Abstract
Paclitaxel has emerged as a front line treatment for aggressive malignancies of the breast, lung, and ovary. Successful therapy of cancer is frequently undermined by the development of paclitaxel resistance. There is a growing need to find other therapeutic targets to facilitate treatment of drug-resistant cancers. Using a proteomics approach, elevated levels of Prohibitin1 (PHB1) and GSTπ were found associated with paclitaxel resistance in discrete subcellular fractions of two drug-resistant sublines relative to their sensitive sublines. Immunofluorescence staining and fractionation studies revealed increased levels of PHB1 on the surface of resistant cell lines. Transiently silencing either PHB1 or GSTπ gene expression using siRNA in the paclitaxel-resistant cancer cell sublines partially sensitized these cells toward paclitaxel. Intriguingly, silencing PHB1 but not GSTπ resulted in activation of the intrinsic apoptosis pathway in response to paclitaxel. Similarly, stably silencing either PHB1 or GSTπ significantly improved paclitaxel sensitivity in A549TR cells both in vitro and in vivo. Our results indicate that PHB1 is a mediator of paclitaxel resistance and that this resistance may depend on the cellular localization of the protein. We suggest PHB1 as a potential target for therapeutic strategies for the treatment of drug-resistant tumors.
Keywords: apoptosis, glutathione-S-transferase Pi, mitochondria, plasma membrane, protein translocation
Resistance to chemotherapy remains one of the principal causes of cancer mortality. This is particularly true of therapy with taxanes, such as paclitaxel and docetaxel, which are being used increasingly in patients with metastatic disease secondary to tumors of the breast, prostate, lung, and other sites (1 –3). Such resistance can be primary, resulting in tumors that never respond to a particular drug, or secondary, where tumors respond initially but then become resistant. Several molecular mechanisms of paclitaxel resistance have been suggested in vitro. These include the expression of the multidrug resistance (MDR) phenotype and alterations in the microtubule system (4, 5). However, attempts to extend these in vitro data to identify useful clinical markers of paclitaxel resistance and to develop other therapeutic strategies to reverse paclitaxel resistance are still ongoing or have yielded disappointing results. It is clear that cancer therapeutics would benefit from alternative strategies to recognize and identify proteins that facilitate resistance and that could be targeted to render cells more sensitive to particular drugs.
In the present study, we used a proteomic approach to identify proteins associated with paclitaxel resistance and to examine their potential use as targets for modulating the resistant phenotype. Our results highlighted two proteins as being altered in paclitaxel-resistant cell lines relative to sensitive cell lines. One protein, GST Pi (GSTπ), has been previously implicated in drug resistance (6) and provides validation of the methodology underlying our approach. The second protein is prohibitin1 (PHB1), which has not previously been implicated in taxane resistance.
Prohibitins are highly conserved proteins with homologs found in organisms ranging from yeast to humans (7). PHB1 has a molecular mass of approximately 30,000 Da, whereas PHB2 has a mass of approximately 37,000; together they form a high molecular mass complex. PHB1 is ubiquitously expressed in all tissues tested to date (7) and has been shown to have significant effects on cell senescence, development, and tumor suppression (for review see refs. 8, 9). It resides at the inner mitochondrial membrane where it serves as a protein chaperone (9, 10). Recently, studies have shown that PHB1 has a role in the regulation of the apoptotic pathway (11 –13). Additionally prohibitins have been found to reside on the cell surface of certain cells, including adipose endothelial cells (14, 15). PHB1 has also been detected in lipid droplets (16) and in lipid raft preparations (17, 18). It has been suggested that PHB1 may be able to translocate between its mitochondrial location and the plasma membrane (8, 14).
Our current results suggest that PHB1 is a mediator of paclitaxel resistance and that this resistance depends on the cellular localization of the protein, rather than on the absolute amount of the protein within the cell. We hypothesize that prohibitin on the surface of tumor cells may represent a tool for localization of taxane-resistant tumors.
Results
PHB1 Is Overexpressed in Microsomal Fractions of Paclitaxel-Resistant Cancer Cells.
Two different pairs of cancer cell lines were used in the study: (i) a paclitaxel-sensitive lung cancer cell line (A549) and its paclitaxel-resistant variant (A549TR) (19), and (ii) a uterine sarcoma cell line (Mes-SA) and its multidrug-resistant variant (Mes-SA DX5-Tx). The resistant sublines differed from the parental lines in their sensitivity to paclitaxel by greater than 100-fold (Fig. 1A and Fig. S1A). We adopted a proteomics-based approach using 2D gel electrophoresis coupled with MS to identify other proteins associated with paclitaxel resistance. Cytoplasmic and microsomal fractions from the parental and paclitaxel-resistant sublines were prepared to enable the identification of low-abundance proteins that would otherwise be undetectable in total protein lysates. Analysis of the gels revealed more than 50 proteins differentially expressed between the parental and the paclitaxel-resistant cell lines. Importantly, we observed a limited number of proteins that were differentially expressed in both paclitaxel-resistant cell models. These included tubulin β-5, annexin I, and GSTπ, that were overexpressed in the cytoplasmic fraction of the resistant cell lines and have been previously reported to have a role in paclitaxel resistance (6, 20, 21). PHB1, a protein not previously associated with taxane resistance, was consistently overexpressed in the microsomal fractions of both of the resistant cell lines. We therefore decided to investigate the role of PHB1 in paclitaxel resistance. GSTπ was used as a positive control due to its previously established role in paclitaxel resistance. GSTπ and PHB1 levels were examined in whole cell lysates and in cellular fractions by Western blot analysis. Increased levels of GSTπ were found in whole cell lysates of both resistant cell lines relative to the paclitaxel-sensitive parental lines (Fig. 1B and Fig. S1B). Interestingly, there was no difference in the total amount of PHB1 protein seen in whole cell lysates or in the mitochondria of both resistant and sensitive sublines (Fig. 1 B and C and Fig. S1 B and C). However, isolation of nonnuclear membrane fractions reveals that PHB1 levels are elevated in these fractions of the two resistant cell lines compared to the sensitive cell lines (Fig. 1D and Fig. S1D). This is intriguing, as PHB1 has been shown to migrate between intracellular locales (22), and suggests that the intracellular distribution of PHB1 may be more relevant to the resistant phenotype than the absolute amount of the protein in the cell.
