Abstract
Binocular rivalry occurs when conflicting images are presented in corresponding locations of the two eyes. Perception alternates between the images at a rate that is relatively stable within individuals but that varies widely between individuals. The determinants of this variation are unknown. In addition, slow binocular rivalry has been demonstrated in bipolar disorder, a psychiatric condition with high heritability. The present study therefore examined whether there is a genetic contribution to individual variation in binocular rivalry rate. We employed the twin method and studied both monozygotic (MZ) twins (n = 128 pairs) who are genetically identical, and dizygotic (DZ) twins (n = 220 pairs) who share roughly half their genes. MZ and DZ twin correlations for binocular rivalry rate were 0.51 and 0.19, respectively. The best-fitting genetic model showed 52% of the variance in binocular rivalry rate was accounted for by additive genetic factors. In contrast, nonshared environmental influences accounted for 18% of the variance, with the remainder attributed to measurement error. This study therefore demonstrates a substantial genetic contribution to individual variation in binocular rivalry rate. The results support the vigorous pursuit of genetic and molecular studies of binocular rivalry and further characterization of slow binocular rivalry as an endophenotype for bipolar disorder.
Keywords: perceptual rivalry, bipolar disorder, endophenotype, genetics, twins
Binocular rivalry has been widely investigated in the visual sciences for more than 100 years. The phenomenon (Fig. 1A) is thought to engage a series of neural processes at different levels of the visual hierarchy (1). However, a detailed understanding of processing at each level and of interactions between levels has yet to be achieved. One aspect of binocular rivalry that has been studied extensively is its temporal dynamics. Several extrinsic factors are known to determine binocular rivalry rate and the strength of one stimulus over its rival (i.e., predominance). These factors include the contrast, spatial frequency, velocity, and semantic context of the stimuli (1, 2). Far less is known about the intrinsic factors that determine binocular rivalry rate when stimulus and ambient conditions are held constant, despite several studies showing that the rate of binocular rivalry varies widely between individuals but is relatively stable within individuals (3 –8). The present study investigated whether there is a genetic contribution to individual variation in binocular rivalry rate.
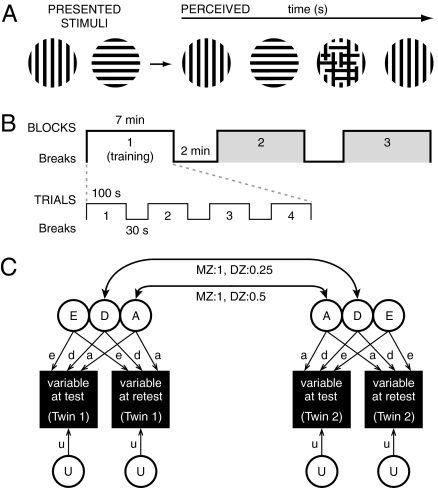
Fig. 1.
Binocular rivalry and genetic modeling. (A) Presenting a different image simultaneously, one to each eye, induces binocular rivalry (with occasional mixed percepts). In the present study, stimuli comprised drifting vertical and horizontal square-wave gratings. Binocular rivalry measures were rate (Hz), predominance (ratio of time spent perceiving one image relative to the other), number of mixed hits, and time associated with mixed hits. (B) Binocular rivalry data were collected for 21 min in three blocks. Block 1 was used for training and was discarded. Data from Blocks 2 and 3 were analyzed and were of most interest because binocular rivalry rates tend to stabilize with viewing time (8). (C) Path modeling of variance (12) into additive (A) and nonadditive (i.e., dominance/epistasis, D) genetic sources, unique environmental sources (E), and measurement error/unreliability (U). An ADEU model was chosen because the MZ twin correlation was more than twice the DZ twin correlation, indicating the importance of the A and D components over C components of variance. Reliable genetic and environmental variance is identified by equating pathways from A, D, and E components to data from the first and second test occasions. The remaining variance (U) is unshared between the two test occasions but represents an equal amount of variance for each variable on each test occasion and therefore is equated. Correlations between cotwins for factors A and D are fixed at Mendelian expectations.
Our interest was motivated by a lack of focus on individual differences in binocular rivalry research and of models addressing such differences. We also were motivated by data (7, 8) showing slow binocular rivalry may be an endophenotype for bipolar disorder, a condition with high heritability (0.59–0.85) (9, 10). A key feature of a putative endophenotype is that it should be a heritable trait (11). To examine the heritability of binocular rivalry, we employed the twin method and studied both monozygotic (MZ) twins who are genetically identical, and dizygotic (DZ) twins who share roughly half their genes. This method enables parsing of familial similarities in a trait into genetic and shared environmental sources, with the remaining variance attributed to unique environmental factors, including measurement errors (Fig. 1C) (12). We report a substantial genetic contribution to individual variation in binocular rivalry rate.
