Abstract
The receptor protein tyrosine phosphatases gamma (PTPRG) and zeta (PTPRZ) are expressed primarily in the nervous system and mediate cell adhesion and signaling events during development. We report here the crystal structures of the carbonic anhydrase-like domains of PTPRZ and PTPRG and show that these domains interact directly with the second and third immunoglobulin repeats of the members of the contactin (CNTN) family of neural recognition molecules. Interestingly, these receptors exhibit distinct specificities: PTPRZ binds only to CNTN1, whereas PTPRG interacts with CNTN3, 4, 5, and 6. Furthermore, we present crystal structures of the four N-terminal immunoglobulin repeats of mouse CNTN4 both alone and in complex with the carbonic anhydrase-like domain of mouse PTPRG. In these structures, the N-terminal region of CNTN4 adopts a horseshoe-like conformation found also in CNTN2 and most likely in all CNTNs. This restrained conformation of the second and third immunoglobulin domains creates a binding site that is conserved among CNTN3, 4, 5, and 6. This site contacts a discrete region of PTPRG composed primarily of an extended β-hairpin loop found in both PTPRG and PTPRZ. Overall, these findings implicate PTPRG, PTPRZ and CNTNs as a group of receptors and ligands involved in the manifold recognition events that underlie the construction of neural networks.
Keywords: cell adhesion, crystal structure, Ig superfamily, receptor protein tyrosine phosphatase
The development and maintenance of the nervous system relies on adhesive interactions between cell surface receptors and the coordinated reversible phosphorylation of downstream protein effectors. The receptor protein tyrosine phosphatases (RPTPs) participate in these two seemingly disparate processes as they combine extracellular domains (ECDs) that resemble those of cell adhesion molecules (CAMs) with one or two intracellular tyrosine phosphatase domains (1). In particular, the proteins PTPRG and PTPRZ make up the type V subgroup of RPTPs and are expressed predominantly in the developing and adult brains of vertebrates (2 –4). However, their expression patterns differ because PTPRG is found almost exclusively on neurons, whereas PTPRZ localizes to glial cells. Each of these type I transmembrane proteins includes a domain homologous to α-carbonic anhydrases (CAs) (5), a single fibronectin type III repeat followed by a cysteine-free spacer, a membrane-spanning region, and tandem cytoplasmic tyrosine phosphatase domains (6, 7).
No ligand for PTPRG has been reported, but several binding partners for PTPRZ have been described and can be grouped according to the portion of the ECD that they recognize. The growth factor pleiotrophin binds to the spacer region as well as a glycosaminoglycan insert found in some isoforms of PTPRZ (8). The Ig superfamily CAMs L1/Ng-CAM, N-CAM, Nr-CAM associate with the spacer region, whereas the IgCAM protein contactin1 (CNTN1/contactin/F3) binds to the CA domain (9, 10). Importantly, the association between PTPRZ on glia and CNTN1 on neurons promotes the outgrowth of neurites and induces bidirectional signaling between glia and neurons suggesting that this interaction plays a role in nervous system development (9, 11). However, the structural basis for these interactions remains unclear.
CNTN1 is a glycophosphatidylinositol (GPI)-anchored protein comprised of 6 N-terminal Ig domains followed by four fibronectin type III domains. It belongs to a family of neural recognition molecules involved in the patterning of neural tissues that includes five additional receptors sharing approximately 40–60% sequence identity (Table S1, 12, 13): CNTN2 (TAG-1/axonin), CNTN3 (PANG/BIG-1), CNTN4 (BIG-2), CNTN5 (NB-2), and CNTN6 (NB-3). As a first step toward illuminating the roles of type V RPTPs and CNTNs in establishing neuronal connections we undertook biochemical and structural studies of PTPRG, PTPRZ, and CNTNs.
Results
Crystal Structures of CA Domains from PTPRG and PTPRZ.
The crystal structures of mouse PTPRGCA, human PTPRGCA and human PTPRZCA were determined by molecular replacement and refined to 1.7 Å (R work = 17.3%, R free = 20.5%), 1.7 Å (R work = 17.8%, R free = 19.3%), and 2.0 Å (R work = 17.5%, R free = 21.9%), respectively (Table S2). These CA-like domains are similar and contain a central 8-strand antiparallel β-sheet surrounded by three to four α-helices and extensive loop regions (Fig. 1 and Fig. S1A and B). Mouse and human PTPRGCA superimpose with a rmsd of 0.5 Å for 257 Cα positions, whereas mouse PTPRGCA and human PTPRZCA superimpose with a rmsd of 1.4 Å for 257 Cα positions. However, unlike PTPRGCA, PTPRZCA includes an additional disulfide bond between C133 and C264, which is conserved in all known orthologs of PTPRZ. The presence of this additional disulfide bridge has little effect on the structure of PTPRZCA when compared to PTPRGCA and its biological significance is currently unknown.