Fig. 1.
Effect of paclitaxel on cell viability. (A) A549 and A549TR cell lines were treated with varying concentrations of paclitaxel 24 h after seeding in 96-well plates. Cell viability was assessed 72 h after paclitaxel addition using the CyQUANT assay. Values are presented as percentage of cell survival in paclitaxel-treated cells relative to untreated cells. Shown are the mean ± SEM of three independent experiments, each performed in triplicate. (B) Total whole cell lysates, (C) mitochondrial fractions and (D) membrane fractions from both cell lines were obtained and separated by 13% SDS/PAGE. The PVDF membrane was cut and immunoblotted for PHB1, GSTπ, Cox IV, and actin as a loading control. Blots representative of three independent experiments are presented.
PHB1 Is Localized in the Mitochondria and the Plasma Membrane of Paclitaxel-Resistant Cell Lines.
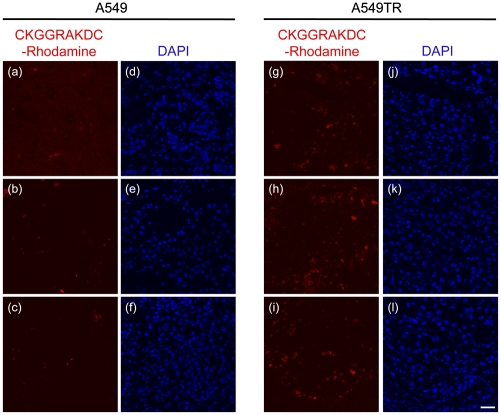
PHB1 has been shown to shuttle between various cellular compartments, including the mitochondria and nucleus (22 –24), and is also present on the cell surface in a variety of cell types (14, 15). Consequently, we examined the subcellular distribution of PHB1 and its localization relative to the mitochondrial resident protein Cytochrome c oxidase IV (Cox IV) using fluorescence confocal microscopy. In both parental sublines, PHB1 was largely localized to the mitochondria as indicated by its colocalization with Cox IV, and this distribution of PHB1 was also unaltered in both paclitaxel-resistant sublines (Fig. 2A and Fig. S2A). Interestingly, PHB1 distribution within the mitochondria was not altered in the paclitaxel-resistant cells compared to the parental cells. More recently, PHB1 was shown to be localized to the surface of endothelial cells in the vasculature of white fat (14). We therefore examined the presence of PHB1 on the surface of both the nonpermeabilized parental and paclitaxel-resistant cell lines. Using an antibody against PHB1, we observed increased surface staining of PHB1 on the paclitaxel-resistant cells compared to the parental cells (Fig. 2B and Fig. S2B). We next examined the cell surface distribution of PHB1 using the PHB1-binding peptide motif (sequence CKGGRAKDC) tagged to rhodamine. This PHB1-binding peptide was identified by Kolonin et al. (14) using a phage display library and shown to home to PHB1-expressing adipose endothelial cells in vivo. As shown with the PHB1 antibody staining, the CKGGRAKDC-Rhodamine peptide was specifically localized to the surface of paclitaxel-resistant but not parental cell lines (Fig. 2C and Fig. S2C). Finally, we initially showed that PHB1 levels are elevated in membrane fractions from resistant cell lines compared to sensitive cell lines (Fig. 1D and Fig. S2C). These fractions, however, contain membranes from multiple membrane species including plasma membrane and mitochondria, as well as the endoplasmic reticulum and golgi. To assess the plasma membrane localization, biotinylated cell surface proteins were isolated and purified from both parental and paclitaxel-resistant cells and probed for PHB1. As shown in Fig. 2D and Fig. S2D, PHB1 levels were significantly increased in the plasma membranes of both paclitaxel-resistant sublines relative to the parental cells. The sodium/potassium ATPase was used as loading control. To strengthen these observations, we used the CKGGRAKDC-Rhodamine peptide to assess whether it would preferentially target taxane-resistant tumors in nude mice bearing s.c. xenografts of A549 or A549TR cells. As in the in vitro observations, i.v. injected CKGGRAKDC-Rhodamine specifically stained the paclitaxel-resistant A549TR xenografts but had minimal staining of the parental A549 xenografts (Fig. 3). CKGGRAKDC-Rhodamine staining did not colocalize with CD31 stained cells (Fig. S3), suggesting that tumor endothelium is not the primary target of the CKGGRAKDC peptides in the tumors.
Fig. 2.
PHB1 colocalizes with Cox IV within the mitochondria and its levels are elevated on the surface of paclitaxel-resistant cells. (A) The cells were fixed with paraformaldehyde, permeabilized then stained for PHB1 (a and e) and Cox IV (b and f) with specific polyclonal antibodies, followed by fluorophore-conjugated secondary antibodies. The nuclei were stained using the DAPI dye (c and g). Composite images of PHB1, Cox IV, and DAPI are presented where yellow indicates regions of colocalization (d and h). For detection of PHB1 at the cell surface, the cells were fixed with paraformaldehyde without permeabilization and processed using (B) anti-PHB1 antibody followed by Alexa488-conjugated donkey anti-goat antibody or (C) CKGGRAKDC-Rhodamine. The images are representative of three experiments. (Scale bar, 40 μm.) (D) Plasma membrane fractions were isolated and probed for PHB1 and the sodium-potassium ATPase by Western blotting. A representative blot of three experiments is presented.
Fig. 3.
