The past decade has seen remarkable advances in the fields of both proteomics and genomics. In addition to basic technical advances that have led to an increased volume of high-quality data, this “-omics” revolution also has begun to provide some interesting insights into the diversity of processes that regulate tumorigenesis in many different types of human cancers. The large roadmaps of gene and protein expression produced by these methods often can be used to classify cancers or predict response to certain types of treatments. However, they often fail to pinpoint specific regulators that may serve as promising targets for the next generation of anticancer drugs, largely because many of the major “druggable” classes of proteins are enzymes that are tightly regulated both at the level of transcription and translation and at the level of enzyme activity. Thus many now-common “-omic” methods fail to provide information on the dynamic regulation of a given enzyme or family of enzymes during the many stages of cancer development. In this issue of PNAS, Shields et al. (1) make use of a relatively new method termed “activity-based proteomics” to identify a protein with serine hydrolase activity that is an essential regulator of tumor cell growth. By using this functional approach, the authors were able to identify a specific enzyme target that may serve as a valuable target for the development of anticancer drugs.
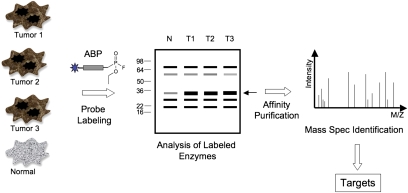
The field of activity-based proteomics or chemical proteomics has emerged as an alternative to standard proteomic methods, which primarily provide information on the overall abundance of proteins (for reviews, see refs. 2 –4). The activity-based proteomic approach makes use of small molecule probes that bind to enzymes in an activity-dependent manner, thus allowing both quantification of the dynamics of enzyme regulation and direct isolation and identification of the targets of interest (Fig. 1). With the development of many new classes of probes (2) as well as new classes of affinity and fluorescent tags (5), activity-based protein profiling (ABPP) has found increasing use in identifying key regulators of human diseases. In particular, a number of recent elegant examples demonstrate the value of ABPP in identifying interesting regulators of cancer progression (4, 6 –8).
Fig. 1.
Activity-based proteomics or activity-based protein profiling (ABPP). In this example, tumor tissue samples are labeled with an activity-based probe (ABP) that contains a reactive fluorophosphonate group. After labeling of target enzymes (in this case serine hydrolases), labeled proteins are separated by SDS/PAGE, and relative activity levels are determined by the intensity of probe labeling. Potentially interesting targets are identified as having increased or reduced levels of activity in tumor samples. Labeled targets are isolated by affinity purification via the probe tag and identified by mass spectrometry.
In the study by Shields et al. (1) in this issue, the authors used a broad-spectrum serine hydrolase probe to profile human pancreatic cancer tissues. These efforts led to the identification of a protein termed retinoblastoma-binding protein 9 (RBBP9) that had elevated hydrolase activity in 40% of the tumor tissues analyzed. Interestingly, this protein had been identified previously as a retinoblastoma (Rb)-binding protein and had no known enzyme activity (9). Prior studies of the function of this protein suggested that its overexpression confers resistance to the effects of TGFβ in suppressing cell growth. However, these effects on TGFβ signaling were thought to be the direct result of binding of RBBP9 to Rb, leading to the release of the eukaryotic translation initiation factor 1 (EIF-1) transcription factor. In their current study, Shields et al. show that RBBP9 has serine hydrolase activity and, more importantly, that this enzyme activity is required for the transforming effects of this protein in cancer cells. Loss of hydrolase activity by mutation of the active-site serine (identified by homology to other serine hydrolases) or RNAi-mediated knock-down of the protein leads to an increase in Smad 2/3 phosphorylation, a decrease in the expression of adhesion molecules such as E-cadherin, and a subsequent reduction in tumor growth. Furthermore, the authors find that RBPP9 activity levels are elevated in a number of other human cancers, suggesting that inhibition of this hydrolase activity may have broad tumor-suppressive effects, making it a potentially valuable target for development of anticancer drugs.
On multiple levels, the study by Shields et al. demonstrates the power of the ABPP approach. First, this approach allowed the identification of an enzyme activity in a protein shown to function in the regulation of cell-growth signaling. By using the ABPP approach, it was possible to monitor the dynamics of regulation of this enzyme activity without the need to identify a native substrate and establish an in vitro assay. Second, levels of expression of RBBP9 were equivalent in both normal and cancer tissues, suggesting that enzyme activity drives the functional contribution of this protein to tumor-cell growth. Thus none of the current genomic or proteomic methods would be capable of identifying this target as a key regulator of disease.
Of course, many questions about the exact mechanistic role of RBBP9 remain. Most importantly, what are the native substrates of this enzyme? Does the enzyme actually hydrolyze its substrates? What is the consequence of substrate hydrolysis? How does substrate processing lead to regulation of Smad2/3 signaling? It will be interesting to see if RBBP9 can be readily inhibited by small molecules so that it may be validated as a potentially viable drug target using more advanced mouse models of human cancer. The answers to these questions most certainly will be forthcoming thanks to the availability of activity-based probes.
Footnotes
The author declares no conflict of interest.
See companion article on page 2189 in issue 5 of volume 107.
References
- 1.Shields DJ, et al. RBBP9—a tumor-associated serine hydrolase activity required for pancreatic neoplasia. Proc Nat. Acad Sci USA. 2010;107:2189–2194. doi: 10.1073/pnas.0911646107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans MJ, Cravatt BF. Mechanism-based profiling of enzyme families. Chem Rev. 2006;106:3279–3301. doi: 10.1021/cr050288g. [DOI] [PubMed] [Google Scholar]
- 3.Fonovic M, Bogyo M. Activity-based probes as a tool for functional proteomic analysis of proteases. Expert. Re. Proteonomics. 2008;5:721–730. doi: 10.1586/14789450.5.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paulick MG, Bogyo M. Application of activity-based probes to the study of enzymes involved in cancer progression. Curr Opin Genet Dev. 2008;18:97–106. doi: 10.1016/j.gde.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadaghiani AM, Verhelst SH, Bogyo M. Tagging and detection strategies for activity-based proteomics. Curr Opin Chem Biol. 2007;11:20–28. doi: 10.1016/j.cbpa.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Chiang KP, Niessen S, Saghatelian A, Cravatt BF. An enzyme that regulates ether lipid signaling pathways in cancer annotated by multidimensional profiling. Chem Biol. 2006;13:1041–1050. doi: 10.1016/j.chembiol.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Conn EM, et al. Cell surface proteomics identifies molecules functionally linked to tumor cell intravasation. J Biol Chem. 2008;283:26518–26527. doi: 10.1074/jbc.M803337200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jessani N, et al. Carcinoma and stromal enzyme activity profiles associated with breast tumor growth in vivo. Proc Natl Acad Sci USA. 2004;101:13756–13761. doi: 10.1073/pnas.0404727101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woitach JT, Zhang M, Niu CH, Thorgeirsson SS. A retinoblastoma-binding protein that affects cell-cycle control and confers transforming ability. Nat Genet. 1998;19:371–374. doi: 10.1038/1258. [DOI] [PubMed] [Google Scholar]