Fig. 1.
Structures of CA domains from type V RPTPs. (A) Ribbon diagram of human PTPRZCA. The letters N and C indicate the N- and C-termini, respectively. Disulfide bonds are shown as orange ball-and-stick models. (B) Human PTPRGCA. The dotted line indicates a disordered β-hairpin loop. (C) Mouse PTPRGCA. (D) Overlay of the structures of human PTPRZCA (blue), human PTPRGCA (brown), and mouse PTPRGCA (magenta) with the structure of mouse CA-XIV (gray). A gray sphere indicates the position of the zinc ion in CA-XIV.
Overall, the CA domains of PTPRG and PTPRZ resemble bona fide α-CAs. Mouse PTPRGCA, human PTPRGCA, and human PTPRZCA superimpose with mouse CA-XIV with rmsd of 1.5–1.7 Å for 253–255 Cα positions and most of the differences reside in the orientation of an extended β-hairpin loop in type V RPTPs (Fig. 1 D, 14). This hairpin loop is disordered in human PTPRGCA and in three of the four molecules found in the asymmetric unit of mouse PTPRGCA crystals, indicating that this region is flexible. In contrast the hairpin is ordered in both copies of human PTPRZCA found in the crystallographic asymmetric unit presumably because it participates in lattice interactions. The catalytic site of α-CAs is characterized by the presence of three histidine residues that coordinate a zinc ion essential for enzymatic activity (5). This cavity is still present in PTPRG and PTPRZ but only one of the histidine residues is conserved (Fig. S1C); consequently no electron density indicative of a bound metal ion was observed.
PTPRG Interacts with CNTN Family Members.
Because the CA domain of PTPRZ binds to CNTN1, we predicted that the CA domain of PTPRG could also mediate protein–protein interactions. Moreover, we predicted that these domains could bind similar molecules, such as members of the CNTN family. Therefore, an in vitro affinity-isolation assay was designed to probe the binding of type V RPTPs to CNTNs. Mouse GPI-anchored CNTNs were fused to human growth hormone (hGH) and expressed transiently in HEK293 cells. Cell lysates containing detergent-solubilized CNTNs were then incubated with mouse PTPRZCA or mouse PTPRGCA immobilized on sepharose. We confirmed the interaction between PTPRZCA and CNTN1 described previously (9), but found no indication of PTPRZCA binding to other CNTNs (Fig. 2 A). In line with previous studies, no interaction between PTPRGCA and CNTN1 was detected (9). However, PTPRGCA bound to CNTN3, 4, 5, and 6 (Fig. 2 B). These interactions have not been described previously.
Fig. 2.
Affinity isolation of GPI-anchored CNTNs. CNTNs were fused to hGH and expressed transiently in HEK293 cells. Cell lysates were incubated with PTPRZCA-resin (A) or with PTPRGCA-resin (B). Bound CNTN fusion proteins were visualized by immunoblotting against hGH.
Because of the sequence similarities between type V RPTPs on one hand and CNTNs on the other hand and because type V RPTPs bind to CNTN family members, we hypothesized that PTPRZCA and PTPRGCA could interact with homologous subdomains of CNTNs. To test this hypothesis, we first identified the region of CNTN1 that mediates binding to PTPRZ. Secreted hGH-tagged fragments of mouse CNTN1 were incubated with a mouse PTPRZCA affinity resin. A complete CNTN1 ECD lacking the GPI anchor, a truncated form of CNTN1 including Ig domains 1–4, and a form of CNTN1 lacking Ig domains 1–4 were tested initially (Fig. 3 A). The results show that the first four Ig domains bind to PTPRZCA. Further dissection showed that single Ig domains are unable to support binding to PTPRZCA, but that a fragment composed of Ig domains 2 and 3 of CNTN1 is necessary and sufficient to interact with immobilized PTPRZCA (Fig. 3 A).
Fig. 3.
Interactions between PTPRZCA, PTPRGCA and fragments of mouse CNTNs. (A–C) Fragments of mouse CNTNs were fused to hGH and expressed transiently in HEK293 cells. Conditioned media were incubated with PTPRZCA-resin (A) or with PTPRGCA-resin (B and C). Bound CNTN fusion proteins were visualized by immunoblotting against hGH. (D) PTPRZCA forms a 1∶1 complex with CNTN1Ig2-3. Comparison of size exclusion chromatograms of CNTN1Ig2-3, PTPRZCA, and CNTN1Ig2-3 mixed with PTPRZCA. Arrows above the chromatogram indicate the elution volumes of molecular weight standards. The insert shows fractions from the complex elution profile resolved by SDS-PAGE. The proteins were visualized by silver staining. (E) PTPRGCA forms a 1∶1 complex with CNTN4Ig1-4. Same as (D), but the experiments were conducted with CNTN4Ig1-4 and PTPRGCA.
