Abstract
Autoimmune encephalomyelitis may be ameliorated experimentally by enhancing NK cell-mediated elimination of activated autoreactive T cells through a mutation that interrupts the interaction between Qa-1b and CD94/NKG2A. Here we evaluate the ability of an anti-NKG2A F(ab′)2 Ab to enhance elimination of autoreactive T cells and reduce experimental autoimmune encephalomyelitis (EAE). Anti-NKG2A F(ab′)2 treatment diminishes progression of both myelin oligodendrocyte glycoprotein (MOG)-induced EAE in intact C57BL/6 mice and after adoptive transfer of disease-causing T cells. Analyses of the underlying mechanism revealed that administration of anti-NKG2A F(ab′)2 Ab reduces CD4+ T recall responses to MOG and skews the proportion of IL-17- and IFNγ-producing CD4+ T cells toward the protective IL-4- and IL-10-secreting CD4+ T cell subpopulations. CD94/NKG2A-dependent inhibition of inflammatory damage to spinal cord is associated with decreased infiltration of T cells and reduced microglia activation in the central nervous system. Because anti-NKG2A F(ab′)2 treatment had no detectable effect on the numbers or activity of T and B lymphocytes and NK cells in peripheral lymphoid tissues, this anti-NKG2A-based approach may represent a safe and effective therapy for this CNS disorder.
Keywords: natural killer, CD94, Qa-1b, multiple sclerosis
Experimental autoimmune encephalomyelitis (EAE) is an experimentally induced inflammatory central nervous system demyelinating disease that mimics many aspects of multiple sclerosis (MS) (1). Hallmarks of active EAE and MS include infiltration of inflammatory cells (i.e., myelin-reactive T cells and macrophages) into the CNS and activation of resident microglia and astrocytes which promote a series of lesions that result in neurological deficits in EAE models and MS patients (2, 3).
There are currently several categories of therapy for the treatment of MS. For example, blockade of the α4β1 integrin came from the observation that entry of pathogenic T cells into the CNS depends on the action of this integrin (4). Other recent approaches depend on relatively new insights into the pathogenic mechanisms that underlie MS (5). Although some of these approaches have shown promise in clinical trials, many may exert a generalized effect on the immune response that results in immune suppression or other deleterious immune side effects.
We have recently reported that genetic disruption of the inhibitory interaction between Qa-1b/Qdm on autoreactive CD4+ T cells and the inhibitory CD94/NKG2A receptor on natural killer (NK) cells enhances lysis of pathogenic T cells and reduces disease (6). Further analysis revealed that a Qa-1b point mutation at R72 prevents binding between CD94/NKG2A receptors on NK cells and its ligand on activated CD4+ T cells. The advantage of this approach over current strategies is that it depends on derepression of lysis of a small subpopulation of autoreactive T cells, rather than a general effect on the T cell repertoire. Thus, releasing the brakes on a lytic attack by a subset of NK cells against pathogenic autoreactive T cells may result in targeted destruction of activated autoreactive T cells without additional perturbation of the T cell repertoire.
Here we derepress the lytic response of NKG2A+ NK cells (representing about 50% of total NK cells) with a F(ab′)2 fragment of the CD94/NKG2A/C/E-specific mAb 20d5 (7). This antibody predominantly targets CD94/NKG2A in C57BL/6 (B6) mice because of its 5-fold higher specificity for CD94/NKG2A than NKG2C and very low NKG2C expression on murine NK cells in this mouse strain. Administration of 20d5 F(ab′)2 Ab to B6 mice that have ongoing MOG-induced EAE promotes complete remission of disease that is associated with a decrease in Qa-1+-activated CD4+ T cells and microglia in spinal cord and increased NK lytic activity in the CNS. Application of F(ab′)2 anti-NKG2A to block CD94/NKG2A-Qa-1b/Qdm (or CD94/NKG2A-HLA-E in humans) interactions may therefore represent an effective strategy to treat ongoing MS.
Results and Discussion
We use two models of disease. The first is the relapsing–remitting EAE model of MS, which entails the use of B6 mice injected with MOG/Complete Freund’s Adjuvant (CFA) followed by two injections of pertussis toxin. This regimen reliably produces a disease course marked by exacerbation and remission for the next 40 days (8, 9). The second, adoptive transfer of MOG-specific CD4+ T cells into Rag2−/− hosts, was used to help define the development of disease in the context of the expansion of pathogenic T cells.
Anti-CD94/NKG2A F(ab′)2 Treatment Ameliorates the Disease Severity of EAE.
