A). Two selective structurally different NF-κB inhibitors were tested: JSH-23 (JSH) and SC-514 (SC) (17, 18). Immediately after immobilization, rats were administered BrdU, a thymidine analogue that is incorporated into dividing cells (2 h post-BrdU injection). Post hoc analysis revealed that immobilization stress significantly decreases the number of BrdU+ cells in the subgranular zone (SGZ) of the dentate gyrus (DG), and this effect is completely blocked by preadministration of either JSH or SC (Fig. 1 B–D).
Fig. 1.
Effects of stress and NF-κB inhibitors on hippocampal neurogenesis in rats. (A) Schematic depicting the experimental procedures for acute stress (aSTR). After 8–10 days of recovery and handling, rats were infused (i.c.v.) with the NF-κB inhibitors JSH or SC or with vehicle (DMSO) before immobilization and were then killed (sac) 2 h after BrdU administration. injt., injection. (B) aSTR decreased the number of BrdU+ cells in the DG of the hippocampus. The effect of aSTR was blocked by either JSH or SC (one-way ANOVA: F 3,26 = 3.527, P < 0.05; n = 7–8 per group). Dividing BrdU+ cells (black arrowheads) in the DG of acute-stressed (C) and JSH-infused stressed (D) rats. GCL, granular cell layer. (Scale bar: 100 μm.) (E) Schematic for CUS. Rats were implanted with a cannula (i.c.v.) and minipump (s.c.), were exposed to two stressors per day for 21 days, received BrdU daily for the last 4 CUS days, and were killed 14 days later. JSH blocked the effects of CUS on BrdU+ cells (F 3,19 = 5.013, P < 0.05; n = 5–6 per group) (F), and immature neurons were measured as the number of BrdU+ (red) DCX+ (green) double-labeled cells per hippocampal DG (F 3,12 = 6.250, P < 0.01; n = 4 per group) (G and H). (Scale bar: 25 μm.) By one-way ANOVA followed by the Fisher’s PLSD test, *P < 0.05 and **P < 0.01 compared with control (DMSO) and #, P < 0.05 and ##, P < 0.01 compared with stressed animals.
We also examined the role of NF-κB in the antiproliferative effects of chronic unpredictable stress (CUS), a model of depression with face, predictive, and construct validity (19, 20). Exposure to CUS significantly decreased the number of BrdU+ cells, and this effect was completely blocked by infusion of JSH (i.c.v., minipump) (Fig. 1F). At the time point examined (14 days after BrdU) (Fig. 1E), the majority of the BrdU+ cells express doublecortin (DCX), a marker of immature neurons (Fig. 1H). Analysis of the BrdU+ DCX+ double-labeled cells demonstrates that CUS also decreases the number of immature neurons, and this effect was blocked by JSH (Fig. 1G).
Acute Stress Decreases the Proliferation of NSCs: Blockade by NF-κB Inhibition.
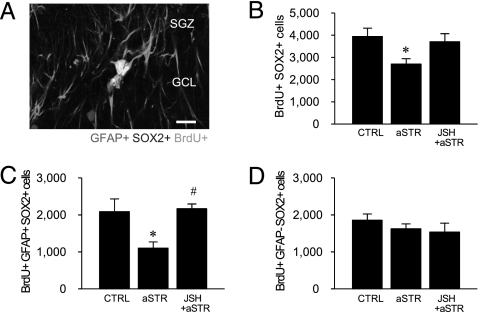
We next examined the influence of acute immobilization stress (45 min) on the two major classes of progenitors. NSCs undergo asymmetric division, are self-renewing, have a radial glial morphology, and express GFAP and the transcription factor sex-determining region Y-box containing gene 2 (SOX2) (21 –23) (Fig. 2A). ANPs undergo symmetric division and express SOX2 but not GFAP (22, 23). In the current study, we found that nearly all BrdU+ cells were SOX2+ (97.1 ± 1.5%), and exposure to acute immobilization stress significantly decreased the number of BrdU+ SOX2+ double-labeled cells. Importantly, this effect of stress was completely blocked by pretreatment with the NF-κB inhibitor JSH (Fig. 2B). We also found that acute stress significantly decreases the number of triple-labeled NSCs (BrdU+ SOX2+ GFAP+) (Fig. 2C) but not the number of ANPs (BrdU+ SOX2+ GFAP−) (Fig. 2D), and this effect is blocked by JSH pretreatment.