CKGGRAKDC-Rhodamine preferentially stains A549TR xenografts after i.v. injection. Three nude mice bearing A549 and A549TR xenografts were i.v. injected with CKGGRAKDC-Rhodamine diluted in PBS at a final concentration of 300 μM. Two hours later, the tumors were resected, frozen, and then sectioned and stained with DAPI. Red fluorescence shows the tumor staining of CKGGRAKDC-Rhodamine (A–C and G–I) and blue fluorescence displays nuclear staining (D–F and J–L). The images are representative of three mice for each cell line. (Scale bar, 30 μm.)
Transient Silencing of GSTπ and PHB1 Partially Rescues Paclitaxel Sensitivity in Vitro.
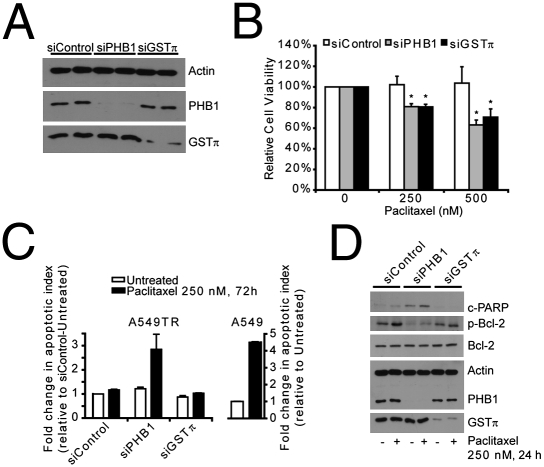
To determine the functional significance of the protein changes that were correlated with paclitaxel resistance, we employed siRNA to selectively reduce the amounts of PHB1 (siPHB1) and GSTπ (siGSTπ) in resistant cell lines. Conditions were chosen in which the protein levels were reduced by 50 to 80% for GSTπ and PHB1 in both A549TR (Fig. 4A) and Mes-SA DX5-Tx (Fig. S4A) cells. Transfection of nontargeting siRNA (siControl) had no effect on GSTπ and PHB1 expression. Moreover, knockdown of PHB1 had no effect on the expression of GSTπ and vice versa. Knockdown of PHB1 significantly decreased cell viability by 19.1% ± 2.9% and 36.9% ± 4.8% when A549TR cells were challenged with 250 nM and 500 nM paclitaxel, respectively (Fig. 4B). A549TR cells transfected with siGSTπ also displayed a decrease in cell viability by 19.3% ± 2.5% and 29.3% ± 7.9% in cells treated with 250 nM and 500 nM paclitaxel, respectively (Fig. 4B). A similar observation was made in Mes-SA DX5-Tx cells transfected with siPHB1 and siGSTπ and treated with 250 nM and 500 nM paclitaxel (Fig. S4B). Interestingly, knockdown of PHB1, but not GSTπ, moderately reduced cell viability of A549TR and Mes-SA DX5-Tx cells in the absence of paclitaxel treatment (Fig. S5 A and B).
Fig. 4.
Effect of silencing GSTπ or PHB1 on paclitaxel sensitivity. A549TR cells transfected with 20 nM siControl, 20 nM siGSTπ, or 20 nM siPHB1 were treated with or without paclitaxel for the times indicated posttransfection. (A) Whole cell lysates were obtained posttransfection and the samples were separated by 13% SDS/PAGE and probed for PHB1, GSTπ, and actin as a loading control. A representative blot loaded with lysates from two independent experiments is presented. (B) Percent cell viability for each siRNA was calculated as percentage of cell survival in paclitaxel-treated cells relative to untreated cells. Shown are the mean ± SEM of at least five independent experiments, each performed in triplicate. *P < 0.05, siPHB1, and siGSTπ relative to siControl at both 250 nM and 500 nM paclitaxel treatment. (C) Apoptosis was assessed by flow cytometry analysis of cells double labeled with annexin V and 7-AminoactinomycinD. The values are presented as a fold change in the apoptotic index relative to untreated A549 or siControl-transfected A549TR cells. Data are presented from a representative experiment performed in triplicates; bars show standard error. (D) Whole cell lysates were also probed for cleaved-PARP, phosphorylated-Bcl-2, Bcl-2, PHB1, GSTπ, and actin. A representative blot of three independent experiments is presented.
PHB1 has been shown to have a role in the regulation of apoptosis (11, 13). We therefore examined whether transient knockdown of PHB1 results in the activation of the apoptotic pathway after treatment with paclitaxel. As shown in Fig. 4C, treatment of siPHB1 transfected A549TR cells with 250 nM paclitaxel for 72 h resulted in a 2.8-fold increase in the apoptotic index compared to untreated and siControl transfected cells. In comparison, treatment of A549 cells with paclitaxel resulted in a 4.5-fold increase in the apoptotic index relative to untreated cells. Interestingly, knockdown of GSTπ did not result in a significant increase in apoptosis when cells were treated with paclitaxel. To further correlate and confirm apoptosis activation, we examined whole cell lysates for the activation of proteins known to participate in the apoptosis pathway. Bcl-2 has been shown to be essential in the regulation of apoptosis and taxane-induced serine phosphorylation is thought to interfere with its antiapoptotic activity (25). Treatment of siControl or siGSTπ transfected A549TR cells with paclitaxel resulted in a significant increase in the serine phosphorylation of bcl-2 (Fig. 4D). PHB1 down-regulation, however, significantly reduced bcl-2 phosphorylation in both untreated and paclitaxel-treated A549TR cells. We also examined whether PHB1 knockdown affects the cleavage of poly(ADP ribose) polymerase (PARP), an established marker of cells undergoing apoptosis (26). Transfection of siPHB1 into A549TR cells resulted in an increase in cleaved PARP in untreated cells as well as cells treated with paclitaxel. PARP cleavage was not observed in cells transfected with siControl or siGSTπ before or after paclitaxel treatment. Caspases also have been shown to be essential in the apoptotic pathway (for review see ref. 27). Caspase 9 is involved in initiating apoptosis by activating effector caspases, such as caspase 3 and caspase 7, which cleave downstream targets and irreversibly commit the cell to the apoptotic fate. Caspase 3/7 and caspase 9 activities were assessed in untreated and paclitaxel-treated A549TR cells transfected with siRNA to PHB1 or GSTπ. PHB1 knockdown notably increased both caspase 3/7 and caspase 9 activity in response to paclitaxel treatment that was determined to be statistically significant (Fig. S5 C and D). However, this effect was not seen in cells transfected with siControl or siGSTπ.