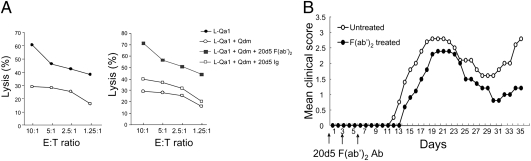
We first determined whether disruption of CD94/NKG2A-Qa-1b/Qdm interaction is effective in the attenuation of EAE in several mouse models of EAE. We observed that the CD94/NKG2A/C/E-blocking rat mAb 20d5 (IgG2a; whole-Ig-containing Fc portion) did not enhance NK lysis in vitro, in contrast to anti-NKG2A F(ab′)2, which enhanced NK lysis in vitro (Fig. 1A). For this reason, but also to avoid unwanted Fc-mediated effector functions in vivo, we used this F(ab′)2 fragment of 20d5 for these studies. Pharmacokinetic studies in mice indicated that complete receptor saturation after 20d5 F(ab′)2 injection (200 μg/mouse) persisted for 4 days (Fig. S1).
Fig. 1.
Rationale for use of the 20d5 F(ab′)2 fragment and determination of the optimal time window for Ab administration. (A) L cells infected with Qa-1 were used as target cells of IL-2–activated NK cells with or without Qdm peptide (30 μM). In some cases, cells were preincubated with 20d5 F(ab′)2 or whole Ig for 1 h at 37 °C. Percentage of lysis is shown at the indicated E:T ratios. (B) EAE was induced in C57BL/6 mice as described in Methods. 20d5 F(ab′)2 was given i.v. at days 0, 3, and 6. Development of EAE was monitored daily.
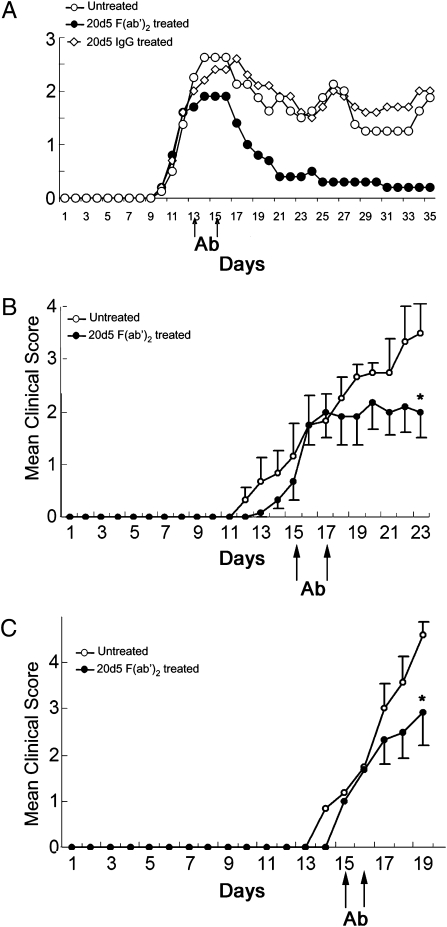
When 20d5 F(ab′)2 was used before clinical onset of symptoms in B6-EAE mice induced by immunization with MOG35–55 peptide, it did not significantly reduce disease severity (Fig. 1B). Notably, 20d5 F(ab′)2 Ab, but not IgG, administered at the peak of disease completely abolished EAE (Fig. 2A and Fig. S2), and treatment late in disease also had an ameliorative effect on this disorder (Fig. S3). 20d5 F(ab′)2 Ab treatment after adoptive transfer of preactivated MOG35–55-reactive CD4+ T cells or after transfer of 2D2 T cell receptor (TCR) transgenic CD4+ T cells into Rag2−/− hosts also reduced this very severe adoptive form of disease (Fig. 2 B and C).
Fig. 2.
Anti-NKG2A F(ab′)2 treatment ameliorates disease severity of EAE. (A) EAE was induced in C57BL/6 mice (n = 6) as described in Methods. Treatment with or without anti-NKG2A F(ab′)2 or whole 20d5 rat IgG2a at days 13 and 15 is shown. Mice were monitored daily for EAE. (B) MOG-reactive CD4+ T cells (106) were purified from EAE-experienced C57BL/6 mice and transferred into Rag2−/− hosts followed by immunization s.c with 200 μg MOG in CFA at day 1 and injection i.p. with 200 ng pertussis toxin at days 1 and 2. EAE assessment was recorded daily. 20d5 F(ab′)2 was administered at days 15 and 17. Data are shown as mean ± SEM (n = 6; *P < 0.05). (C) 2D2 transgenic CD4+ T cells (104) were transferred into Rag2−/− hosts (n = 6) and EAE was induced as described in B. 20d5 F(ab′)2 was injected at days 15 and 16(*P <0.05).
20d5 F(ab′)2 Treatment Reduces Cellular Infiltration into Spinal Cord.