Fig. 2.
Role of NF-κB signaling in the regulation of NSC and ANP cell proliferation in acute stress. (A) Representative photograph of proliferating NSCs [GFAP+ (red) SOX2+ (blue) BrdU+ (green) cells] in the SGZ. (B) Acute stress decreased the proliferation of total progenitors (SOX2+ BrdU+ cells), and there was no significant effect in CUS animals receiving JSH (F 2,11 = 4.215, P < 0.05; n = 4–5 per group). aSTR, acute stress; CTRL, control. Stress-induced impairment of NSC (F 2,11 = 5.814, P < 0.05) (C) but not ANP (F 2,11 = 0.852, P = n.s.) (D) proliferation was blocked by JSH. (Scale bar: 25 μm.) By the Fisher’s PLSD test, *, P < 0.05 compared with CTRL group and # , P < 0.05 compared with aSTR group.
Previously, we have shown that the antineurogenic effects of stress occur via IL-1β/IL-1receptor type I (RI) signaling, both in vivo and in vitro (6). We found that IL-1RI is expressed in both NSCs and ANPs, albeit at different levels (27.6 ± 2.2% and 49.2 ± 6.3%, respectively) (Fig. S1). Because the majority of the SOX2+ cells are GFAP+ NSCs (81.3 ± 1.4%), there are more NSCs than ANPs that express IL-1RI.
Acute Stress Activates NF-κB Signaling in NSCs.
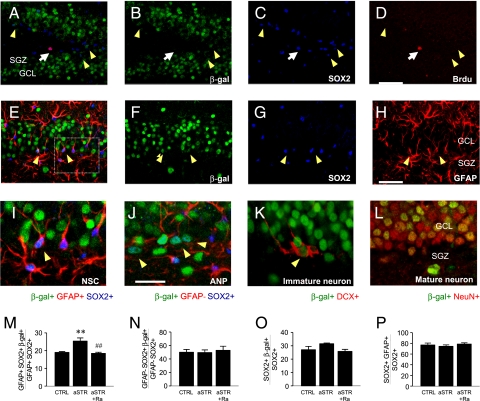
We examined the cellular localization of NF-κB activation using transgenic NF-κB/LacZ reporter mice. Immunohistochemical analysis demonstrates that β-gal is expressed in the granule cell layer of the DG (Fig. 3 A and B), as previously reported (24). Triple-labeling studies demonstrate that β-gal is expressed in both NSCs (GFAP+ SOX2+) (Fig. 3 E–I) and ANPs (GFAP− SOX2+) (Fig. 3J) in the SGZ as well as in cells in the SGZ/DG that express markers of immature (DCX) (Fig. 3K) or mature (NeuN) (Fig. 3L) neurons. Notably, proliferating newborn cells labeled with BrdU (2 h after injection) did not express β-gal (Fig. 3 A–D), suggesting that NF-κB activation blocks proliferation.
Fig. 3.