The PTPRG-binding site on CNTN3, 4, 5, and 6 was localized using a similar approach. We confirmed that PTPRGCA bound to Ig domains 1–4 of CNTN3, 4, 5, and 6 but not those of CNTN1 or 2 (Fig. S2). Fragments of CNTN4 containing single and overlapping pairs of Ig domains within the first four Ig repeats were then analyzed (Fig. 3 B). As was observed in the case of PTPRZ and CNTN1, Ig repeats 2 and 3 make up the minimal PTPRGCA-binding site on CNTN4. These domains are also sufficient to bind to PTPRGCA in the case of CNTN3, 5, and 6 (Fig. 3 C). Taken together, these results indicate that CNTN proteins bind to distinct type V RPTPs, but that the binding site spans the second and third Ig repeats in all cases tested.
Because interactions detected by affinity-isolation assays may depend on additional factors found in cell lysates or conditioned media, we tested whether type V RPTPs associate directly with CNTNs. The interaction between PTPRZCA and CNTN1Ig2-3 was thus further studied by analytical size-exclusion chromatography (Fig. 3 D). This experiment showed that the two molecules form a 1∶1 complex at a concentration of 10 μM. It was not possible to purify significant amounts of CNTN4Ig2-3 to perform a similar experiment with PTPRGCA so we used CNTN4Ig1-4 instead. As was observed for PTPRZ and CNTN1, PTPRGCA and CNTN4Ig1-4 form a 1∶1 complex (Fig. 3 E). No evidence of higher oligomer formation was observed in either case.
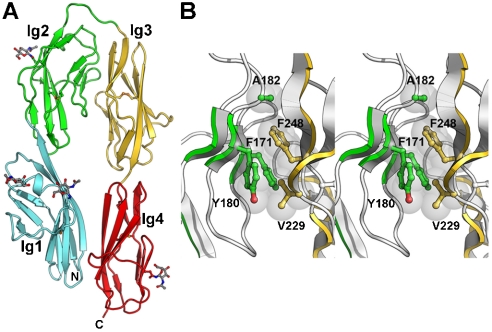
Ig Domains 1–4 of Mouse CNTN4 Adopt a Horseshoe-Like Conformation.
CNTN2, the only member of the CNTN family that does not bind to the CA domains of either PTPRZ or PTPRG, adopts a horseshoe-like conformation (15, 16). To determine if significant structural differences between CNTN2Ig1-4 and CA-binding CNTNs could account for this observation, we determined the crystal structure of mouse CNTN4Ig1-4 to 2.4 Å resolution (R work = 19.2%, R free = 25.2%, Table S2). As was observed for CNTN2, contacts between Ig domains 1 and 4 on one hand and 2 and 3 on the other hand lock CNTN4Ig1-4 into a horseshoe-like conformation (Fig. 4 A and Fig. S3). The structures of human and chicken CNTN2Ig1-4 and mouse CNTN4Ig1-4 in fact resemble each other fairly closely despite limited sequence identity (rmsd of 1.6–2.3 Å, Table S1). Similar conformations have been reported for the insect immune protein hemolin and isoforms of the Down syndrome cell adhesion molecule (DSCAM) (17 –19).
Fig. 4.
Structure of mouse CNTN4Ig1-4. (A) Ribbon diagram of mouse CNTN4Ig1-4. The letters N and C indicate the N- and C-termini, respectively. Disulfide bonds are shown as orange ball-and-stick models. Asparagine-linked N-acetylglucosamine residues are depicted as gray ball-and-stick models along with the asparagine side chain. Ig domains 1, 2, 3, and 4 are colored cyan, green, gold, and red, respectively. (B) Stereo view of the interface between Ig domains 2 and 3 in CNTN4Ig1-4. Residues at the interface between the two domains are shown as ball-and-sticks with transparent spheres (gray) and colored green (Ig2) or gold (Ig3).
The interface between Ig pairs 1-2 and 3-4 is mostly apolar and buries a surface area of 2,233 Å 2 with a shape complementarity coefficient of 0.72, both of which compare favorably to those of known biological interfaces (20, 21). Its core includes residues F171, Y180, and A182 in Ig2 and V229 and F248 in Ig3 (Fig. 4 B). Residues F171, Y180, and F248 are absolutely conserved in human and mouse CNTNs (Fig. S4); A182 is changed to S in CNTN5 and V229 to A in CNTN2, which still adopts an identical horseshoe-like conformation. Taken together, these observations suggest that all CNTN family members may adopt a similarly constrained conformation.
The β-hairpin Loop of PTPRGCA Mediates Binding to Ig Domains 2 and 3 of CNTN4.