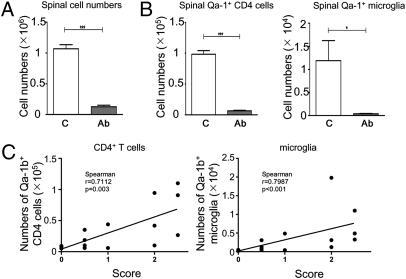
The inhibitory effects of 20d5 F(ab′)2 in different EAE models prompted us to examine the presence of leukocytes in the spinal cord in 20d5 F(ab′)2-treated and untreated mice. In the MOG35–55-induced EAE model in B6 mice, ≈10-fold fewer leukocytes were found in spinal cord of 20d5 F(ab′)2-treated mice compared with untreated mice (Fig. 3A). Treatment with 20d5 F(ab′)2 Ab decreased the numbers of activated Qa-1b-expressing microglia and CD4+ T cells in direct proportion to the reduction of total numbers of leukocytes in spinal cord (Fig. 3B). A similar reduction in leukocyte numbers was observed after blocking the CD94/NKG2A-Qa-1b interaction in the adoptive transfer models. We observed a linear correlation between reduction in 20d5 F(ab′)2-induced clinical scores and number of CD4+ T cells and microglia in spinal cord (Fig. 3C), suggesting that 20d5 F(ab′)2 treatment alleviates destructive immune responses in the CNS through a decrease in pathogenic immune cells in diseased tissues.
Fig. 3.
20d5 F(ab′)2 Ab treatment reduces cell infiltration and activation status of microglia and CD4+ T cells in spinal cord. EAE was induced in B6 mice and treated as described in Methods. (A) Mean cell counts in spinal cord were determined by hemocytometer and graphed with standard errors. (B) The absolute cell numbers of Qa-1b-expressing CD4 cells and microglia were determined by flow cytometry by gating on CD45hiCD4+ and CD45medCD11b+ populations, respectively. *P < 0.05; **P < 0.01; ***P < 0.001. (C) Correlation of Qa-1b-expressing CD4 cells and microglia to clinical score in mice was measured at the day of sacrifice.
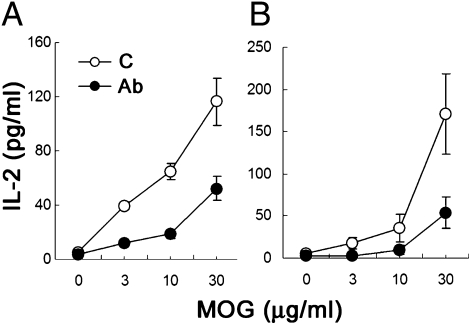
20d5 F(ab′)2 Treatment Decreases Splenic CD4+ T Cell Recall Responses.
Microglia and CD4+ T cells participate in autoimmune responses in both MS and EAE models. Unlike microglia, pathogenic CD4+ T cells originate from outside the brain. We analyzed whether 20d5 F(ab′)2 treatment affected CD4+ T cell responses in the spleen of MOG35–55-induced EAE mice. Upon restimulation, CD4+ T cells produced IL-2 in response to MOG in a dose-dependent fashion. Noticeably, CD4+ T cells from 20d5 F(ab′)2-treated EAE mice displayed more than a 50% reduction in their IL-2 response to MOG35–55 peptide (Fig. 4 A and B). This may reflect either a qualitative reduction in MOG35–55-specific splenic CD4+ T cell responses or a decrease in the number of MOG35–55-specific CD4+ T cells in the spleen after 20d5 F(ab′)2 treatment. In either case, these data suggest that 20d5 F(ab′)2 treatment directly affects antigen-specific CD4+ T cells in the spleen.
Fig. 4.
20d5 F(ab′)2 Ab treatment decreases splenic CD4+ T cell recall responses. Splenocytes from B6-EAE mice (A) or Rag2−/− mice transferred with MOG-reactive CD4 cells (B) were incubated with the indicated concentrations of MOG peptide in the presence of irradiated splenocytes from B6 mice. IL-2 secretion was measured by ELISA 48 h after culture. Data are shown as mean ± SEM (n = 4; *P < 0.05).
20d5 F(ab′)2 Treatment Alters Cytokine Profiles of CD4+ T Cells.
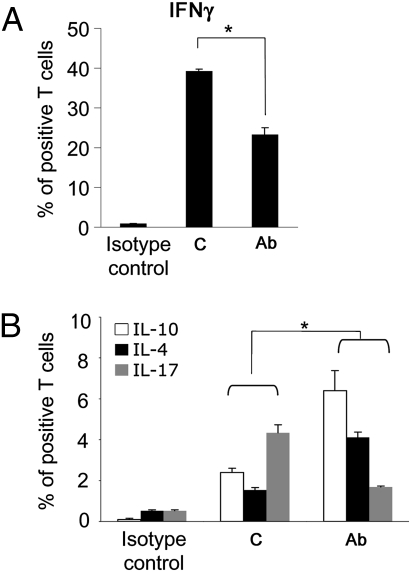
Th1 and Th17 cells may contribute to the initiation and progression of EAE, whereas Th2-type cytokines IL-4 and IL-10 may dampen autoimmune responses in EAE. CD4+ T cells isolated from spleen and draining cervical lymph nodes of treated mice secreted less IFNγ but more IL-4 compared to untreated animals upon in vitro restimulation with phorbol myristate acetate (PMA) and ionomycin. This altered cytokine profile was noted in spinal cord (Fig. 5 A and B). 20d5 F(ab′)2 treatment significantly decreased the proportion of IL-17-producing but increased the proportion of IL-10-secreting spinal CD4+ T cells (Fig. 5B), suggesting that Ab-dependent blockade of CD94/NKG2A-Qa-1b-Qdm interaction may favor inhibitory CD4+ T cell expansion and ease local inflammation.