Acute stress effects on NF-κB reporter expression in the hippocampus in NF-κB/LacZ reporter mice. (A–D) Most hippocampal progenitors [∼94%; BrdU+ (red), SOX2+ (blue), white arrows] did not express the NF-κB reporter [β-gal+ (green), yellow arrowheads]. (Scale bar: 50 μm.) (E–H) Expression of NF-κB reporter in NSCs [β-gal+ (F, green) SOX2+ (G, blue) GFAP+ (H, red)]. (Scale bar: 50 μm.) (I) Enlarged view of NSCs from E (Inset). (J) NF-κB/β-gal was observed in ANPs (β-gal+ SOX2+ GFAP−) as well as NSCs in the SGZ. Immature [β-gal+ DCX+ (red)] (K) and mature [β-gal+ NeuN+ (red)] (I) neurons also expressed the NF-κB reporter. (M–P) Acute immobilization stress (aSTR) resulted in more NF-κB activation (β-gal+) in NSCs but not in ANPs or in total progenitors compared with control (CTRL) [NSCs, F 2,9 = 11.381, P < 0.01 (M); ANPs, F 2,9 = 0.134, P = n.s. (N); total progenitors, F 2,9 = 2.994, P = n.s. (O); n = 4 per group]. (Scale bar: 25 μm.) By the Fisher’s PLSD test, **, P < 0.01 compared with CTRL and ## , P < 0.01 compared with aSTR group.
We also found that acute stress significantly increased the number of NF-κB/β-gal+ NSCs but not ANPs in the SGZ, and this effect was blocked by pretreatment with the IL-1 receptor antagonist (Ra) (Fig. 3 M and N). This suggests that acute stress preferentially activates NF-κB in NSCs. Stress did not change the ratio of NSCs to total progenitors in the SGZ [F 2,9 = 0.537, P = not significant (n.s.)]. Together, these data demonstrate that acute stress stimulates NF-κB signaling in NSCs and that this effect is mediated by IL-1β.
NF-κB Signaling Underlies Depressive-Like Behavioral Effects of CUS.
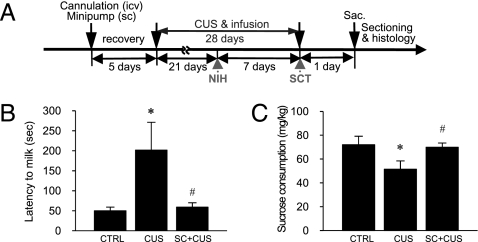
To determine if NF-κB signaling underlies the effect of CUS on depressive-like behaviors, SC was administered during 4 weeks of exposure to CUS (Fig. 4A). The novelty-induced hypophagia (NIH) test is a model of anxiety that is responsive to chronic antidepressant administration (25). CUS exposure significantly increased the latency to drink relative to nonstressed control mice in the novel cage, as determined on day 21 (Fig. 4B), and this effect is blocked by continuous infusion of SC (i.c.v., minipump). There was no difference in latency to drink in the home cage, a control for this paradigm (F 2,38 = 0.315, P = n.s.; n = 13–15 per group) and no difference between nonstressed SC-infused (66.2 ± 14.4 s) and control (75.0 ± 19.6 s) groups in the novel cage (t 9 = 0.345, P = n.s.; n = 5–6 per group). We next tested the animals on sucrose consumption, a measure of anhedonia, which is a core symptom of depression (19, 20). CUS significantly decreased sucrose consumption, and this effect was also blocked by SC infusion (Fig. 4C). There was no difference in the consumption of tap water, a control for this test (F 2,21 = 0.578, P = n.s.; n = 5–13 per group) and no difference in the sucrose consumption between nonstressed SC-infused (75.9 ± 2.8 mg/kg) and control (73.3 ± 3.8 mg/kg) groups (t 12 = 0.517, P = n.s.; n = 6–8 per group).
Fig. 4.
Effects of CUS on depressive-like behaviors in NIH and sucrose consumption test (SCT) in mice. (A) Experimental procedures for CUS in mice. Mice were exposed to two or three stressors per day for 28 days and received BrdU daily for the last 4 days of CUS. On days 21 and 28, NIH and the SCT, respectively, were conducted. Mice were killed (sac) 24 h after the last BrdU administration. (B) CUS significantly increased latency to drink, and this effect was blocked by continuous administration of SC (F 2,38 = 3.748, P < 0.05; n = 13–15 per group). CTRL, control. (C) SC administration also blocked the effect of CUS on sucrose consumption (F 2,21 = 3.497, P < 0.05; n = 5–13 per group). By the Fisher’s PLSD test, *, P < 0.05 compared with CTRL and # , P < 0.05 compared with CUS.