Overexpression of MDR transporters belonging to the ABC family of transporters is widely considered to be the primary mechanism of resistance to paclitaxel (4). P-glycoprotein or MDR1 is the best known and most widely studied transporter within this family (28). We therefore examined whether PHB1 or GSTπ knockdown improves paclitaxel sensitivity in a MDR-dependent manner. As shown in Fig. S6, siPHB1 or siGSTπ did not affect the total expression of MDR1 or total MDR activity. Moreover, β-tubulin levels (Fig. S6A) were unaffected by knockdown of PHB1 or GSTπ. Our results suggest that transient down-regulation of PHB1 in paclitaxel-resistant cells improves their sensitivity to paclitaxel by initiating the intrinsic apoptosis pathway. Interestingly, transient knockdown of GSTπ seems to improve paclitaxel sensitivity in A549TR and Mes-SA DX5-Tx cells despite a lack of activation of the intrinsic apoptosis pathway. This observation may be a reflection of the extent of GSTπ knockdown or an indication that GSTπ repression may improve paclitaxel sensitivity via a mechanism alternative to the intrinsic apoptosis pathway.
Stable Knockdown of GST-π and PHB1 Partially Rescues Paclitaxel Sensitivity in Vivo.
To demonstrate that PHB1, like GSTπ, can be a valid target to improve paclitaxel sensitivity of drug-resistant tumors in vivo, we generated stable A549TR clones with reduced expression of GSTπ or PHB1 using lentiviral particles expressing two unique shRNA sequences against the respective proteins. As shown in Fig. 5 A and B, three A549TR clones expressing PHB1 or GSTπ shRNA were selected for and display a significant knockdown of PHB1 or GSTπ levels, respectively, relative to clones expressing nonspecific control shRNA. Like the A549TR cells (Fig. 1A), the control clones were significantly resistant to paclitaxel at all concentrations tested. However, stable knockdown of PHB1 or GSTπ resulted in a significant improvement in paclitaxel sensitivity (Fig. 5C and Fig. S7 A and B). Because the role of GSTπ in multidrug resistance is well established, we examined whether PHB1 silencing would also improve sensitivity to other chemotherapeutic agents. As shown in Fig. S8, stable repression of PHB1 in the resistant sublines also improved their sensitivity to other chemotherapeutic agents, including docetaxel, doxorubicin and etoposide. Sensitivity to camptothecin was not improved at the concentrations tested. These results suggest that stable repression of PHB1 can improve sensitivity to multiple chemotherapeutic agents but not to all such agents. As with the transient knockdown, stable knockdown of PHB1 resulted in a significant increase in caspase 3/7 activity (Fig. S7C). This increase in activity is comparable to that seen in the paclitaxel-sensitive A549 cells. Transient knockdown of GSTπ did not result in the activation of caspase 3/7 (Fig. S5C), whereas stable reduction of GSTπ expression did induce such activity (Fig. S7C), perhaps as a compensation to the stable repression. These results suggest that stable repression of PHB1 or GSTπ may improve paclitaxel-sensitivity as a result of increased activation of the intrinsic apoptosis pathway as well as by reducing MDR activity (Fig. S7D).
Fig. 5.
Stable repression of PHB1 or GSTπ improves paclitaxel sensitivity in vitro. Whole cell lysates from A549TR cells stably expressing control, PHB1 or GSTπ shRNAs were obtained from three clones for each shRNA and probed for actin as a loading control and (A) PHB1 or (B) GSTπ. Blots representative of three independent experiments are presented. (C) One clone expressing control, PHB1, or GSTπ shRNA was chosen and assessed for cell viability when treated with varying concentrations of paclitaxel for 72 h. Shown are the mean ± SEM of three independent experiments, each performed in triplicate.
To strengthen the conclusions from the in vitro observations, nude mice bearing s.c. xenografts of the stable transfectants were injected i.p. with either vehicle or paclitaxel at a final concentration of 12 mg/kg to assess whether stable repression of PHB1 or GSTπ in paclitaxel-resistant tumors would improve sensitivity to paclitaxel in vivo. This concentration of paclitaxel was very effective in inhibiting the growth of A549 xenografts compared to vehicle-injected xenografts and had minimal effect on mouse morbidity as measured by mouse weight (Fig. S9 A and B). As shown in Fig. 6A and Fig. S10, vehicle or paclitaxel treatment had no effect on the growth of two taxol-resistant xenografts. Interestingly, one of these (CON3) actually grew better during paclitaxel treatment compared to vehicle treatment. As shown in Fig. 6 B and C and Fig. S10, PHB1 knockdown restored paclitaxel sensitivity to previously resistant cells. The effect observed with PHB1 knockdown was equivalent or greater than that observed with GSTπ knockdown, as shown by analysis of tumor volumes (Fig. 6 D and E). The mean tumor volumes of the vehicle-treated PHB07-7 xenografts were significantly smaller compared to CON4 and GST06-9 xenografts (Fig. 6 D and E), consistent with our finding that transient knockdown of PHB1 but not GSTπ reduced cell proliferation in vitro (Fig. S5). These results collectively indicate that cellular PHB1 contributes to the onset or maintenance of paclitaxel resistance and suggests that prohibitins may be a valid target for both localization and for other treatment modalities for paclitaxel-resistant tumors.
Fig. 6.