Fig. 5.
20d5 F(ab′)2 Ab treatment alters CD4+ T cell cytokine profiles. EAE was induced in Rag2−/− hosts by adoptive transfer of MOG-reactive CD4 cells. Intracellular levels of IFNγ (A) and IL-10, IL-4, and IL-17 (B) from spinal cord were assessed by FACS analyses as described in Methods. The percent of cytokine-secreting CD4 cells is shown with standard errors (n = 3–4; *P < 0.05).
20d5 F(ab′)2 Treatment Enhanced the Numbers of Lytic NK Cells in Spinal Cord.
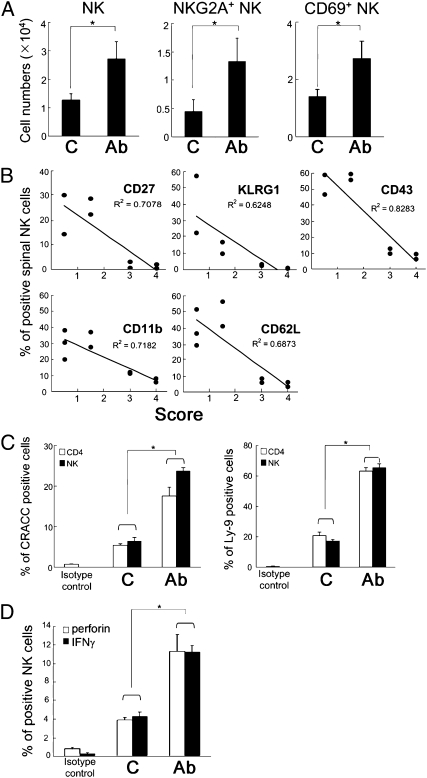
Because amelioration of EAE development by interruption of the CD94/NKG2A-Qa-1b-Qdm interaction is associated with NK-dependent elimination of pathogenic T cells (6), we determined the number and phenotype of spinal cord NK cells. 20d5 F(ab′)2-treated mice showed increased numbers of NK cells in the CNS, including CD94/NKG2A+ NK cells (Fig. 6A, left and center). In contrast to reduced autoreactive CD4+ T cells in spinal cord, there was an increase in NK cell accumulation in response to treatment with 20d5 F(ab′)2 (Fig. 6A, left). Analyses of the NK cell phenotype showed up-regulated expression of activation markers, including CD69, CD27, CD11b, CD43, and KLRG1, suggesting that 20d5 F(ab′)2 might activate NK cells (Fig. 6A, right and Fig. 6B). Moreover, low clinical disease scores correlated with increased frequency of activated NK cells, whereas high clinical scores were associated with diminished NK cell activation (Fig. 6B).
Fig. 6.
20d5 F(ab′)2 Ab treatment accumulates activated NK cells into spinal cord. (A) EAE was induced in B6 mice followed by treatment with anti-NKG2A F(ab′)2 Ab. The numbers of spinal CD69-expressing and NKG2A+ NK cells were determined by FACS analyses on CD45hiDX5+ cells (n = 3–4; *P < 0.05). (B) 2D2 CD4 cells were transferred into Rag2−/− hosts and EAE was induced as described in Fig. 2C. The levels of different activation markers on spinal cord NK cells were determined by FACS analyses on CD45hiNK1.1+ cells. The levels of NK activation markers were correlated with EAE scores at termination. (C) The percent of CRACC and Ly-9-expressing spinal cord CD4 and NK cells was determined on CD45hiCD4+ and CD45hiNK1.1+ populations, respectively. (D) EAE was induced in Rag2−/− hosts by adoptive transfer of MOG-reactive CD4 cells. Spinal cord cells were plated on anti-NK1.1-coated plates and stimulated for 8 h with BD Golgiplug (BD Biosciences) for the last 7 h. The levels of intracellular perforin and IFNγ were determined on a CD45hiDX5+ population. Data are shown as mean ± SEM (n = 4; *P < 0.05).