We determined the crystal structure of a complex of PTPRGCA and CNTN4Ig1-4 to 2.0 Å resolution (R work = 17.9%, R free = 23.2%, Table S2) to provide a structural basis for the interactions between type V RPTPs and CNTNs (Fig. 5). The asymmetric unit contains a single 1∶1 complex of PTPRGCA and CNTN4Ig1-4 and analysis of the lattice contacts did not indicate the presence of higher order oligomers, which is consistent with our size exclusion chromatography experiments (Fig. 3 E). In PTPRGCA, the flexible β-hairpin (residues 288–301) accounts for approximately 80% of the interactions with CNTN4; the remainder of the contacts are mediated by residues 225–229. In line with our affinity-isolation assays (Fig. 3 B), contacts between CNTN4 and PTPRG are limited to Ig domains 2 and 3. The structures of Ig domains 2 and 3 in both the free and bound states superimpose with a rmsd of 0.9 Å for 191 Cα atoms, indicating that only minimal structural changes occur upon binding of PTPRG. Interestingly, the PTPRG-binding site in CNTN4 involves essentially two discrete segments, residues 129–142 in Ig2 and 220–228 in Ig3, which partially overlap with the regions that mediate homophilic binding in DSCAMs (Fig. S5). This suggests that the horseshoe-like scaffold can support homophilic and heterophilic binding modes using homologous surfaces.
Fig. 5.
Structure of the PTPRGCA·CNTN4Ig1-4 complex. The view on the left is obtained from the one shown in Fig. 4 A by a counterclockwise rotation of approximately 60° along a vertical axis. In the right view, only residues 225–229 and 288–301 (β-hairpin) of PTPRGCA are shown for the sake of clarity. The letters N and C indicate the N- and C-termini, respectively. Disulfide bonds are shown as orange ball-and-stick models. Asparagine-linked N-acetylglucosamine residues are depicted as gray ball-and-stick models along with the asparagine side chain. Ig domains 1, 2, 3, and 4 are colored cyan, green, gold, and red, respectively. PTPRGCA is colored magenta. Dotted lines indicate disordered regions. The β-hairpin region is well ordered with average B factors of 32.1 Å 2 versus 33.8 Å 2 for the entire chain of PTPRGCA.
The interface between PTPRGCA and CNTN4Ig1-4 buries a total of 1,702 Å 2 with a shape complementarity coefficient of 0.67, values similar to these of antibody–antigen complexes (1,680 Å 2 and 0.64–0.68, respectively, 20, 21). The two strands of the β-hairpin in PTPRGCA complement the 3-strand antiparallel β-sheet in CNTN4Ig2 to form a 5-strand antiparallel β-sheet with the main chain atoms of residues 295–299 of PTPRG forming hydrogen bonds with the main chain atoms of residues 139–143 in CNTN4 (Fig. 5). The interface contains a hydrophobic region consisting of V132, L142, M220, Y223, and L250 in CNTN4 and F288, V296, and V299 in PTPRG as well as a hydrophilic region that includes R135, Q138, E228, and the main chain of S130 in CNTN4 and D294, K297, and E300 in PTPRG (Fig. 6). In addition to the contacts mediated by the β-hairpin, the side chain of H226 in PTPRG abuts the aliphatic portion of K226 in CNTN4 and K229 mediates potential salt bridges and hydrogen bonds with CNTN4 residues E224 and N304 (Fig. 6). All the CNTN4 residues that interact with PTPRG are conserved in CNTN3, 5, and 6, thus explaining why PTPRG binds specifically to these four CNTN family members (Fig. S4).
Fig. 6.
Stereo view of the PTPRGCA·CNTN4Ig1-4 interface. This view is in the same orientation as the right view in Fig. 5. Residues are shown as ball-and-sticks with transparent gray spheres for those involved in van der Waals contacts. Dashed lines indicate potential hydrogen bonds and salt bridges. Residues from CNTN4Ig2, CNTN4Ig3, and PTPRGCA are colored green, gold, and magenta, respectively.
The structure of the PTPRG·CNTN4 complex likely approximates the complex between PTPRZ and CNTN1. Indeed, because Ig2 and Ig3 in CNTN1 probably adopt a restrained conformation similar to the one of CNTN4 and because these domains are necessary and sufficient to mediate binding to PTPRZCA, the PTPRZ-binding site on CNTN1 likely spans a region similar to the PTPRG-binding site on CNTN4. Amino acid changes at key positions help rationalize the absence of detectable binding between PTPRZ and CNTN3, 4, 5, and 6. Residues G273 and M276 in PTPRZ replace residues D294 and K297 in PTPRG resulting in the loss of polar interactions with CNTN4 residues S130 and E228 (Fig. 6 and Fig. S1). Sequence variations within the CNTN family also explain, in part, why PTPRG does not interact with CNTN1 or 2. M220 is replaced by T in CNTN1 and by P in CNTN2; the hydrogen bond/salt bridge between CNTN4 residues E228 and K297 in PTPRG is not possible in CNTN1 (E is changed to V) or CNTN2 (E is changed to K). CNTN4 residue Q138 is replaced by W in CNTN2 and therefore unable to form a hydrogen bond with E300 of PTPRG. In addition, CNTN4 residue S130 is changed to D in CNTN2, possibly resulting in charge repulsion with D294 in PTPRG (Fig. 6).