Inhibition of NF-κB Blocks the Antiproliferative Effects of IL-1β in Neural Progenitor Cells in Vitro.
To investigate further the mechanisms by which stress inhibits neurogenesis, studies were conducted on cultured adult hippocampal progenitors (AHPs). Under the culture conditions used (20 ng/mL FGF-2), ∼90% of DAPI+ cells expressed nestin, a marker of AHPs (Fig. S2B). Most of the nestin+ cells (∼90%) also incorporate BrdU, indicating that the majority of the AHPs are actively proliferating (6). Incubation with IL-1β (2 h) significantly decreases AHP proliferation compared with vehicle, and this effect is blocked by coincubation with JSH or IL-1Ra (Fig. 5 A–C). The effect of IL-1β was not influenced by inhibitors of corticosterone (CORT; RU486) or p38 MAPK (SB203580) (Fig. 5C). IL-1β and CORT decreased the ratio of BrdU+ to nestin+ cells but did not influence the total number of DAPI+ cells or the ratio of nestin+ to DAPI+ cells (Fig. S2 A-C). In contrast, the antiproliferative effect of CORT was not influenced by JSH but was blocked by RU486 as expected and by a p38 MAPK inhibitor (SP600125; Fig. S3A). These data suggest that CORT-mediated suppression of AHP proliferation is mediated by p38 MAPK signaling and that different mechanisms underlie the antiproliferative effects of CORT and IL-1β. Incubation with IL-1β or CORT did not alter the ratio of TUNEL+ to DAPI+ cells (Fig. S3 B and C), indicating that the decrease in cell number is not a result of cell death.
Fig. 5.
Analysis of IL-1β signaling in cultured AHPs. (A and B) Representative images of proliferating AHPs [BrdU+ (red) nestin+ (green)] in the presence of IL-1β (10 ng/mL) ± JSH (25 μM). (C) Coincubation with IL-1Ra (Ra, 100 ng/mL) or JSH blocked the effect of IL-1β on the ratio of BrdU+ to nestin+ compared with control (CTRL) (F 5,16 = 11.174, P < 0.001; n = 3–4 per group). By the Fisher’s PLSD test, **, P < 0.01 and ***, P < 0.001 compared with CTRL and ## , P < 0.01 and ### , P < 0.001 compared with IL-1β. (Scale bar: 50 μm.)
Discussion
The results of the current study demonstrate that inhibition of neurogenesis by acute or chronic stress is blocked by inhibition of NF-κB signaling. A requirement for NF-κB is shown using two different selective inhibitors, one that blocks IkB kinase (IKK) (SC) and the dissociation of IκB and NF-κB and one that directly inhibits NF-κB (JSH) (17, 18). Moreover, the results show that the antineurogenic effects of acute stress result from inhibition of NSC proliferation (Fig. 6). Triple-labeling studies show that the number of GFAP+ SOX2+ BrdU+ NSCs but not ANPs was significantly decreased by acute stress. These effects were confirmed in cultured hippocampal progenitor cells. Because NSCs undergo asymmetric division, giving rise to another NSC and an intermediate progenitor (i.e., ANP), the long-term consequences of inhibiting NSC proliferation (i.e., in response to CUS) would be a reduction in the pool of NSCs as well as intermediate progenitors. The ANPs then give rise to additional neurons, and decreased numbers of these cells could account for the reduction in immature neurons observed after exposure to long-term stress in the CUS paradigm.