Increased numbers of effector cells and decreased numbers of target cells support the idea that disruption of the CD94/NKG2A-Qa-1b-Qdm interaction by 20d5 F(ab′)2 Ab promotes NK cell-mediated killing of pathogenic CD4+ T cells and microglia. Because ectopic expression of SLAM family receptors, such as CRACC and Ly-9, on B16 cells enhances NK lysis of these cells (10), we investigated the expression of ligands for SLAM family receptors on spinal cord NK and CD4+ T cells. 20d5 F(ab′)2 treatment increased the proportion of CD4+ T and NK cells expressing CRACC and Ly-9 (Fig. 6C). Moreover, spinal NK cells from mice given 20d5 F(ab′)2 Ab expressed greater amounts of intracellular perforin and IFN-γ after stimulation with anti-NK1.1 in vitro (Fig. 6D), suggesting that F(ab′)2 Ab treatment induced a boost in NK cell lytic potential.
20d5 F(ab′)2 Does Not Affect the Proportion of CD94/NKG2A-Expressing CD8+ T and Peripheral NK Cells, nor the Activation Status of Dendritic and B Cells.
Because CD94/NKG2A is expressed primarily on NK cells and a subset of CD8+ T cells (11), we asked whether 20d5 F(ab′)2 Ab might alter the proportion of CD94/NKG2A+ subsets. Unlike spinal NK cells, the numbers of peripheral CD94/NKG2A+ NK cells (spleen and liver) from the treated group remained similar to untreated cells, as did the NKG2A+CD8+ T cells (Table 1). Although surface expression of the CD94/NKG2A ligand, Qa-1b/Qdm surface protein, is restricted to activated T and B lymphocytes and dendritic cells (DC), blockade of this interaction by 20d5 F(ab′)2 Ab did not affect the numbers or activation status of DC and B cells, as indicated by surface expression of Qa-1b, MHC II (I-Ab), and CD86 (Table 2). These unaffected cell subsets were found in spleen and draining lymph nodes as well as local inflammation sites, including spinal cord and brain.
Table 1.
Absolute numbers of NKG2A-expressing CD8 and NK cells
| CD8 | NK | |||||
| Spleen | Lymph nodes | Spinal cord | Brain | Spleen | Liver | |
| Untreated | 5.9 (2.8) | 6.6 (1.2) | 0.4 (0.1) | 25.0 (9.7) | 9.8 (1.3) | 9.1 (2.4) |
| 20d5 F(ab′)2 | 7.9 (2.0) | 8.5 (0.6) | 0.06 (0.0) | 29.1 (6.6) | 5.2 (2.6) | 7.6 (4.6) |
20d5 F(ab’)2 treatment does not affect the numbers of NKG2A-expressing CD8+ T and peripheral NK cells. EAE was induced in B6 mice and treated with different sources of anti-NKG2A F(ab′)2 Ab. The percentages of NKG2A-positive CD8+ T and NK cells were determined by flow cytometry on CD45+CD8+ and CD45+DX5+ populations, respectively. Cell yields were calculated by multiplying the percentages by total cell counts. Mean cell counts (×104) are given with SEs indicated in parentheses (n = 4).
Table 2.
Activation status of B and dendritic cells
| DC | B | |||||
| Qa-1b | I-Ab | CD86 | Qa-1b | I-Ab | CD86 | |
| Untreated | 29.3 (7.6) | 123 (15.3) | 9.2 (1.4) | 41.2 (3.7) | 388.5 (20.5) | 8.2 (0.7) |
| 20d5 F(ab′)2 | 28.3 (2.1) | 117.3 (3.9) | 8.5 (0.3) | 42.8 (1.2) | 371.5 (10.5) | 8.7 (0.2) |
20d5 F(ab′)2 treatment does not influence the activation status of DC and B cells. Mean fluorescence intensity of Qa-1b, I-Ab, and CD86 on DC and B cells was determined by flow cytometry on CD45+CD11c+ and CD45+B220+ cells, respectively. Data shown represent mean values with SEs indicated in parentheses (n = 4).
In sum, we show that treatment with anti-CD94/NKG2A 20d5 F(ab′)2 Ab, associated inhibition of autoreactive T cells, and activation of NK cells may represent a useful therapy for the treatment of ongoing EAE as evidenced by diminished local inflammatory responses in spinal cord without systemic immunosuppression. This is advantageous compared with other monoclonal Ab therapies, such as anti-CD3, -CD28, or -CD40L, which can block activation receptors on T cells but often retain stimulatory activity that can lead to massive release of cytokines (12). Other antibodies aimed at removal of lymphocytes, such as anti-CD52 or anti-CD20, can cause generalized immunodeficiency and severe adverse effects (13). Furthermore, drugs for MS treatment such as Copaxone increase NK cytotoxic activity in vitro (14), whereas IFN-β-1a or anti-CD25 (Zenapax [daclizumab]) therapy may also correlate with CD56bright NK cell expansion and disease remission (15, 16). These observations further underscore the potential importance of NK cells in the regulation of MS. However, it should be noted that the period of NKG2A-based therapy may also inhibit the induction of protective immune responses in the context of, for example, vaccination, and so protective immunization of these patients should not be performed during active NKG2A therapy.