Discussion
The interactions Between PTPRG and CNTN3, 4, 5, and 6 Identified In Vitro May Occur In Vivo.
The biochemical and structural data presented here suggest that PTPRG and PTPRZ are potential ligands for all but one member of the CNTN family of IgCAMs. The binding between PTPRZ and CNTN1 has already been described (9), but the interactions between PTPRG and CNTN3, 4, 5, and 6 have not. Although indirect, several lines of evidence suggest that these interactions are relevant in vivo.
PTPRG is expressed in the nervous system of mouse embryos and in the adult brain (4). Expression of PTPRG is restricted to neurons and is found in adult mouse pyramidal cells; it is also detected in all sensory organs, including the glomeruli of the olfactory bulb. Recently it was demonstrated that CNTN4 is expressed in some but not all olfactory sensory neurons in mouse and is important for their migrating axons to target the glomeruli of the olfactory bulb (22). Furthermore, these same studies detected but did not identify a heterophilic binding partner for CNTN4 expressed on the glomeruli. The data presented here in addition to the expression pattern of PTPRG suggest that PTPRG could be the CNTN4-binding protein found in the olfactory bulb. Although less is known about the function of CNTN3, its expression in the olfactory bulb of adult mice matches the expression pattern of PTPRG indicating that a physical interaction is possible in vivo (4, 23).
Both PTPRG and CNTN6 are expressed in layer V of the cerebral cortex and in the pyramidal field CA1 of the hippocampus. Interestingly, mice deficient in either PTPRG or CNTN6 exhibit impaired motor coordination during rod walking and string tests (4, 24). These similar phenotypes suggest that the physical association of PTPRG and CNTN6 may be important for acquiring proper motor functions. CNTN5 is expressed early postbirth and its expression is the strongest in regions involved in the auditory pathway, in particular in the cochlear nuclei (25, 26). This localization of CNTN5 matches that of PTPRG (4), which is also found, at least at the embryonic stage, in the nuclei of the vestibulocochlear nerve responsible for carrying signals about hearing and balance.
An Indirect Control of Dephosphorylation?
One of the most important questions about RPTPs concerns the functional relationship between the ECD and the control of the phosphatase activity. Biochemical and structural lines of evidence suggest that dimerization of RPTPs is linked to inhibition of the phosphatase activity. In particular, PTPRZ is a catalytically active monomer on the cell surface in the absence of the growth factor pleiotrophin but becomes inactive after treatment with pleiotrophin induces dimerization of the ECD (27). Furthermore, the tandem phosphatase domains of PTPRG form stable dimers in solution, which occludes the catalytic site (28). Taken together these observations provide strong evidence that dimerization of type V RPTPs can control their catalytic activity.
Interestingly, our experiments show that the formation of the PTPRZCA·CNTN1Ig2-3 and PTPRGCA·CNTN4Ig1-4 complexes does not induce dimerization of either PTPRZ or PTPRG (Fig. 3 D, E and Fig. 5). One reason could be that larger fragments of PTPRG, PTPRZ, or CNTNs are required to bring about changes in oligomeric state upon extracellular binding. However, an alternative explanation is that binding by CNTNs does not control phosphatase activity directly but rather specifies the location of dephosphorylation reactions. Indeed, PTPRM (RPTPμ), a type IIa RPTP, forms head-to-tail dimers that localize specifically to regions of cell–cell contacts where the intercellular spacing matches the length of the PTPRM dimer (29). A possible role for CNTNs could then be to guide PTPRG and PTPRZ toward specific cellular regions, thus providing a spatial control of dephosphorylation (30).
Materials and Methods
Detailed descriptions are given in SI Materials and Methods.
Protein Expression and Purification.
Human and mouse PTPRGCA (residues 56–320) and PTPRZCA (residues 34–302) were expressed a fusion proteins with thioredoxin, a hexahistidine tag, and a human rhinovirus 3C protease site in E. coli strain Origami 2(DE3) or Origami B(DE3). Fusion proteins were purified by immobilized-metal affinity chromatography. After cleavage with human rhinovirus 3C protease, proteins were purified by ion exchange and size-exclusion chromatography.
GPI-anchored and secreted fragments of CNTN proteins were transiently expressed in HEK293 cells as fusion proteins with hGH, a octahistidine tag, and a human rhinovirus 3C protease site. Mouse CNTN1Ig2-3 (residues 133–329) and CNTN4Ig1-4 (residues 25–404) used for analytical size exclusion chromatography experiments were purified from the conditioned media of transiently transfected HEK293 cells by immobilized-metal affinity chromatography followed by proteolytic removal of the hGH fusion partner and ion exchange chromatography. Deglycosylated CNTN4Ig1-4 was prepared for structural analyses by substituting N-acetylglucosaminyltransferase I-negative HEK293S cells for HEK293 cells. N-linked carbohydrates were removed using endoglycosidase H.