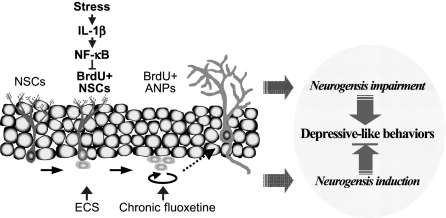
Fig. 6.
Model of stress-induced impairment of NSC proliferation in the hippocampus. Stress activates IL-1β/NF-κB signaling, resulting in impairment of hippocampal neurogenesis and thereby contributing to depressive-like behaviors. Notably, the results demonstrate that acute stress decreases NSC proliferation and that this effect is mediated by IL-1β/NF-κB signaling. In contrast, antidepressant treatment increases neurogenesis by inducing NSC [electroconvulsive seizure (ECS)] or ANP (chronic fluoxetine) proliferation.
The results also demonstrate that acute stress activates NF-κB signaling in GFAP+ NSCs in adult hippocampus (Fig. 6), consistent with the hypothesis that NF-κB signaling in NSCs but not in ANPs underlies the reduction in cell proliferation. However, the relatively small percentage of NSCs that exhibit NF-κB/β-gal staining (20–25%) raises a question as to whether inhibition of this population could account for a 50% reduction in NSC proliferation? There are several potential explanations for this discrepancy. One is that NF-κB-induced gene expression is not an accurate reflection of the total level of NF-κB activity in NSCs. This could result from low sensitivity of β-gal gene expression (i.e., NF-κB is activated but without an increase in β-gal expression) and/or other gene transcription-independent signaling mechanisms that could influence NSC proliferation. Stress could also activate NF-κB in surrounding cells (e.g., ANPs, neurons, glia) that indirectly influence NSC proliferation. For example, stress-induced NF-κB activation results in generation of nitric oxide (NO) (26 –28), and NO has been shown to inhibit neurogenesis in the adult hippocampus and to contribute to the inhibition of neurogenesis by stress (29, 30).
Our current results also demonstrate that activation of NF-κB in response to acute stress is blocked by an inhibitor of IL-1RI. This is consistent with previous reports that IL-1β/IL-1RI signaling is rapidly activated (31) and is necessary and sufficient for suppression of neurogenesis by acute stress (6). Localization studies demonstrate that IL-1RI is expressed in both NSCs and ANPs in adult hippocampus. In addition, although the percentage of NSCs that express IL-1RI is lower than that of ANPs (∼25% and 50%, respectively), the ratio of IL-1RI+ NSCs to IL-1RI+ ANPs is ∼3:1, because the total number of NSCs is much greater than the total number of ANPs. However, given the expression of IL-1RI in intermediate progenitors, it is surprising that stress selectively activates NF-κB and inhibits proliferation of NSCs. This could result from differential localization/availability of IL-1β in proximity to NSCs vs. ANPs or from expression of different signaling in these two populations of progenitors.
Previous studies report that NF-κB differentially affects proliferation, maturation, and survival depending on cell type, localization, and conditions. Much of this work has focused on immune and cancer/tumor cells, in which NF-κB is associated with cell proliferation and antiapoptotic pathways but also with proapoptotic pathways (32, 33). There is also evidence that NF-κB activation has either prosurvival or cell death effects in the brain depending on the conditions (34 –36). These effects could be explained by differential expression and activation of the multiple subtypes and components of the NF-κB family. For example, the proapoptotic actions of NF-κB that occur postischemia involve p50/p65, whereas c-Rel-containing dimers increase resistance to ischemia by activating antiapoptotic pathways (36). There is one report that RelA is expressed in NSCs in the SVZ, whereas p50 is expressed in migrating neural precursors (37). In p50 deletion mice, there is no effect on cell proliferation in adult hippocampus under nonstress conditions (38), consistent with the negative results of the current study. Additional studies of stress in p50 mutant mice, or in conditional deletion mutants, would further characterize the role of NF-κB family members.