20d5 F(ab′)2 Ab treatment reduced splenic CD4+ T cell recall responses and infiltration of Qa-1b-expressing activated CD4+ T cells into spinal cord, providing support for the findings that CD94/NKG2A-Qa-1b engagement that inhibits NK activity is essential for T cell expansion and development of immunological memory (6). The ability to lyse activated CD4+ T cells through NK-mediated sensing of Qa-1b/Qdm complexes on these target cells relies on the NKG2A+ NK subset. Although the levels of NKG2A on peripheral NK cells were not affected by 20d5 F(ab′)2 Ab (Table 1), its selective targeting of NKG2A+ cells allowed mobilization of an NK-dependent regulatory mechanism. The resolution of autoimmune responses by F(ab′)2 Ab treatment may also reflect enhanced Qa-1b-restricted CD8 suppressor activity (17). As shown in Fig. 3, the increased frequency of spinal Qa-1b-expressing CD4+ T cells and microglia in mice with high clinical scores may stimulate the generation of this regulatory subset (18, 19). Elevated CD94/NKG2A receptors on CD8 Treg, as reported for relapsing MS patients (20), may also limit their regulatory capacity and lead to disease exacerbation. Indeed, interruption of the NKG2A/Qa-1b interaction by the Qa-1b R72 mutation unleashes CD8 Treg activity and abolishes disease development (17). Although antibody staining did not reveal significant expression of NKG2A-expressing CD8 cells in 20d5 F(ab′)2-treated mice (2 weeks after immunization; Table 1), further analyses using more sensitive methods, such as Qa-1b/Qdm tetramer staining, are necessary to fully evaluate their NKG2A phenotype.
It is presently unclear how 20d5 F(ab′)2 Ab decreases IFNγ- and IL-17-producing CD4 cells but spares IL-10- and IL-4-secreting CD4+ T cells. Increased NK-dependent lysis of the former subsets or creation of a favorable milieu for the latter by NK cells are two possibilities. The heterogeneity of EAE and MS reflects the action of distinct autoreactive T cell subsets, including Th1, Th17, and Th9, but not Th2 cells (21). In response to different polarizing factors they express distinct surface receptors, which may confer differential susceptibility to lysis by NK cells. In fact, our analyses of surface Qa-1b expression on in vitro differentiated Th1, Th2, and Th17 cells showed that ≈20% of Th1 and Th17 cells expressed Qa-1b whereas Qa-1b was 2-fold higher (40–50%) at the surface of Th2 cells. Increased expression of SLAM family receptors on CD4+ T cells, as shown here, may provide an alternative explanation. Data from knockout mouse models of SLAM family receptors have shown that almost every SLAM-related member promotes Th1-mediated autoimmune responses (22). In particular, Ly9 deficiency resulted in a mild Th2 defect, suggesting that SLAM family receptors may be involved in regulation of the Th1/Th17 versus Th2 cell balance (23). Homotypic interaction of SLAM receptor ligands between NK cells and target cells helps fulfill the responsibility of NK cells in immunosurveillance to eliminate unwanted cells (10). Indeed, CRACC was shown to activate NK-mediated cytotoxicity (24). Analogous to Th1 and Th2 subsets, NK cells are able to polarize into IFNγ-producing type 1 or IL-5- and IL-13-secreting type 2 NK cells (NK1/NK2). The remission of MS patients is associated with a strong bias of NK cells toward NK2 subsets (25). To what extent 20d5 F(ab′)2 Ab treatment might induce type 2 NK cells in mice remains to be investigated (25).
Blockade of the CD94/NKG2A-Qa-1b interaction inhibits transmission of inhibitory signaling into NK cells, which may lower the threshold for NK activation (26). This is supported by the finding that NK cells from 20d5 F(ab′)2-treated mice produced higher levels of intracellular IFN-γ and perforin (Fig. 6D). Furthermore, increased frequency of activated spinal NK cells correlated with lower clinical scores (Fig. 6B). Activation markers on these NK cells, including CD11b, CD27, CD43, and KLRG1, have been reported as hallmarks of mature, cytotoxic NK cells (27 –30). Moreover, synergistic coengagement of activating NK cell receptors is required to induce cytotoxic granule release (31). 20d5 F(ab′)2 Ab treatment is therefore a powerful tool to enable NK-dependent removal of unwanted cells. Increased adhesion molecule expression, such as CD62L, after 20d5 F(ab′)2 treatment (Fig. 6B) may enhance recruitment of NK cells into inflamed spinal cords. Although CD62L expression on effector cells is required for myelin damage in EAE (32), its expression on NK cells may also promote resolution of disease progression, accounting for the poor efficacy of some antibodies designed to block lymphocyte trafficking. The possibility that up-regulation of these receptors on NK cells may result from diminished inhibitory signaling remains to be investigated.