Affinity-Isolation Assays.
Purified mouse PTPRGCA and PTPRZCA were coupled to CNBr-activated sepharose at a density of 1 mg of protein per ml of resin and 2 mg of protein per ml of resin, respectively. For each reaction 250 μL of conditioned medium containing a secreted mouse CNTN fragment was mixed with 250 μL of 50 mM Tris-HCl pH 7.4, 200 mM NaCl, and 0.2% (vol/vol) Tween-20 and incubated for 1 h at room temperature with 30 μL of a 50% (vol/vol) slurry of quenched CNBr-activated sepharose to remove nonspecific binding material. The beads were removed by centrifugation and the conditioned medium was then incubated with 20 μL of a 50% (vol/vol) slurry of PTPRGCA or PTPRZCA resin for 2 h at room temperature. Resins were washed with 25 mM Tris-HCl pH 7.4, 100 mM NaCl and 0.1% (vol/vol) Tween-20. Samples were resolved by SDS-PAGE and bound CNTN fragments were detected by immunoblotting using a rabbit polyclonal antibody against hGH (Fitzgerald).
For affinity-isolation assays using membrane-bound CNTNs, transfected cells were detached from the plastic plate in PBS containing 5 mM EDTA, harvested by centrifugation and suspended in lysis buffer (100 mM NaCl, 10% (vol/vol) glycerol, 1% (vol/vol) Triton X-100, 1% (wt/vol) sodium deoxycholate, 50 mM Tris-HCl pH 8.0, protease inhibitors). After lysis on ice, cellular debris were removed by centrifugation and lysates were cleared with 30 μL of a 50% (vol/vol) slurry of quenched CNBr-activated sepharose for 1 h at room temperature. Lysates were then incubated with 20 μL of a 50% (vol/vol) slurry of PTPRGCA or PTPRZCA resins for 2 h at room temperature. Resins were washed with 25 mM Tris-HCl pH 7.4 and 100 mM NaCl. Samples were resolved by SDS-PAGE and mouse CNTNs were detected by immunoblotting against hGH.
Characterization of Binding Interactions Using Size Exclusion Chromatography.
A mixture of mouse PTPRZCA (3 nmol) and mouse CNTN1Ig2-3 (3 nmol) was adjusted to 300 μL with PBS and incubated for 2 h at 4 °C. An aliquot (200 μL) of this mixture was analyzed by size exclusion chromatography using a Superdex 200 10/30 HR column (GE Healthcare) in PBS. The same protocol was used to probe the binding between PTPRGCA and CNTN4Ig1-4.
Crystallization and Structure Determination.
All crystals were grown at 20 °C by hanging drop vapor diffusion. Oscillation diffraction data were collected at 1.00 Å at beamlines 22-ID or 22-BM of the Advanced Photon Source of Argonne National Laboratory and processed with HKL2000 (31).
The structure of mouse PTPRGCA was solved by molecular replacement with PHASER as implemented in PHENIX using the structures of human CA-XII and mouse CA-XIV in a single ensemble as a search model (14, 32, 33). The final model was obtained after manual building using COOT and refinement in PHENIX (32, 34). It consists of four chains with residues 58–181, 185–293, and 295–320 in molecule A, residues 58–292 and 297–320 in molecule B, residues 57–320 in molecule C, residues 58–291 and 298–320 in molecule D, and 689 water molecules.
The structures of human PTPRGCA and human PTPRZCA were solved by molecular replacement using the structure of mouse PTPRGCA as a search model. The final model for human PTPRGCA consists of residues 58–291, 298–320, 145 water molecules, and one sulfate ion. The final model for human PTPRZCA includes residues 34–301 in molecules A and B as well as 222 water molecules.
The structure of CNTN4Ig1-4 was determined by molecular replacement using chicken CNTN2Ig1-2 and CNTN2Ig3-4 as search models (15). The final model consists of two protein chains with residues 25–40, 45–404 in molecule A, residues 25–404 in molecule B, 8 N-acetylglucosamine residues, and 189 water molecules. Structural descriptions in the text refer to molecule A because it has overall lower B factors than molecule B.
The structure of a complex of mouse PTPRGCA and mouse CNTN4Ig1-4 was determined by molecular replacement with PHENIX using the structures of mouse PTPRGCA, mouse CNTN4Ig1,4, and mouse CNTN4Ig2-3 as independent search models (15). The final model consists of residues 25–71, 76–402, and 4 N-acetylglucosamine residues in CNTN4Ig1-4, residues 56–95 and 99–320 in PTPRGCA, and 415 water molecules.