Results
Participants were adolescent twins (722 individuals; 48% male) with a mean age of 14 years. A subsample (97 individuals) was retested approximately 2 years later. Fig. 1 shows the binocular rivalry measures, data collection protocol, and genetic modeling procedure. The binocular rivalry stimuli comprised drifting vertical and horizontal square-wave gratings, viewed through liquid crystal display goggles, with no training in fixation required. Participants pressed one key for the vertical percept, an adjacent key for the horizontal percept, and a third response option (“mixed”) for mixed percepts, unusual or uncertain percepts, or a previously incorrect response. Binocular rivalry rate was calculated by dividing the number of perceptual switches by the total viewing period in Blocks 2 and 3, excluding the periods immediately preceding and following a mixed response. The resulting value for binocular rivalry rate is the number of perceptual switches per second (expressed in Hz). Predominance was calculated by dividing the total time spent perceiving the vertical grating by the total time spent perceiving the horizontal grating in Blocks 2 and 3 (with the same mixed-response exclusions). The resulting ratio then was log transformed.
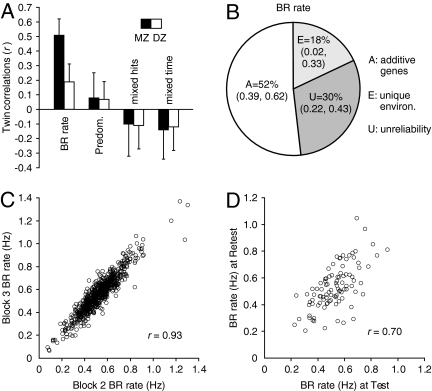
Exclusive binocular rivalry (i.e., minimal mixed percepts) was achieved successfully (Table 1). Binocular rivalry rates varied widely between individuals (0.08–1.32 Hz; mean, 0.54 ± 0.15 SD), as did predominance (Table 1). The main finding is that the twin correlation for binocular rivalry rate was significantly higher for MZ (0.51) than for DZ twins (0.19), indicating genetic influence on this measure (Fig. 2A). In contrast, neither MZ or DZ twin correlations were significant for predominance or nonexclusive (mixed) rivalry periods (Table 1 and Fig. 2A), so these measures were not included in genetic modeling analyses.
Table 1.
Binocular rivalry summary statistics and correlations
| Variable | Test | Retest | Reliability | Twin correlations | ||||
| Mean (SD) | Range | Mean (SD) | Range | Within-test r (95% CI) | Between-test r (95% CI) | MZ r (95% CI) | DZ r (95% CI) | |
| Binocular rivalry rate, Hz | 0.54 (0.15) | 0.08–1.32 | 0.53 (0.16) | 0.21–1.04 | 0.93 (0.92–0.94) | 0.70 (0.58–0.78) | 0.51 (0.37–0.62) | 0.19 (0.07–0.31) |
| log predominance | 0.17 (0.09) | −0.44–0.48 | 0.14 (0.08) | −0.24–0.22 | 0.71 (0.67–0.75) | 0.43 (0.23–0.59) | 0.08 (−0.10–0.25) | 0.07 (−0.06–0.19) |
| Mixed hits | 18.4 (25.4) | 0–156 | 12.5 (18.0) | 0–83 | 0.94 (0.92–0.95) | 0.30 (0.03–0.53) | −0.10 (−0.32–0.13) | −0.11 (−0.27–0.05) |
| Mixed time, seconds | 33.9 (50.2) | 0–372.1 | 25.3 (42.8) | 0–243.0 | 0.91 (0.89–0.93) | 0.39 (0.13–0.60) | −0.14 (−0.34–0.08) | −0.12 (−0.28–0.04) |
All mean, SD, and range values are before winsorization. Data were winsorized to ± 3.3 SD before (i) reliability and twin maximum likelihood correlation analyses, (ii) preliminary analyses for genetic modeling, and (iii) genetic modeling. Mixed hits and mixed time were categorized for correlation analyses because of non-normal distribution (thresholds were set that divided each variable into three categories of approximately equal size based on scores from the first test occasion). Exclusive binocular rivalry was achieved successfully, indicated by comparison of mixed hits/time with the hits/time associated with vertical (V) and horizontal (H) grating percepts, for which the Test group means, SD, and ranges are, respectively: V hits, 189.0, 64.3, 18–516; V time, 353.0, 61.8, 84.1–547.1; H hits, 188.9, 64.3, 19–514; H time, 339.1, 61.8, 109.3–549.8.
Fig. 2.