Reduced numbers of Qa-1b-expressing spinal cord CD4+ T cells and microglia that indicate amelioration of disease suggest that 20d5 F(ab′)2 Ab therapy is an effective treatment of EAE. HLA-E, the human homolog of murine Qa-1b, exhibits similar expression patterns as murine Qa-1b and its receptors on human NK cells are CD94/NKG2A (33). Although there may be potential differences in the interaction between CD94/NKG2A and Qa-1b versus HLA-E, these studies of a murine model of EAE suggest that Ab-dependent derepression of NK cells may underlie new therapeutic approaches to treatment of MS, and may also be useful in other Th1-mediated autoimmune diseases, such as rheumatoid arthritis and inflammatory bowel disease.
Methods
Mice.
C57BL/6 and B6.Rag2 −/− mice were purchased from Charles River and Taconic Laboratories, respectively. C57BL/6 2D2 TCR transgenic mice express a TCR recognizing the CNS autoantigen MOG (34). Mice were housed in a specific pathogen-free, viral Ab-free animal facility at the Dana-Farber Cancer Institute. All experiments were done in compliance with federal laws and institutional guidelines and have been approved by the Dana Farber Cancer Institute Animal Care and Use Committee.
Reagents.
The peptide MOG35–55 (MEVGWYRSPFSRVVHLYRNGK) was synthesized by New England Peptide. Anti-NKG2A 20d5 F(ab′)2 antibodies were produced from 20d5 rat IgG2a Ab by using a F(ab′)2 preparation kit (Pierce), according to the manufacturer’s instructions. Antibodies used for flow cytometry were purchased from BD Biosciences or eBioscience.
NK Cytotoxic Assay.
Purified NK cells were incubated with 1000 U/mL of hIL-2 (Peprotech) for 5 days and used as effector cells. Target cells were labeled with 50 μCi of Na2[51Cr]O4 for 1 h at 37 °C, and washed three times with PBS before mixing (1 × 104/well) with effector cells in U-bottomed 96-well plates at different E:T ratios as indicated, in triplicate. After 4 h of incubation, cell-free supernatants were collected and radioactivity was measured by Micro β counter (Wallac). Percent lysis is calculated by (sample release − spontaneous release)/(maximum release − spontaneous release) × 100.
EAE Induction and Assessment.
To induce EAE in C57BL/6 mice, mice were immunized s.c. with 200 μg of MOG 35–55 emulsified in CFA (supplemented with 4 mg/mL of Mycobacterium tuberculosis) and injected i.p. on days 0 and 2 with 200 ng pertussis toxin to induce EAE, as described (6). Purified CD4 cells from EAE-experienced B6 mice were used as a source for MOG-reactive CD4 transfer into Rag2−/− hosts. Purified 2D2 CD4 cells were transferred into Rag2−/− hosts to establish the EAE model followed by immunization as described above. Clinical assessment of EAE was performed daily and scoring was as follows: 0, no disease; 1, decreased tail tone; 2, hind limb weakness or partial paralysis; 3, complete hind limb paralysis; 4, front and hind limb paralysis; 5, moribund state.
Preparation of Cell Suspensions.
Spleen and draining cervical lymph nodes were excised and cell suspensions were prepared as previously described. To isolate mononuclear cells from spinal cord and brain, neural tissues were digested in collagenase/dispase (Roche) for 30 min at 37 °C and then separated on a 30%/70% of percoll gradient by centrifuging at 500 × g for 20 min. Cells at the 30%:70% interface were collected for further analyses.
Flow Cytometry.
Single-cell suspensions were incubated with Fc block for 15 min followed by staining with various antibodies against surface markers. To compare CD94/NKG2A levels between untreated and treated groups, cells were first incubated with 20d5 F(ab′)2 Ab (1 μg/mL) for 30 min to saturate surface CD94/NKG2A and further stained with FITC- or PE-anti-rat Ig, κ light-chain monoclonal Ab (clone MRK-1) (BD Pharmingen). For intracellular staining, cells were restimulated with leukocyte activation mixture (BD Biosciences) for 5 h, stained with surface markers, fixed, and permeabilized followed by incubation with cytokine antibodies. All experiments were performed on a FACSCalibur and analyzed by FlowJo software.
In Vitro Recall Response.
Splenocytes were isolated from immunized mice and incubated with different concentrations of MOG peptide and irradiated splenocytes from B6 mice in RPMI medium 1640 supplemented with 10% FCS and 50 μM β-mercaptoethanol. Culture supernatants were collected after 48 h of culture, and cytokine concentrations in supernatants were determined by ELISA kit (BD Pharmingen).
Statistical Analyses.
Statistical analysis was obtained using GraphPad Prism software. Values are indicated as mean ± SEM.
Supplementary Material
Acknowledgments
This work was supported in part by NIH Research Grant AI 037562 and a gift from The Leroy and Shoshana Schecter Research Foundation to H.C., and a Collaborative Sponsored Research Agreement with Novo Nordisk and T32 CA070083 to J.W.L.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914732107/DCSupplemental.