Ramachandran statistics were calculated using RAMPAGE as implemented in CCP4 (35). Core residues from protein structures were superimposed using the DaliLite server (36). Buried surface areas and lists of contact residues were calculated in CCP4; surface complementarity coefficients were obtained using the program SC (20). Figures were prepared with PyMOL (www.pymol.org).
Supplementary Material
Acknowledgments.
We are grateful to the staff of Southeast Regional Collaborative Access Team at the Advanced Photon Source sector 22 for assistance with data collection. We also thank Dan Leahy and Brian Geisbrecht for helpful comments on the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 3JXA, 3JXF, 3JXG, 3JXH, 3KLD).
This article contains supporting information online at www.pnas.org/cgi/content/full/0911235107/DCSupplemental.
References
- 1.Johnson KG, Van Vactor D. Receptor protein tyrosine phosphatases in nervous system development. Physiol Rev. 2003;83:1–24. doi: 10.1152/physrev.00016.2002. [DOI] [PubMed] [Google Scholar]
- 2.Andersen JN, et al. Structural and evolutionary relationships among protein tyrosine phosphatase domains. Mol Cell Biol. 2001;21:7117–7136. doi: 10.1128/MCB.21.21.7117-7136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harroch S, et al. No obvious abnormality in mice deficient in receptor protein tyrosine phosphatase beta. Mol Cell Biol. 2000;20:7706–7715. doi: 10.1128/mcb.20.20.7706-7715.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamprianou S, et al. Receptor protein tyrosine phosphatase gamma is a marker for pyramidal cells and sensory neurons in the nervous system and is not necessary for normal development. Mol Cell Biol. 2006;26:5106–5119. doi: 10.1128/MCB.00101-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tripp BC, Smith K, Ferry JG. Carbonic anhydrase: new insights for an ancient enzyme. J Biol Chem. 2001;276:48615–48618. doi: 10.1074/jbc.R100045200. [DOI] [PubMed] [Google Scholar]
- 6.Barnea G, et al. Identification of a carbonic anhydrase-like domain in the extracellular region of RPTP gamma defines a new subfamily of receptor tyrosine phosphatases. Mol Cell Biol. 1993;13:1497–1506. doi: 10.1128/mcb.13.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krueger NX, Saito H. A human transmembrane protein-tyrosine-phosphatase, PTP zeta, is expressed in brain and has an N-terminal receptor domain homologous to carbonic anhydrases. Proc Natl Acad Sci USA. 1992;89:7417–7421. doi: 10.1073/pnas.89.16.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maeda N, Nishiwaki T, Shintani T, Hamanaka H, Noda M. 6B4 proteoglycan/phosphacan, an extracellular variant of receptor-like protein-tyrosine phosphatase zeta/RPTPbeta, binds pleiotrophin/heparin-binding growth-associated molecule (HB-GAM) J Biol Chem. 1996;271:21446–21452. doi: 10.1074/jbc.271.35.21446. [DOI] [PubMed] [Google Scholar]
- 9.Peles E, et al. The carbonic anhydrase domain of receptor tyrosine phosphatase beta is a functional ligand for the axonal cell recognition molecule contactin. Cell. 1995;82:251–260. doi: 10.1016/0092-8674(95)90312-7. [DOI] [PubMed] [Google Scholar]
- 10.Peles E, Schlessinger J, Grumet M. Multi-ligand interactions with receptor-like protein tyrosine phosphatase beta: implications for intercellular signaling. Trends Biochem Sci. 1998;23:121–124. doi: 10.1016/s0968-0004(98)01195-5. [DOI] [PubMed] [Google Scholar]
- 11.Revest JM, et al. The interaction between F3 immunoglobulin domains and protein tyrosine phosphatases zeta/beta triggers bidirectional signalling between neurons and glial cells. Eur J Neurosci. 1999;11:1134–1147. doi: 10.1046/j.1460-9568.1999.00521.x. [DOI] [PubMed] [Google Scholar]
- 12.Katidou M, Vidaki M, Strigini M, Karagogeos D. The immunoglobulin superfamily of neuronal cell adhesion molecules: Lessons from animal models and correlation with human disease. Biotechnol J. 2008;3:1564–1580. doi: 10.1002/biot.200800281. [DOI] [PubMed] [Google Scholar]
- 13.Kamei Y, et al. Human NB-2 of the contactin subgroup molecules: Chromosomal localization of the gene (CNTN5) and distinct expression pattern from other subgroup members. Genomics. 2000;69:113–119. doi: 10.1006/geno.2000.6310. [DOI] [PubMed] [Google Scholar]
- 14.Whittington DA, et al. Expression, assay, and structure of the extracellular domain of murine carbonic anhydrase XIV: Implications for selective inhibition of membrane-associated isozymes. J Biol Chem. 2003;279:7223–7228. doi: 10.1074/jbc.M310809200. [DOI] [PubMed] [Google Scholar]