Population binocular rivalry and genetic modeling data. (A) MZ and DZ twin maximum likelihood correlations for binocular rivalry measures (error bars indicate 95% CI). BR, binocular rivalry; Predom, predominance. (B) The variance in binocular rivalry rate, estimated from the AEU (best-fitting) model, was accounted for by a substantial additive genetic component; plus unique environmental influences and measurement unreliability over a period of 2 years. The 30% unreliable variance splits 7% within tests and 23% between occasions. (C) Scatterplot showing very high within-test reliability of binocular rivalry rate (95% CI, 0.92–0.94; n = 722) in 14-year-old twins. (D) Scatterplot showing high retest reliability of binocular rivalry rate (95% CI, 0.58–0.78; n = 97) in twins retested at 16 years of age.
The within-test reliability for binocular rivalry rate was very high (Fig. 2C), and reliability over time (retest) was high (Fig. 2D). Within-test reliability for mixed hits and mixed time was very high; within-test reliability for predominance was lower but was still high (Table 1). Reliability over time was moderate for predominance, mixed hits, and mixed time (Table 1). Measures of between-block change in predominance showed poor reliability over time (retest correlations were nonsignificant) and therefore are not reported.
Preliminary analyses of binocular rivalry rate before genetic modeling showed homogeneity of sampling, with no birth order, zygosity, or sex effects for means or variances. Further, no mean effect was found for age. However, a significant mean effect was found for acuity (Δχ2 1 = 17.0), such that 37 individuals with acuity of 6/9 in either eye had a marginally slower binocular rivalry rate than the rest of the sample for whom acuity was 6/6 or better in both eyes. Therefore, acuity was included as a covariate in all further analyses.
Variation in binocular rivalry rate can be influenced by additive (A) and nonadditive (dominance/epistasis, D) genetic factors and by common (C) and unique (E) environmental factors. Similarity between cotwins can be influenced only by A, D, and C factors. If only C is influential, MZ and DZ correlations would be similar, because both cotwins are influenced similarly. If only A is influential, MZ correlations would be approximately twice DZ correlations, because MZ pairs share 100% of their genes, whereas DZ pairs share, on average, only 50%. Dominant (D) genes increase similarity between MZ twins (who inherit the same combination of parental genotypes) but can decrease similarity between DZ pairs depending on the combination of parental genotypes they inherit. Thus, if both A and D factors are influential, MZ correlations will be more than twice the DZ correlations. In contrast, if both A and C factors are influential, the greater degree of shared influences across MZ and DZ pairs results in an MZ correlation that is less than twice the DZ correlation. While both D and C factors may be influential, they are confounded and cannot be modeled simultaneously if twins are raised together.
For binocular rivalry rate, the pattern of twin correlations indicated that both A and D factors are influential, that is, the MZ correlation (0.51) was more than twice the DZ correlation (0.19). Further, because the MZ correlation was less than 1.0, unique environmental influences and/or measurement error/unreliability were indicated. The availability of retest data allowed measurement unreliability to be estimated. Binocular rivalry rate therefore was examined in a model including additive (A) and nonadditive (D) genetic influences, unique environmental influences (E), and measurement unreliability (U). In this full model, A was estimated to account for 45% of the variance [95% confidence interval (CI), 24–62%], D for 6% (CI, 0–22%), E for 19% (CI, 3–35%), and U for 30% (CI, 22–42%); thus, genetic factors (i.e., A and D factors) accounted for 51% of the total variance. Dropping D did not worsen model fit (Δχ2 1 = 0.6), showing that D was not significant, as indicated also by the CI for D in the full ADEU model (i.e., the CI includes 0). Thus the most parsimonious and best-fitting model was an AEU model (Fig. 2B), in which 52% of the total variance (CI, 39–62%) was accounted for by additive genetic influences (genetic variance represents 74% of reliable variance), with the remainder of the variance attributed to unique environmental influences (18%) and measurement error (30%).
Discussion
This study represents the largest binocular rivalry population dataset yet published and provides confirmation of reports that binocular rivalry rate varies widely between individuals (3 –8). The data also confirm reports of very high within-test (8) and high retest reliability of binocular rivalry rate (4, 5, 7, 8). We have demonstrated a substantial genetic contribution to individual variation in binocular rivalry rate. The mechanisms of this genetic influence remain to be determined.
Studies of binocular rivalry mechanisms have focused on the level at which the phenomenon occurs in the brain, with perception-dependent neural activity reported as early as the lateral geniculate nucleus (13, 14), in the primary visual cortex (15), as late as the inferotemporal cortex (16), and also in high-level nonvisual regions (17). Similarly, modulation of binocular rivalry can occur with brain stimulation applied at low (18) or high (19, 20) levels. Proposed mechanistic models have included rivalry between specific populations of neurons (21), rivalry between stimulus representations at a high level of visual processing (22), hierarchical computational models (23, 24), and rivalry between independent attentional selection mechanisms in each cerebral hemisphere (19, 20, 25).