Activation of the hypothalamic-pituitary-adrenal axis and elevation of glucocorticoids are typically associated with antiinflammatory responses, including inhibition of NF-κB signaling, via inhibition of IKK and inhibition of NF-κB transcription (17, 18). However, there are also reports that stress can increase the effects of NF-κB, including enhancement of inflammatory responses (13, 14, 27). The current study provides another example wherein stress results in selective activation of NF-κB signaling in a discrete population of cells, the NSCs.
We also examined the role of NF-κB in anxiety- and depression-related behaviors after exposure to CUS. Inhibition of NF-κB blocked the increase in latency to feed in response to CUS in the novelty suppressed feeding test (NSF) paradigm, consistent with a previous report that anxiety-related behaviors are decreased in NF-κB/p50 null mice (39). We also found that inhibition of NF-κB blocks the reduction in sucrose consumption resulting from CUS, an antidepressant-like effect. These findings are consistent with human studies, which demonstrate that social stress/anxiety increases NF-κB signaling and that this effect is enhanced in depressed patients (16). Although we have focused on models that are responsive to chronic antidepressants, which are more relevant to the therapeutical response times, it would be interesting to examine other models predictive of antidepressant response (e.g., forced swim test, learned helplessness paradigm). It would also be interesting to determine if blockade of NF-κB after exposure to stress rather than before stress, as was done in the current study, would be sufficient to reverse NF-κB signaling and produce antidepressant behavioral effects.
In summary, the results demonstrate that IL-1β/NF-κB signaling is activated by stress and indicate that this signaling pathway is required for the antineurogenic and anhedonic effects of repeated stress (Fig. 6). Blockade of NF-κB could inhibit the actions of other proinflammatory cytokines implicated in stress and depression, including IL-6 and TNF-α. Also, blockade of NF-κB in both peripheral immune cells and the brain could provide beneficial antiinflammatory and antistress actions. The diverse and cell type-dependent actions of NF-κB make it a complex drug target, although there may be specific cases of elevated inflammatory processes in which NF-κB inhibitors would be useful and efficacious for the treatment of depression.
Methods
Animals.
Adult male Sprague–Dawley (SD) rats (Charles River) with initial weights of 230–250 g and adult male NF-κB/LacZ transgenic reporter mice (24, 40) and their WT littermates with initial weights of 20–30 g were used for experiments. Animals were housed, two to five per cage, under standard illumination parameters (12-h light/dark cycle) and with ad libitum access to food and water. All experiments were conducted in accordance with guidelines of the Society for Neuroscience and National Institutes of Health as well as those of the institutional animal care and use committees at Yale University and Mount Sinai Schools of Medicine.
Cannula Implantation and Microinjections.
A 26-gauge guide cannula (Plastics One) was implanted for i.c.v. infusion of the NF-κB inhibitors JSH and SC (15 μg per 2.5 μL per rat; Calbiochem) or 100% (vol/vol) DMSO (vehicle) into either the left or right (randomly assigned) lateral ventricle of the rats and for i.c.v. infusion of IL-1Ra (0.25 μg per 1 μL per mouse; R&D Systems) or 0.1% BSA/PBS (vehicle) into the mouse lateral ventricle. For details, see SI Methods.
Acute Stress Exposure.
For acute immobilization stress, SD rats were restrained in a Plexiglas immobilization tube (3.5-in diameter × 7-in length) for 45 min. For acute stress experiments with mice, animals were stressed for 50 min with a combination of restraint stress and rotation stress on an orbital shaker. All experiments for acute stress exposure were performed between 1100 and 1400 hours.
BrdU Labeling and Quantitative Analysis.
BrdU immunohistochemistry and quantitative analysis using a modified unbiased stereology protocol was conducted as described in SI Methods.
Surgical Procedure and Treatments for CUS.