- 15.Freigang J, et al. The crystal structure of the ligand binding module of axonin-1/TAG-1 suggests a zipper mechanism for neural cell adhesion. Cell. 2000;101:425–433. doi: 10.1016/s0092-8674(00)80852-1. [DOI] [PubMed] [Google Scholar]
- 16.Mortl M, Sonderegger P, Diederichs K, Welte W. The crystal structure of the ligand-binding module of human TAG-1 suggests a new mode of homophilic interaction. Protein Sci. 2007;16:2174–2183. doi: 10.1110/ps.072802707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meijers R, et al. Structural basis of Dscam isoform specificity. Nature. 2007;449:487–491. doi: 10.1038/nature06147. [DOI] [PubMed] [Google Scholar]
- 18.Sawaya MR, et al. A double S shape provides the structural basis for the extraordinary binding specificity of Dscam isoforms. Cell. 2008;134:1007–1018. doi: 10.1016/j.cell.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su XD, et al. Crystal structure of hemolin: a horseshoe shape with implications for homophilic adhesion. Science. 1998;281:991–995. doi: 10.1126/science.281.5379.991. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence MC, Colman PM. Shape complementarity at protein/protein interfaces. J Mol Biol. 1993;234:946–950. doi: 10.1006/jmbi.1993.1648. [DOI] [PubMed] [Google Scholar]
- 21.Lo Conte L, Chothia C, Janin J. The atomic structure of protein–protein recognition sites. J Mol Biol. 1999;285:2177–2198. doi: 10.1006/jmbi.1998.2439. [DOI] [PubMed] [Google Scholar]
- 22.Kaneko-Goto T, Yoshihara S, Miyazaki H, Yoshihara Y. BIG-2 mediates olfactory axon convergence to target glomeruli. Neuron. 2008;57:834–846. doi: 10.1016/j.neuron.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 23.Yoshihara Y, et al. BIG-1: a new TAG-1/F3-related member of the immunoglobulin superfamily with neurite outgrowth-promoting activity. Neuron. 1994;13:415–426. doi: 10.1016/0896-6273(94)90357-3. [DOI] [PubMed] [Google Scholar]
- 24.Takeda Y, et al. Impaired motor coordination in mice lacking neural recognition molecule NB-3 of the contactin/F3 subgroup. J Neurobiol. 2003;56:252–265. doi: 10.1002/neu.10222. [DOI] [PubMed] [Google Scholar]
- 25.Li H, et al. Aberrant responses to acoustic stimuli in mice deficient for neural recognition molecule NB-2. Eur J Neurosci. 2003;17:929–936. doi: 10.1046/j.1460-9568.2003.02514.x. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa J, et al. Neural recognition molecule NB-2 of the contactin/F3 subgroup in rat: Specificity in neurite outgrowth-promoting activity and restricted expression in the brain regions. J Neurosci Res. 2001;65:100–110. doi: 10.1002/jnr.1133. [DOI] [PubMed] [Google Scholar]
- 27.Fukada M, et al. Protein tyrosine phosphatase receptor type Z is inactivated by ligand-induced oligomerization. FEBS Lett. 2006;580:4051–4056. doi: 10.1016/j.febslet.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 28.Barr AJ, et al. Large-scale structural analysis of the classical human protein tyrosine phosphatome. Cell. 2009;136:352–363. doi: 10.1016/j.cell.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aricescu AR, et al. Structure of a tyrosine phosphatase adhesive interaction reveals a spacer-clamp mechanism. Science. 2007;317:1217–1220. doi: 10.1126/science.1144646. [DOI] [PubMed] [Google Scholar]
- 30.Yudushkin IA, et al. Live-cell imaging of enzyme-substrate interaction reveals spatial regulation of PTP1B. Science. 2007;315:115–119. doi: 10.1126/science.1134966. [DOI] [PubMed] [Google Scholar]
- 31.Otwinowski Z, Minor W. Processing of x-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 32.Adams PD, et al. PHENIX: Building new software for automated crystallographic structure determination. Acta Crystallogr Sect D Biol Crystallogr. 2002;58:1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- 33.Whittington DA, et al. Crystal structure of the dimeric extracellular domain of human carbonic anhydrase XII, a bitopic membrane protein overexpressed in certain cancer tumor cells. Proc Natl Acad Sci USA. 2001;98:9545–9550. doi: 10.1073/pnas.161301298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr Sect D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 35.Lovell SC, et al. Structure validation by calpha geometry: Phi, psi and Cbeta deviation. Proteins. 2003;50:437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- 36.Holm L, Kääriäinen S, Rosenström P, Schenkel A. Searching protein structure databases with DaliLite v.3. Bioinformatics. 2008;24:2780–2781. doi: 10.1093/bioinformatics/btn507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.