Early studies argued against an influence of peripheral factors, such as eye movements, on binocular rivalry (26, 27; notwithstanding central control of peripheral factors). More recently, an influence of eye movements (that induce retinal image shifts) has been reported (28; see also ref. 29 in regard to blinking). However, perceptual alternations also were shown to occur independently of peripheral factors, thus implicating a central binocular rivalry process (28; see also ref. 30). Further studies are required to identify the precise role of peripheral and central factors in determining binocular rivalry rate. An influence of voluntary attention on binocular rivalry has been reported also, but such influence is limited (31, 32) and is much less than the order of magnitude of individual variation reported here and in previous studies (3 –8). Nonetheless, the emerging genetics of attentional networks (33) may be relevant to the present finding, because mechanisms of involuntary attention are thought to be engaged during binocular rivalry (1, 20, 25). Few studies have examined the role of neurotransmitter systems and the effects of pharmacological agents on binocular rivalry; however, recent reports suggest involvement of serotonergic (34, 35) and noradrenergic (30) systems. Such studies may provide clues to molecular mechanisms underlying individual variation in binocular rivalry rate. Indeed, the present finding warrants expanding the focus of binocular rivalry research from levels-based investigation to genetic and molecular aspects of the phenomenon.
Also warranted is a focus on models of binocular rivalry that accommodate individual variation in binocular rivalry rate. One such model (7, 19, 25, 36), which remains under investigation, suggested a role for cationic channel levels in determining binocular rivalry rate. This suggestion was based on demonstrating slow binocular rivalry in bipolar disorder (7), a finding that has since been replicated (8, 37) and also has been shown with different types of perceptual rivalry (38 –40). The present finding supports the use of slow binocular rivalry as an endophenotype for bipolar disorder because endophenotypes for heritable conditions must themselves be heritable traits (11). The heritability of binocular rivalry rate reported here is comparable to that reported for other neuropsychiatric endophenotypes, such as P300 event-related potential amplitude (heritability range, 0.3–0.8) in subjects with alcohol dependency (41, 42). The present finding has the potential to reveal not only mechanisms of binocular rivalry but also mechanisms of bipolar disorder and suggests gene-finding approaches to both. It also suggests use of the trait to help overcome challenges posed by the heterogeneity of the bipolar clinical phenotype (7 –11, 37).
However, further characterization of the trait is required, including assessment of binocular rivalry rate in other psychiatric conditions, particularly schizophrenia and major (unipolar) depression, to establish the trait’s specificity (8, 10). Also required is assessment of binocular rivalry rate in first-degree relatives of bipolar probands and of possible effects of medication and clinical state. However, effects of medication or clinical state are unlikely to account for slow binocular rivalry in bipolar disorder (7, 8, 37), a suggestion that is consistent with the present finding of a substantial genetic contribution to binocular rivalry rate. Similarly, eye movements are unlikely to account for the slowing of binocular rivalry in bipolar disorder because the limited number of studies of saccades during smooth pursuit in this clinical population (e.g., 43, 44) show either no significant difference from controls or an increased saccade frequency (which should cause a faster rather than slower binocular rivalry rate). Moreover, slow binocular rivalry in bipolar disorder also has been demonstrated with stationary gratings (8) that do not elicit pursuit eye movements.
If further studies confirm that endogenous binocular rivalry rate is fundamentally determined by central processing factors, the present finding also represents demonstration of, in a large sample, a substantial genetic contribution to individual variation in a postretinal visual processing phenomenon (45). Although there have been previous reports of genetic contributions to illusory movement (46), flicker fusion thresholds (47), and Rorschach indices (47), the sample sizes in those studies were small. A recent twin study, which did employ a large sample, assessed contrast sensitivity in middle-aged males and found only a modest heritability estimate (0.14–0.38) (48). Moreover, it is not known whether deficient contrast sensitivity occurs in the lens, retina, or postretinal processing (48). Inspection time for line-length discrimination also has been examined in a large twin sample and was shown to have substantial genetic influence (heritability estimate, 0.57), but this perceptual task is thought to reflect attentional and decision processes used in response monitoring (49). Although a role for decision-making during binocular rivalry has been proposed (30, 50), the perceptual alternations cannot be prevented, and, as discussed above, voluntary attention has only a limited effect on the phenomenon (1, 31, 32).
Finally, the heritability and molecular basis of individual variation in human color vision—a retinal phenomenon—is well understood (51, 52). We therefore propose that if individual variation in binocular rivalry rate is indeed a predominantly central processing phenomenon, then, as for color vision and the retina, binocular rivalry may serve as a paradigm case to unravel the genetics, physiology, and pathophysiology of postretinal vision and perception. Although binocular rivalry is likely to be a complex trait with complex inheritance (unlike color vision), it is a phenomenon that has proven highly amenable to research in animals and humans using a wide variety of investigative methods (1). It is a phenomenon that also may provide a unique window into the science, and indeed genetics, of visual consciousness (53, 54).