Relative improvement of paclitaxel sensitivity in vivo after repression of PHB1 or GSTπ. Nude mice were inoculated s.c. with 4 × 106 A549TR cells stably expressing (A) control, (B) PHB1, or (C) GSTπ shRNA. The animals were divided randomly into groups consisting of at least eight mice per group. Once the average tumor volume within each group was at least 120 mm3, vehicle-control and paclitaxel (12 mg/kg) were administered for the times indicated. Tumor growth was determined as the tumor volume on the day of treatment relative to the tumor volume at the start of treatment and presented as a percentage. Each curve represents the average tumor growth ± SEM of at least eight mice per group. (D) The actual tumor volumes ± SEM and (E) the resected tumors at the end of the treatment are presented. (Scale bar, 10 mm.)
Discussion
Paclitaxel is a widely used agent that is effective in the treatment of a variety of human cancers. As with many other chemotherapeutic agents, however, resistance to paclitaxel remains a limiting factor to its efficiency in the clinic. Despite this limitation, paclitaxel remains at the frontline of cancer therapy and has stimulated a concerted effort to understand the molecular mechanisms of paclitaxel resistance. In the current study, we used a proteomic approach to identify biomarkers and therapeutic targets of paclitaxel resistance. Our approach revealed proteins that have been previously shown to participate in the onset or maintenance of resistance to paclitaxel, such as tubulin β-5, annexin I, and GSTπ. We also observed that PHB1 levels were significantly elevated in microsomal membrane fractions in paclitaxel-resistant cells from two different human cancers. Interestingly, GSTπ has been employed as a target to reverse drug resistance in human cancers. We therefore used GSTπ as a proof of principle and as a positive control for our methodology to assess whether PHB1 has a role in paclitaxel resistance. Our results suggest that PHB1 may represent a target for the disruption of paclitaxel resistance.
Several studies have examined the expression patterns of PHB1 in tumor cell lines and many of these suggest that PHB1 levels are elevated in transformed cells or tumors when compared to their normal cell counterparts (13, 15, 29 –36). In the present study, we show that PHB1 levels are significantly elevated in membrane fractions isolated from paclitaxel-resistant sublines compared to normal drug-sensitive cells. PHB1 has been shown to have an important role in the regulation of a variety of cellular functions. In addition to its potential role as a chaperone in the inner mitochondrial membrane, there is a growing body of evidence suggesting that PHB1 may have an antiapoptotic function within the cell. Several studies have shown that PHB1 expression correlates with the initial events of apoptosis (11, 12, 37, 38) and that transient or stable overexpression of PHB1 can protect cells from apoptosis. This can occur via modulation of transcription (12, 22) or by inhibition of activation of the intrinsic apoptosis pathway (11, 13).
Recently, Gregory-Bass et al. (13) showed that repression of PHB1 in ovarian cancer cells improved their sensitivity to staurosporine. We now show that stable and transient knockdown of PHB1 in paclitaxel-resistant sublines significantly improves their sensitivity to paclitaxel as well as to other chemotherapeutic agents in vitro and in vivo. The improved sensitivity to paclitaxel after PHB1 knockdown is a result of the activation of the intrinsic apoptosis pathway. Interestingly, we did not observe a complete improvement in paclitaxel sensitivity after repression of PHB1 in paclitaxel-resistant cells suggesting that PHB1 alone may not be sufficient and that other proteins may be necessary for the onset and maintenance of paclitaxel resistance. This is supported by our observation that A549TR cells stably transfected with siGSTπ also showed improved paclitaxel sensitivity as a result of paclitaxel-induced activation of the intrinsic apoptosis pathway. Together our results suggest that reduction of PHB1 can improve paclitaxel sensitivity in taxane-resistant cells.
We have provided evidence that PHB1 expression is elevated in specific subcellular fractions in paclitaxel-resistant cells. PHB1 levels were elevated in microsomal membrane fractions in taxane-resistant cells, but whole cell levels of the protein were unchanged. This differs from a recent report by Cicchillitti et al. (39), who reported that PHB1 levels are significantly elevated in total cell lysates from a paclitaxel-resistant ovarian cancer cell line compared to its sensitive counterpart. This difference in protein expression may be cell line specific. Moreover, we have shown that PHB1 levels are elevated in the microsomal membrane fractions of at least two taxane-resistant cell lines (A549TR and Mes-SA DX5-Tx). Specifically, PHB1 was shown to accumulate on the cell surface of paclitaxel-resistant cells. We conclude from these results that it is the redirection of PHB1 from an intracellular locale to one on the cell surface that mediates the drug-resistant phenotype. We do not at this time know whether this is due specifically to the presence of PHB1 on the cell surface or to its removal from intracellular sites. PHB1 was previously shown to be present on the cell surface of both B lymphocytes (15), intestinal epithelial cells (40), and on adipose endothelial cells, where, in the latter case, it can facilitate the targeting of cytotoxic agents (14).
PHB1 is a member of a family of proteins that are known to be localized to lipid-raft microdomains in diverse cellular membranes. Such microdomains are thought to be specialized and compartmentalized platforms where key cellular processes such as membrane transport and signal transduction occur. Indeed, Rajalingam et al. (18) showed recently that PHB1 may act as an important signaling scaffold at the plasma membrane where it is necessary for the recruitment of C-Raf to caveolin-1 rich lipid rafts and the activation of the Ras-Raf-MEK-ERK pathway. Interestingly, bcl-2 has been shown to be phosphorylated by multiple kinases, including Raf (for review see ref. 41), and we have shown that PHB1 knockdown in taxane-resistant cells results in diminished bcl-2 phosphorylation. It is plausible that PHB1 repression may result in diminished Raf activation, which results in diminished bcl-2 activation and increased activation of intrinsic apoptosis pathway following paclitaxel treatment. Future studies will be necessary to reveal the precise relationships among Raf localization, bcl-2 activation, and prohibitin-mediated drug resistance.