An i.c.v. cannula (Brain Infusion Kit 2 for rats, Brain Infusion Kit 3 for mice; Alzet) was inserted into the lateral ventricle of the rats or mice for prolonged infusions of either vehicle (50% (vol/vol) DMSO in PBS), JSH (for rats, concentration of 10 μg/μL), or SC (for mice, concentration of 20 μg/μL) during CUS. A miniosmotic pump (Alzet 2004 for rats, Alzet 1004 for mice), with a flow rate of 60 μg/day for rats and 48 μg/day for mice, was implanted s.c. and connected to the cannula. For details, see SI Methods.
CUS Procedure.
For our CUS procedure, we used various stressors of which the sequence was intentionally designed to maximize unpredictability. All CUS rats were exposed to two stressors a day for 21 days (for details, see Table S1). All rats received BrdU injection (i.p., 75 mg/kg) for 4 days of the last CUS period. Two weeks after BrdU injection, rats were perfused, BrdU immunohistochemistry was performed, and BrdU+ cells were quantified in the hippocampus using a modified unbiased stereology protocol. BrdU+ cells colabeled with DCX were also quantified in DG (three to four sections per animal) using confocal laser microscopy (Zeiss). In the case of mice, two or three stressors a day were used for 28 days (for details, see Table S2).
NIH and Sucrose Consumption Test.
All mice were singly housed 2 days before the start of NIH habituation (CUS day 14). On CUS days 21 and 28, respectively, NIH and the sucrose consumption test were performed. For details of the tests, see SI Methods.
Neural Progenitor Cell Isolation and Culture.
Neural progenitor cells were isolated from the hippocampi of adult female SD rats (body weight of 160–200 g; Charles River) and cultured as previously described (6, 41). For details, see SI Methods.
Statistical Analysis.
All data are expressed as the mean ± SEM. Experiments with two groups were analyzed statistically using an unpaired t test. Experiments with three or more groups were subjected to one-way ANOVA, followed by the Fisher’s probable least-squares difference (PLSD) post hoc test. The level of statistical significance for all analyses was set at P < 0.05.
Additional Details.
The reader is referred to SI Methods for additional routine procedures, including immunofluorescence and TUNEL assays.
Supplementary Material
Acknowledgments
We thank A. Bhakar for NF-κB/LacZ reporter mice and E. Mouzon and X. Li for technical help. This work was supported by US Public Health Service Grants MH45481, 2 PO1 MH25642, and R01 MH51399; a Veterans Administration National Center Grant for Post-Traumatic Stress Disorder; and the Connecticut Mental Health Center.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910658107/DCSupplemental.
References
- 1.Kessler RC, et al. National Comorbidity Survey Replication The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg PE, et al. The economic burden of depression in the United States: How did it change between 1990 and 2000? J Clin Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- 3.Anisman H, Matheson K. Stress, depression, and anhedonia: Caveats concerning animal models. Neurosci Biobehav Rev. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 4.McEwen BS. Understanding the potency of stressful early life experiences on brain and body function. Metabolism. 2008;57(Suppl 2):S11–S15. doi: 10.1016/j.metabol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koo JW, Duman RS. IL-1β is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 10.Campbell S, MacQueen G. An update on regional brain volume differences associated with mood disorders. Curr Opin Psychiatry. 2006;19:25–33. doi: 10.1097/01.yco.0000194371.47685.f2. [DOI] [PubMed] [Google Scholar]
- 11.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: Opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 13.Munhoz CD, et al. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci. 2006;26:3813–3820. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaPlant Q, et al. Role of nuclear factor kappaB in ovarian hormone-mediated stress hypersensitivity in female mice. Biol Psychiatry. 2009;65:874–880. doi: 10.1016/j.biopsych.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bierhaus A, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci USA. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pace TW, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 17.Shin HM, et al. Inhibitory action of novel aromatic diamine compound on lipopolysaccharide-induced nuclear translocation of NF-kappaB without affecting IkappaB degradation. FEBS Lett. 2004;571:50–54. doi: 10.1016/j.febslet.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 18.Kishore N, et al. A selective IKK-2 inhibitor blocks NF-κ B-dependent gene expression in interleukin-1 beta-stimulated synovial fibroblasts. J Biol Chem. 2003;278:32861–32871. doi: 10.1074/jbc.M211439200. [DOI] [PubMed] [Google Scholar]