Several hundred years since binocular rivalry was discovered, and more than a century since early reports that the rate of binocular rivalry varies widely between individuals, we have shown this aspect of the phenomenon to be under substantial genetic influence. This result suggests a range of novel approaches to investigate binocular rivalry further in both general and clinical populations, with a focus on genetic, molecular, and endophenotype studies.
Materials and Methods
Participants.
A population sample of twins was recruited by the Queensland Institute of Medical Research (QIMR) for a genetic study of melanocytic nevi (moles) (55) through mailings to schools in South-East Queensland between 2000 and 2009. Binocular rivalry data were collected during a routine mole-count visit scheduled when twins turned 14 years of age. The sample included 722 individuals (48% male; 128 MZ pairs, 220 DZ pairs, and 26 unpaired cotwins; mean age, 14.1 ± 0.1 SD; range, 14–15 years). Zygosity was determined by typing nine independent polymorphic DNA markers using the AmpFLSTR Profiler PCR Amplification Kit (Applied Biosystems) and crosschecked with ABO, MN, and Rh blood groups and/or phenotypic information (hair, skin, and eye color), with an extremely low probability of error (<10−4). At ≈16 years of age, twins were invited to participate in a study of cognition (55), and a subset (n = 97; 53% male) was retested 1.9–2.8 years (mean, 2.1 ± 0.2 SD) after the first test. The retest sample comprised 11 MZ pairs, 35 DZ pairs, and 5 unpaired cotwins aged 16.0–16.9 years (mean, 16.1 ± 0.2 SD). Individuals at age 14 or 16 years were excluded if they (i) reported a history of, or medication for, bipolar disorder, depression, or attention deficit hyperactivity disorder, (ii) reported a history of brain injury or other neurological condition, (iii) reported a history of uncorrected strabismus, (iv) had visual acuity worse than 6/9 in either eye (acuity was measured using a Snellen chart at 3 m), or (v) there were problems with data collection. There were 26 exclusions at the first test and 11 exclusions at retest. Written, informed consent was obtained from all participants and a parent or guardian. The study conformed to the National Statement on Ethical Conduct in Human Research (2007) issued by the National Health and Medical Research Council (NHMRC) of Australia and was approved by the QIMR Human Research Ethics Committee.
Binocular Rivalry Stimuli, Recording Procedure, and Measures.
Binocular rivalry stimuli were presented on a monochrome (green) computer monitor situated 3 m from the participant, in a dimly lit room. The orthogonal stimuli were vertical gratings drifting left-to-right, always presented to the left eye, and horizontal gratings drifting downward, always presented to the right eye. The gratings had a spatial frequency of 8 cycles/degree, were drifting at 4 cycles/second and were presented in a circular patch subtending 1.5° of visual angle. Contrast of the gratings was 0.9. Participants were instructed not to consume caffeinated beverages for 2 hours before the binocular rivalry test session. To assist with training, they received an explanatory sheet showing the various possible perceptions and explaining how to respond in each scenario. Participants were instructed to view the stimuli passively rather than to attempt to influence their perceptions. They were supervised by a research assistant at all times during data collection. Block 1 recording was used to train the participant, checking that the patient understood the instructions and was performing the task correctly. Questions could be asked during this period. Participants used the left hand to press a raised key in response to the vertical percept and the right hand to press an adjacent raised key in response to the horizontal percept. Mixed percepts were indicated by pressing the space bar, using either hand, and this response option also was used to indicate unusual percepts and incorrect or undecided responses. Although the mixed hits and mixed time values provide an approximate indication of nonexclusive rivalry (i.e., mixed percepts), this value is likely to be an overestimation because the mixed response option also was used to indicate unusual percepts and incorrect or undecided responses. Analyses were performed with specialized software (BiReme Systems) and PASW Statistics 17.0 (SPSS Inc). Within- and between-test reliability and genetic analyses used the structural equation modeling package Mx (www.vcu.edu/mx) which employs maximum likelihood estimation from raw data observations.
Genetic Model Fitting.
Genetic modeling (56) can estimate additive (A) and nonadditive (i.e., dominance/epistasis, D) genetic effects, common (C) and unique (E) environmental effects, and, when retest data are available, the influence of measurement error/unreliability (U). Because D and C components are negatively confounded in twins reared together, we use the twin correlations to indicate which component, if either, is most influential. For binocular rivalry rate, the MZ twin correlation was more than twice the DZ correlation, indicating the importance of the A component and of the D component over C (57), so an ADEU model was fitted (Fig. 1C). Further confirmation was indicated by the poorer fit, as indicated by the lower Akaike Information Criterion (AIC) goodness of fit index of an ACEU (AIC = 557.9) versus an ADEU (AIC = 554.4) model. In addition, exploratory analyses allowing for common environmental (C) influences resulted in a zero estimate for the C component. To test whether the ADEU model was the most parsimonious, a constrained AEU model was compared with the fully saturated ADEU model, within which it is nested, by examining the difference in the −2 log likelihood, which is distributed as a χ2 for given degrees of freedom.