We have shown that i.v. inoculation of a rhodamine-tagged PHB1-binding peptide results in a significant accumulation of this peptide in paclitaxel-resistant but not in paclitaxel-sensitive tumor xenografts in vivo. Moreover, we observed that this peptide specifically labeled tumor cells and not the tumor endothelium, raising the possibility that therapeutic agents could be targeted directly to taxane-resistant tumors through the use of reagents that bind to cell-surface PHB1. The increased expression of cell-surface PHB1 on drug-resistant cells further suggests that cell-surface PHB1 on cells in solid tumors or particularly on circulating tumor cells could provide a marker for the detection of taxane resistance in cancer patients either before or during the course of chemotherapy. PHB1 can also circulate independently and has been shown to be increased in the serum of patients with colorectal cancer (42). The relevance of this increased level of circulating PHB1 to any form of drug-resistance has not yet been investigated.
Our results clearly demonstrate a function for PHB1 in the initiation and maintenance of resistance to paclitaxel in at least two types of human cancer cells. Silencing of PHB1 renders cells sensitive to paclitaxel both in vitro and in vivo. We do not propose that PHB1 is the only modulator of taxane sensitivity in human cancers as other mediators have been reported including increased levels of GSTπ (6, 43, 44) (confirmed in this study), tubulin mutations, and alterations in the MDR pathway (4, 5). For some tumors, however, alterations in PHB1 levels or interference with PHB1 localization may result in increased sensitivity of human tumors to taxane treatment.
Materials and Methods
Cell Culture.
The human nonsmall cell lung carcinoma cell line, A549, and its paclitaxel-resistant derivative cell line, A549TR, were cultured in F-12K medium (ATCC) supplemented with 10% FBS (FBS, Invitrogen) at 37 °C in a humidified atmosphere with 5% CO2. The uterine sarcoma cells (Mes-SA) and the multidrug-resistant derivative (Mes-SA DX5) were maintained in McCoy’s 5A media (ATCC) supplemented with 10% FBS. The paclitaxel-resistant cell lines were grown under selective pressure (100 nM paclitaxel) and placed in paclitaxel-free culture medium 5–7 d before experiments were performed. Paclitaxel-resistant cells stably expressing control, PHB1, or GSTπ shRNAs were generated by transducing A549TR cells with the respective lentiviral transduction particles as per manufacturer’s instructions (Sigma-Aldrich). Resistant clones were selected in 2.5 μg/mL puromycin (Invivogen) for 12 d, isolated using cloning cylinders, and subsequently expanded and maintained in puromycin-containing medium.
Immunofluorescence Assay of Cell-Surface and Intracellular PHB1.
Immunofluorescence for cell-surface PHB1 was carried out on intact cells seeded on round glass coverslips coated with 10 μg/mL fibronectin (BD Biosciences) in 24-well plates. The cells were washed with ice-cold PBS containing 1 mM MgCl2 and 1 mM CaCl2 on ice. The subsequent steps were performed at 4 °C, unless otherwise indicated. The cells were mildly fixed for 3 min with 3% paraformaldehyde (PFA, Electron Microscopy Sciences) and then stained for PHB1 using either (i) the goat polyclonal anti-PHB1 antibody (1:50) in 1% (wt/vol) BSA for 1 h followed by extensive washing and incubation with secondary antibody (Alexa 488-conjugated donkey anti-goat IgG, 1:500) in the dark for 1 h or (ii) the CKGGRAKDC-Rhodamine peptide (250 nM) in PBS for 2 h. The coverslips were further fixed with 3% PFA for 15 min and then mounted with ProLong antifade reagent with DAPI (Invitrogen). To assess the intracellular localization of PHB1, the cells were immediately fixed at 4 °C with 3% PFA. The cells were then permeabilized with ice-cold 100% methanol for 10 min at −20 °C followed by incubation with anti-PHB1 antibody (1:50) and anti-Cox IV (1:500) for 1 h. The cells were extensively washed and incubated with secondary antibody (Alexa 488-conjugated donkey anti-goat and Cy3-conjugated donkey anti-rabbit IgGs, 1:500) in the dark for 1 h. For confocal fluorescence microscopy, cells were examined using an inverted Leica DM-IRE2 microscope (Leica Microsystems). Acquisition parameters were adjusted to exclude saturation of the pixels. For quantification, such parameters were kept constant across the various conditions.
Animal Xenograft Studies.
For xenograft studies, the hind flanks of 8-wk-old male athymic (nu/nu) nude mice (Taconic Farms, Inc.) were injected s.c. with 4 × 106 cells resuspended in Matrigel (BD Biosciences). When the tumors reached 120–200 mm3 in volume (approximately 30 d), the animals were randomly divided into test groups consisting of at least eight mice per group (day 0). Tumor-bearing animals were administered either vehicle-control or 12 mg/kg paclitaxel (Bristol-Myers Squibb Co.) i.p. every Monday, Wednesday, and Friday and then humanely killed once morbidity was evident. The length and width of the tumor mass as well as the mouse weight were measured on the day of treatment and tumor volume was calculated with the equation 1/2(l × w2). Tumor growth was determined as the tumor volume on the day of treatment relative to the tumor volume on day 0 and presented as a percentage. Xenografts were excised, photographed, fixed in 10% formalin, and embedded in paraffin. For CKGGRAKDC-Rhodamine peptide localization in vivo, mice bearing A549 or A549TR s.c. xenografts were i.v. injected with the peptide at a final concentration of 300 μM in PBS. Two hours later, the tumors were then excised, frozen in optimum cutting temperature medium (Sakura Finetek), and sectioned. The sections were then either mounted with ProLong antifade reagent with DAPI (Invitrogen) or processed for immunofluorescence as described above. All animals were kept in specific pathogen-free housing with abundant food and water under guidelines approved by the Children’s Hospital Boston Animal Care and Use Committee and Animal Resources Com-mittee (Boston, MA).
Supplementary Methods.
SI Materials and Methods include all materials and protocols for whole cell lysis and subcellular fractionation, in vitro transfection with siRNA, cell viability assays, and flow cytometry analysis.