- 19.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 20.Willner P. Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 21.Seri B, García-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filippov V, et al. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci. 2003;23:373–382. doi: 10.1016/s1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- 23.Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci USA. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhakar AL, et al. Constitutive nuclear factor-kappa B activity is required for central neuron survival. J Neurosci. 2002;22:8466–8475. doi: 10.1523/JNEUROSCI.22-19-08466.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 26.Leza JC, Salas E, Sawicki G, Russell JC, Radomski MW. The effects of stress on homeostasis in JCR-LA-cp rats: The role of nitric oxide. J Pharmacol Exp Ther. 1998;286:1397–1403. [PubMed] [Google Scholar]
- 27.Madrigal JL, et al. Inducible nitric oxide synthase expression in brain cortex after acute restraint stress is regulated by nuclear factor kappaB-mediated mechanisms. J Neurochem. 2001;76:532–538. doi: 10.1046/j.1471-4159.2001.00108.x. [DOI] [PubMed] [Google Scholar]
- 28.Madrigal JL, et al. Induction of cyclooxygenase-2 accounts for restraint stress-induced oxidative status in rat brain. Neuropsychopharmacology. 2003;28:1579–1588. doi: 10.1038/sj.npp.1300187. [DOI] [PubMed] [Google Scholar]
- 29.Packer MA, et al. Nitric oxide negatively regulates mammalian adult neurogenesis. Proc Natl Acad Sci USA. 2003;100:9566–9571. doi: 10.1073/pnas.1633579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou QG, et al. Neuronal nitric oxide synthase contributes to chronic stress-induced depression by suppressing hippocampal neurogenesis. J Neurochem. 2007;103:1843–1854. doi: 10.1111/j.1471-4159.2007.04914.x. [DOI] [PubMed] [Google Scholar]
- 31.Johnson JD, et al. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- 32.Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 33.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discovery. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kassed CA, Willing AE, Garbuzova-Davis S, Sanberg PR, Pennypacker KR. Lack of NF-kappaB p50 exacerbates degeneration of hippocampal neurons after chemical exposure and impairs learning. Exp Neurol. 2002;176:277–288. doi: 10.1006/exnr.2002.7967. [DOI] [PubMed] [Google Scholar]
- 35.Mattson MP. NF-kappaB in the survival and plasticity of neurons. Neurochem Res. 2005;30:883–893. doi: 10.1007/s11064-005-6961-x. [DOI] [PubMed] [Google Scholar]
- 36.Pizzi M, Sarnico I, Lanzillotta A, Battistin L, Spano P. Post-ischemic brain damage: NF-kappaB dimer heterogeneity as a molecular determinant of neuron vulnerability. FEBS J. 2009;276:27–35. doi: 10.1111/j.1742-4658.2008.06767.x. [DOI] [PubMed] [Google Scholar]
- 37.Denis-Donini S, Caprini A, Frassoni C, Grilli M. Members of the NF-kappaB family expressed in zones of active neurogenesis in the postnatal and adult mouse brain. Brain Res Dev Brain Res. 2005;154:81–89. doi: 10.1016/j.devbrainres.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Denis-Donini S, et al. Impaired adult neurogenesis associated with short-term memory defects in NF-kappaB p50-deficient mice. J Neurosci. 2008;28:3911–3919. doi: 10.1523/JNEUROSCI.0148-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kassed CA, Herkenham M. NF-kappaB p50-deficient mice show reduced anxiety-like behaviors in tests of exploratory drive and anxiety. Behav Brain Res. 2004;154:577–584. doi: 10.1016/j.bbr.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 40.Russo SJ, et al. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J Neurosci. 2009;29:3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.