Before genetic modeling, birth order, zygosity, and sex effects for means and variances were examined. An initially fully saturated model, in which means and variances were free to vary, was compared with successively more constrained models that tested means and variances for these effects (for a more detailed explanation of this procedure, see ref. 58). In addition, mean effects for age and visual acuity were examined. Twin correlations were examined to see if data could be pooled across sex for the genetic analyses; that is, whether correlations could be set equal for (i) male and female MZ pairs, (ii) same-sex male and female DZ pairs, and, subsequently, (iii) same-sex and opposite-sex DZ pairs. Data from the first test occasion were divided into two collection blocks and modeled to estimate within-test unreliability. All correlations are based on Pearson’s formula.
Acknowledgments
We thank A. Eldridge, M. Grace, M. Caffrey, and K. McAloney for assistance with data collection, D. Park and S. Gordon for technical assistance, A. Hannan, J. Hohwy, and two anonymous reviewers for comments on the manuscript, and the twins and their family members for participating in the study. S.M.M. was supported by the National Health and Medical Research Council (NHMRC) of Australia and currently is supported by the Victorian Neurotrauma Initiative. T.T.N. is supported by the NHMRC. This project was funded in part by the NHMRC.
Footnotes
Conflict of interest statement: S.M.M. and J.D.P. are co-inventors on a granted University of Queensland, national and international patent concerning slow binocular rivalry in bipolar disorder. All other authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Blake R, Logothetis NK. Visual competition. Nat Rev Neurosci. 2002;3:13–21. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- 2.Levelt WJM. On Binocular Rivalry. Assen, The Netherlands: Van Gorcum; 1965. [Google Scholar]
- 3.McDougall W. Physiological factors of the attention-process (IV) Mind. 1906;15:329–359. [Google Scholar]
- 4.George RW. The significance of the fluctuations experienced in observing ambiguous figures and in binocular rivalry. J Gen Psychol. 1936;15:39–61. [Google Scholar]
- 5.Enoksson P. Binocular rivalry and monocular dominance studied with optokinetic nystagmus. Acta Ophthalmol (Copenh) 1963;41:544–563. doi: 10.1111/j.1755-3768.1963.tb03568.x. [DOI] [PubMed] [Google Scholar]
- 6.Aafjes M, Hueting JE, Visser P. Individual and interindividual differences in binocular retinal rivalry in man. Psychophysiology. 1966;3:18–22. doi: 10.1111/j.1469-8986.1966.tb02674.x. [DOI] [PubMed] [Google Scholar]
- 7.Pettigrew JD, Miller SM. A ‘sticky’ interhemispheric switch in bipolar disorder? Proc Biol Sci. 1998;265:2141–2148. doi: 10.1098/rspb.1998.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller SM, et al. Slow binocular rivalry in bipolar disorder. Psychol Med. 2003;33:683–692. doi: 10.1017/s0033291703007475. [DOI] [PubMed] [Google Scholar]
- 9.Smoller JW, Finn CT. Family, twin, and adoption studies of bipolar disorder. Am J Med Genet C Semin Med Genet. 2003;123C:48–58. doi: 10.1002/ajmg.c.20013. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenstein P, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: A population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 12.Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- 13.Haynes J-D, Deichmann R, Rees G. Eye-specific effects of binocular rivalry in the human lateral geniculate nucleus. Nature. 2005;438:496–499. doi: 10.1038/nature04169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wunderlich K, Schneider KA, Kastner S. Neural correlates of binocular rivalry in the human lateral geniculate nucleus. Nat Neurosci. 2005;8:1595–1602. doi: 10.1038/nn1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S-H, Blake R, Heeger DJ. Hierarchy of cortical responses underlying binocular rivalry. Nat Neurosci. 2007;10:1048–1054. doi: 10.1038/nn1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheinberg DL, Logothetis NK. The role of temporal cortical areas in perceptual organization. Proc Natl Acad Sci USA. 1997;94:3408–3413. doi: 10.1073/pnas.94.7.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lumer ED, Friston KJ, Rees G. Neural correlates of perceptual rivalry in the human brain. Science. 1998;280:1930–1934. doi: 10.1126/science.280.5371.1930. [DOI] [PubMed] [Google Scholar]