Supplementary Material
Acknowledgments
We sincerely thank Dr. John Heymach of M.D. Anderson Cancer Institute, Houston, TX, and Jeremy Force, Children’s Hospital, Boston, MA, for kindly providing us with the A549TR cells. We also thank Drs. Renata Pasqualini and Wadih Arap of M.D. Anderson Cancer Institute, Houston, TX, for providing information regarding the use of the CKGGRAKDC peptide. This work was supported by Grant CA37393 from the National Institutes of Health. N.P. was supported by a postdoctoral fellowship from the Natural Sciences and Engineering Research Council of Canada.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910649107/DCSupplemental.
References
- 1.Holmes FA, et al. Phase II trial of taxol, an active drug in the treatment of metastatic breast cancer. J Natl Cancer Inst. 1991;83:1797–1805. doi: 10.1093/jnci/83.24.1797-a. [DOI] [PubMed] [Google Scholar]
- 2.McGuire WP, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 3.Murphy WK, et al. Phase II study of taxol in patients with untreated advanced non-small-cell lung cancer. J Natl Cancer Inst. 1993;85:384–388. doi: 10.1093/jnci/85.5.384. [DOI] [PubMed] [Google Scholar]
- 4.Galletti E, Magnani M, Renzulli ML, Botta M. Paclitaxel and docetaxel resistance: Molecular mechanisms and development of new generation taxanes. ChemMedChem. 2007;2:920–942. doi: 10.1002/cmdc.200600308. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf RZ, Duan Z, Lamendola DE, Penson RT, Seiden MV. Paclitaxel resistance: Molecular mechanisms and pharmacologic manipulation. Curr Cancer Drug Targets. 2003;3:1–19. doi: 10.2174/1568009033333754. [DOI] [PubMed] [Google Scholar]
- 6.Townsend DM, Tew KD. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene. 2003;22:7369–7375. doi: 10.1038/sj.onc.1206940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McClung JK, Jupe ER, Liu XT, Dell’Orco RT. Prohibitin: Potential role in senescence, development, and tumor suppression. Exp Gerontol. 1995;30:99–124. doi: 10.1016/0531-5565(94)00069-7. [DOI] [PubMed] [Google Scholar]
- 8.Mishra S, Murphy LC, Nyomba BL, Murphy LJ. Prohibitin: A potential target for new therapeutics. Trends Mol Med. 2005;11:192–197. doi: 10.1016/j.molmed.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Nijtmans LG, Artal SM, Grivell LA, Coates PJ. The mitochondrial PHB complex: Roles in mitochondrial respiratory complex assembly, ageing and degenerative disease. Cell Mol Life Sci. 2002;59:143–155. doi: 10.1007/s00018-002-8411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nijtmans LG, et al. Prohibitins act as a membrane-bound chaperone for the stabilization of mitochondrial proteins. EMBO J. 2000;19:2444–2451. doi: 10.1093/emboj/19.11.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowdhury I, et al. Apoptosis of rat granulosa cells after staurosporine and serum withdrawal is suppressed by adenovirus-directed overexpression of prohibitin. Endocrinology. 2007;148:206–217. doi: 10.1210/en.2006-0187. [DOI] [PubMed] [Google Scholar]
- 12.Fusaro G, Wang S, Chellappan S. Differential regulation of Rb family proteins and prohibitin during camptothecin-induced apoptosis. Oncogene. 2002;21:4539–4548. doi: 10.1038/sj.onc.1205551. [DOI] [PubMed] [Google Scholar]
- 13.Gregory-Bass RC, et al. Prohibitin silencing reverses stabilization of mitochondrial integrity and chemoresistance in ovarian cancer cells by increasing their sensitivity to apoptosis. Int J Cancer. 2008;122:1923–1930. doi: 10.1002/ijc.23351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat Med. 2004;10:625–632. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- 15.Terashima M, et al. The IgM antigen receptor of B lymphocytes is associated with prohibitin and a prohibitin-related protein. EMBO J. 1994;13:3782–3792. doi: 10.1002/j.1460-2075.1994.tb06689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brasaemle DL, Dolios G, Shapiro L, Wang R. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem. 2004;279:46835–46842. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- 17.Garin J, et al. The phagosome proteome: Insight into phagosome functions. J Cell Biol. 2001;152:165–180. doi: 10.1083/jcb.152.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajalingam K, et al. Prohibitin is required for Ras-induced Raf-MEK-ERK activation and epithelial cell migration. Nat Cell Biol. 2005;7:837–843. doi: 10.1038/ncb1283. [DOI] [PubMed] [Google Scholar]
- 19.Chou TC, Dong H, Zhang X, Tong WP, Danishefsky SJ. Therapeutic cure against human tumor xenografts in nude mice by a microtubule stabilization agent, fludelone, via parenteral or oral route. Cancer Res. 2005;65:9445–9454. doi: 10.1158/0008-5472.CAN-05-1014. [DOI] [PubMed] [Google Scholar]
- 20.Kavallaris M, et al. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J Clin Invest. 1997;100:1282–1293. doi: 10.1172/JCI119642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, et al. Annexin-I expression modulates drug resistance in tumor cells. Biochem Biophys Res Commun. 2004;314:565–570. doi: 10.1016/j.bbrc.2003.12.117. [DOI] [PubMed] [Google Scholar]
- 22.Fusaro G, Dasgupta P, Rastogi S, Joshi B, Chellappan S. Prohibitin induces the transcriptional activity of p53 and is exported from the nucleus upon apoptotic signaling. J Biol Chem. 2003;278:47853–47861. doi: 10.1074/jbc.M305171200. [DOI] [PubMed] [Google Scholar]