- 18.Pearson J, Tadin D, Blake R. The effects of transcranial magnetic stimulation on visual rivalry. J Vis. 2007 doi: 10.1167/7.7.2. 10.1167/7.7.2. [DOI] [PubMed] [Google Scholar]
- 19.Miller SM, et al. Interhemispheric switching mediates perceptual rivalry. Curr Biol. 2000;10:383–392. doi: 10.1016/s0960-9822(00)00416-4. [DOI] [PubMed] [Google Scholar]
- 20.Ngo TT, Liu GB, Tilley AJ, Pettigrew JD, Miller SM. Caloric vestibular stimulation reveals discrete neural mechanisms for coherence rivalry and eye rivalry: A meta-rivalry model. Vision Res. 2007;47:2685–2699. doi: 10.1016/j.visres.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 21.Blake R. A neural theory of binocular rivalry. Psychol Rev. 1989;96:145–167. doi: 10.1037/0033-295x.96.1.145. [DOI] [PubMed] [Google Scholar]
- 22.Logothetis NK, Leopold DA, Sheinberg DL. What is rivalling during binocular rivalry? Nature. 1996;380:621–624. doi: 10.1038/380621a0. [DOI] [PubMed] [Google Scholar]
- 23.Wilson HR. Computational evidence for a rivalry hierarchy in vision. Proc Natl Acad Sci USA. 2003;100:14499–14503. doi: 10.1073/pnas.2333622100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freeman AW. Multistage model for binocular rivalry. J Neurophysiol. 2005;94:4412–4420. doi: 10.1152/jn.00557.2005. [DOI] [PubMed] [Google Scholar]
- 25.Miller SM. Binocular rivalry and the cerebral hemispheres. With a note on the correlates and constitution of visual consciousness. Brain Mind. 2001;2:119–149. [Google Scholar]
- 26.Blake RR, Fox R, McIntyre C. Stochastic properties of stabilized-image binocular rivalry alternations. J Exp Psychol. 1971;88:327–332. doi: 10.1037/h0030877. [DOI] [PubMed] [Google Scholar]
- 27.Lack LC. The role of accommodation in the control of binocular rivalry. Percept Psychophys. 1971;10:38–42. [Google Scholar]
- 28.van Dam LCJ, van Ee R. Retinal image shifts, but not eye movements per se, cause alternations in awareness during binocular rivalry. J Vis. 2006 doi: 10.1167/6.11.3. 10.1167/6.11.3. [DOI] [PubMed] [Google Scholar]
- 29.van Dam LCJ, van Ee R. The role of saccades in exerting voluntary control in perceptual and binocular rivalry. Vision Res. 2006;46:787–799. doi: 10.1016/j.visres.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Einhäuser W, Stout J, Koch C, Carter O. Pupil dilation reflects perceptual selection and predicts subsequent stability in perceptual rivalry. Proc Natl Acad Sci USA. 2008;105:1704–1709. doi: 10.1073/pnas.0707727105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng M, Tong F. Can attention selectively bias bistable perception? Differences between binocular rivalry and ambiguous figures. J Vis. 2004 doi: 10.1167/4.7.2. 10.1167/4.7.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Ee R, van Dam LCJ, Brouwer GJ. Voluntary control and the dynamics of perceptual bi-stability. Vision Res. 2005;45:41–55. doi: 10.1016/j.visres.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 33.Posner MI, Rothbart MK, Sheese BE. Attention genes. Dev Sci. 2007;10:24–29. doi: 10.1111/j.1467-7687.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- 34.Carter OL, et al. Modulating the rate and rhythmicity of perceptual rivalry alternations with the mixed 5-HT2A and 5-HT1A agonist psilocybin. Neuropsychopharmacology. 2005;30:1154–1162. doi: 10.1038/sj.npp.1300621. [DOI] [PubMed] [Google Scholar]
- 35.Nagamine M, Yoshino A, Miyazaki M, Takahashi Y, Nomura S. Effects of selective 5-HT1A agonist tandospirone on the rate and rhythmicity of binocular rivalry. Psychopharmacology (Berl) 2008;198:279–286. doi: 10.1007/s00213-008-1139-2. [DOI] [PubMed] [Google Scholar]
- 36.Pettigrew JD. Searching for the switch: Neural bases for perceptual rivalry alternations. Brain Mind. 2001;2:85–118. [Google Scholar]
- 37.Nagamine M, Yoshino A, Miyazaki M, Takahashi Y, Nomura S. Difference in binocular rivalry rate between patients with bipolar I and bipolar II disorders. Bipolar Disord. 2009;11:539–546. doi: 10.1111/j.1399-5618.2009.00719.x. [DOI] [PubMed] [Google Scholar]
- 38.Ewen JH. The psychological estimation of the effects of certain drugs upon the syntonic and schizophrenic psychoses. With a brief enquiry into a physiological basis of temperament. J Ment Sci. 1931;77:742–766. [Google Scholar]
- 39.Hunt J, Guilford JP. Fluctuation of an ambiguous figure in dementia praecox and in manic-depressive patients. J Abnorm Soc Psychol. 1933;27:443–452. [Google Scholar]
- 40.Krug K, Brunskill E, Scarna A, Goodwin GM, Parker AJ. Perceptual switch rates with ambiguous structure-from-motion figures in bipolar disorder. Proc Biol Sci. 2008;275:1839–1848. doi: 10.1098/rspb.2008.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Beijsterveldt CEM, van Baal GCM, Molenaar PCM, Boomsma DI, de Geus EJC. Stability of genetic and environmental influences on P300 amplitude: A longitudinal study in adolescent twins. Behav Genet. 2001;31:533–543. doi: 10.1023/a:1013389226795. [DOI] [PubMed] [Google Scholar]
- 42.Strat YL, Ramoz N, Schumann G, Gorwood P. Molecular genetics of alcohol dependence and related endophenotypes. Curr Genomics. 2008;9:444–451. doi: 10.2174/138920208786241252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holzman PS, O’Brian C, Waternaux C. Effects of lithium treatment on eye movements. Biol Psychiatry. 1991;29:1001–1015. doi: 10.1016/0006-3223(91)90357-r. [DOI] [PubMed] [Google Scholar]
- 44.Martin LF, et al. Physiology of schizophrenia, bipolar disorder, and schizoaffective disorder. Am J Psychiatry. 2007;164:1900–1906. doi: 10.1176/appi.ajp.2007.06010017. [DOI] [PubMed] [Google Scholar]
- 45.Wilmer JB. How to use individual differences to isolate functional organization, biology, and utility of visual functions; with illustrative proposals for stereopsis. Spat Vis. 2008;21:561–579. doi: 10.1163/156856808786451408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fraser A, Wilcox KJ. Perception of illusory movement. Nature. 1979;281:565–566. doi: 10.1038/281565a0. [DOI] [PubMed] [Google Scholar]
- 47.Murawski BJ. Genetic factors in tests of perception and the Rorschach. J Genet Psychol. 1971;119:43–52. doi: 10.1080/00221325.1971.10532625. [DOI] [PubMed] [Google Scholar]
- 48.Cronin-Golomb A, et al. Genetic influence on contrast sensitivity in middle-aged male twins. Vision Res. 2007;47:2179–2186. doi: 10.1016/j.visres.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luciano M, et al. Perceptual speed does not cause intelligence, and intelligence does not cause perceptual speed. Biol Psychol. 2005;70:1–8. doi: 10.1016/j.biopsycho.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 50.Leopold DA, Logothetis NK. Multistable phenomena: Changing views in perception. Trends Cogn Sci. 1999;3:254–264. doi: 10.1016/s1364-6613(99)01332-7. [DOI] [PubMed] [Google Scholar]
- 51.Solomon SG, Lennie P. The machinery of colour vision. Nat Rev Neurosci. 2007;8:276–286. doi: 10.1038/nrn2094. [DOI] [PubMed] [Google Scholar]
- 52.Mancuso K, et al. Gene therapy for red–green colour blindness in adult primates. Nature. 2009;461:784–787. doi: 10.1038/nature08401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crick F, Koch C. Consciousness and neuroscience. Cereb Cortex. 1998;8:97–107. doi: 10.1093/cercor/8.2.97. [DOI] [PubMed] [Google Scholar]
- 54.Miller SM. On the correlation/constitution distinction problem (and other hard problems) in the scientific study of consciousness. Acta Neuropsychiatr. 2007;19:159–176. doi: 10.1111/j.1601-5215.2007.00207.x. [DOI] [PubMed] [Google Scholar]
- 55.Wright MJ, Martin NG. The Brisbane Adolescent Twin Study: Outline of study methods and research projects. Aust J Psychol. 2004;56:65–78. [Google Scholar]
- 56.Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6th Ed. Richmond, VA: Department of Psychiatry, Virginia Commonwealth University; 2003. [Google Scholar]
- 57.Martin NG, Jardine R, Andrews G, Heath AC. Anxiety disorders and neuroticism: Are there genetic factors specific to panic? Acta Psychiatr Scand. 1988;77:698–706. doi: 10.1111/j.1600-0447.1988.tb05190.x. [DOI] [PubMed] [Google Scholar]
- 58.McGregor B, et al. Genetic and environmental contributions to size, color, shape, and other characteristics of melanocytic naevi in a sample of adolescent twins. Genet Epidemiol. 1999;16:40–53. doi: 10.1002/(SICI)1098-2272(1999)16:1<40::AID-GEPI4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]