- 23.Rastogi S, Joshi B, Fusaro G, Chellappan S. Camptothecin induces nuclear export of prohibitin preferentially in transformed cells through a CRM-1 dependent mechanism. J Biol Chem. 2006;281:2951–2959. doi: 10.1074/jbc.M508669200. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Fusaro G, Padmanabhan J, Chellappan SP. Prohibitin co-localizes with Rb in the nucleus and recruits N-CoR and HDAC1 for transcriptional repression. Oncogene. 2002;21:8388–8396. doi: 10.1038/sj.onc.1205944. [DOI] [PubMed] [Google Scholar]
- 25.Ganansia-Leymarie V, Bischoff P, Bergerat JP, Holl V. Signal transduction pathways of taxanes-induced apoptosis. Curr Med Chem Anticancer Agents. 2003;3:291–306. doi: 10.2174/1568011033482422. [DOI] [PubMed] [Google Scholar]
- 26.Oliver FJ, et al. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J Biol Chem. 1998;273:33533–33539. doi: 10.1074/jbc.273.50.33533. [DOI] [PubMed] [Google Scholar]
- 27.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Higgins CF. Multiple molecular mechanisms for multidrug resistance transporters. Nature. 2007;446:749–757. doi: 10.1038/nature05630. [DOI] [PubMed] [Google Scholar]
- 29.Coates PJ, et al. Mammalian prohibitin proteins respond to mitochondrial stress and decrease during cellular senescence. Exp Cell Res. 2001;265:262–273. doi: 10.1006/excr.2001.5166. [DOI] [PubMed] [Google Scholar]
- 30.Asamoto M, Cohen SM. Prohibitin gene is overexpressed but not mutated in rat bladder carcinomas and cell lines. Cancer Lett. 1994;83:201–207. doi: 10.1016/0304-3835(94)90320-4. [DOI] [PubMed] [Google Scholar]
- 31.Byrjalsen I, et al. Two-dimensional gel analysis of human endometrial proteins: Characterization of proteins with increased expression in hyperplasia and adeno-carcinoma. Mol Hum Reprod. 1999;5:748–756. doi: 10.1093/molehr/5.8.748. [DOI] [PubMed] [Google Scholar]
- 32.Jupe ER, Liu XT, Kiehlbauch JL, McClung JK, Dell’Orco RT. Prohibitin antiproliferative activity and lack of heterozygosity in immortalized cell lines. Exp Cell Res. 1995;218:577–580. doi: 10.1006/excr.1995.1194. [DOI] [PubMed] [Google Scholar]
- 33.Jupe ER, Liu XT, Kiehlbauch JL, McClung JK, Dell’Orco RT. Prohibitin in breast cancer cell lines: Loss of antiproliferative activity is linked to 3′ untranslated region mutations. Cell Growth Differ. 1996;7:871–878. [PubMed] [Google Scholar]
- 34.Jupe ER, Liu XT, Kiehlbauch JL, McClung JK, Dell’Orco RT. The 3′ untranslated region of prohibitin and cellular immortalization. Exp Cell Res. 1996;224:128–135. doi: 10.1006/excr.1996.0120. [DOI] [PubMed] [Google Scholar]
- 35.Williams K, Chubb C, Huberman E, Giometti CS. Analysis of differential protein expression in normal and neoplastic human breast epithelial cell lines. Electrophoresis. 1998;19:333–343. doi: 10.1002/elps.1150190231. [DOI] [PubMed] [Google Scholar]
- 36.Ummanni R, et al. Prohibitin identified by proteomic analysis of prostate biopsies distinguishes hyperplasia and cancer. Cancer Lett. 2008;266:171–185. doi: 10.1016/j.canlet.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 37.Thompson WE, et al. Characterization of prohibitin in a newly established rat ovarian granulosa cell line. Endocrinology. 2001;142:4076–4085. doi: 10.1210/endo.142.9.8354. [DOI] [PubMed] [Google Scholar]
- 38.Thompson WE, Powell JM, Whittaker JA, Sridaran R, Thomas KH. Immunolocalization and expression of prohibitin, a mitochondrial associated protein within the rat ovaries. Anat Rec. 1999;256:40–48. doi: 10.1002/(SICI)1097-0185(19990901)256:1<40::AID-AR6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 39.Cicchillitti L, et al. Comparative proteomic analysis of paclitaxel sensitive A2780 epithelial ovarian cancer cell line and its resistant counterpart A2780TC1 by 2D-DIGE: The role of ERp57. J Proteome Res. 2009;8:1902–1912. doi: 10.1021/pr800856b. [DOI] [PubMed] [Google Scholar]
- 40.Sharma A, Qadri A. Vi polysaccharide of Salmonella typhi targets the prohibitin family of molecules in intestinal epithelial cells and suppresses early inflammatory responses. Proc Natl Acad Sci USA. 2004;101:17492–17497. doi: 10.1073/pnas.0407536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basu A, DuBois G, Haldar S. Posttranslational modifications of Bcl2 family members—a potential therapeutic target for human malignancy. Front Biosci. 2006;11:1508–1521. doi: 10.2741/1900. [DOI] [PubMed] [Google Scholar]
- 42.Mengwasser J, Piau A, Schlag P, Sleeman JP. Differential immunization identifies PHB1/PHB2 as blood-borne tumor antigens. Oncogene. 2004;23:7430–7435. doi: 10.1038/sj.onc.1207987. [DOI] [PubMed] [Google Scholar]
- 43.Arai T, et al. Association of GSTP1 expression with resistance to docetaxel and paclitaxel in human breast cancers. Eur J Surg Oncol. 2008;34:734–738. doi: 10.1016/j.ejso.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Mathieu A, et al. Development of a chemoresistant orthotopic human nonsmall cell lung carcinoma model in nude mice: Analyses of tumor heterogenity in relation to the immunohistochemical levels of expression of cyclooxygenase-2, ornithine decarboxylase, lung-related resistance protein, prostaglandin E synthetase, and glutathione-S-transferase-alpha (GST)-alpha, GST-mu, and GST-pi. Cancer. 2004;101:1908–1918. doi: 10.1002/cncr.20571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.