References
- 1.Lublin FD. Adoptive transfer of murine relapsing experimental allergic encephalomyelitis. Ann Neurol. 1985;17:188–190. doi: 10.1002/ana.410170214. [DOI] [PubMed] [Google Scholar]
- 2.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 3.Hafler DA. Multiple sclerosis. J Clin Invest. 2004;113:788–794. doi: 10.1172/JCI21357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yednock TA, et al. Prevention of experimental autoimmune encephalomyelitis by antibodies against α 4 β 1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 5.Han MH, et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature. 2008;451:1076–1081. doi: 10.1038/nature06559. [DOI] [PubMed] [Google Scholar]
- 6.Lu L, et al. Regulation of activated CD4+ T cells by NK cells via the Qa-1–NKG2A inhibitory pathway. Immunity. 2007;26:593–604. doi: 10.1016/j.immuni.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vance RE, Kraft JR, Altman JD, Jensen PE, Raulet DH. Mouse CD94/NKG2A is a natural killer cell receptor for the nonclassical major histocompatibility complex (MHC) class I molecule Qa-1b . J Exp Med. 1998;188:1841–1847. doi: 10.1084/jem.188.10.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinman L. Multiple sclerosis: A two-stage disease. Nat Immunol. 2001;2:762–764. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- 9.Steinman L, Martin R, Bernard C, Conlon P, Oksenberg JR. Multiple sclerosis: Deeper understanding of its pathogenesis reveals new targets for therapy. Annu Rev Neurosci. 2002;25:491–505. doi: 10.1146/annurev.neuro.25.112701.142913. [DOI] [PubMed] [Google Scholar]
- 10.Dong Z, et al. Essential function for SAP family adaptors in the surveillance of hematopoietic cells by natural killer cells. Nat Immunol. 2009;10:973–980. doi: 10.1038/ni.1763. [DOI] [PubMed] [Google Scholar]
- 11.McMahon CW, Raulet DH. Expression and function of NK cell receptors in CD8+ T cells. Curr Opin Immunol. 2001;13:465–470. doi: 10.1016/s0952-7915(00)00242-9. [DOI] [PubMed] [Google Scholar]
- 12.Suntharalingam G, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 13.Major EO. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med. 2010;61:35–47. doi: 10.1146/annurev.med.080708.082655. [DOI] [PubMed] [Google Scholar]
- 14.Sand KL, Knudsen E, Rolin J, Al-Falahi Y, Maghazachi AA. Modulation of natural killer cell cytotoxicity and cytokine release by the drug glatiramer acetate. Cell Mol Life Sci. 2009;66:1446–1456. doi: 10.1007/s00018-009-8726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bielekova B, et al. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Rα-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci USA. 2006;103:5941–5946. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandenbark AA, et al. Interferon-β-1a treatment increases CD56bright natural killer cells and CD4+CD25+ Foxp3 expression in subjects with multiple sclerosis. J Neuroimmunol. 2009;215:125–128. doi: 10.1016/j.jneuroim.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Lu L, Kim HJ, Werneck MB, Cantor H. Regulation of CD8+ regulatory T cells: Interruption of the NKG2A-Qa-1 interaction allows robust suppressive activity and resolution of autoimmune disease. Proc Natl Acad Sci USA. 2008;105:19420–19425. doi: 10.1073/pnas.0810383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang H, Chess L. An integrated view of suppressor T cell subsets in immunoregulation. J Clin Invest. 2004;114:1198–1208. doi: 10.1172/JCI23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu D, et al. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5:516–523. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 20.Correale J, Villa A. Isolation and characterization of CD8+ regulatory T cells in multiple sclerosis. J Neuroimmunol. 2008;195:121–134. doi: 10.1016/j.jneuroim.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Jäger A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan AY, Westcott JM, Mooney JM, Wakeland EK, Schatzle JD. The role of SAP and the SLAM family in autoimmunity. Curr Opin Immunol. 2006;18:656–664. doi: 10.1016/j.coi.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Graham DB, et al. Ly9 (CD229)-deficient mice exhibit T cell defects yet do not share several phenotypic characteristics associated with SLAM- and SAP-deficient mice. J Immunol. 2006;176:291–300. doi: 10.4049/jimmunol.176.1.291. [DOI] [PubMed] [Google Scholar]
- 24.Bouchon A, Cella M, Grierson HL, Cohen JI, Colonna M. Activation of NK cell-mediated cytotoxicity by a SAP-independent receptor of the CD2 family. J Immunol. 2001;167:5517–5521. doi: 10.4049/jimmunol.167.10.5517. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi K, et al. Natural killer type 2 bias in remission of multiple sclerosis. J Clin Invest. 2001;107:R23–R29. doi: 10.1172/JCI11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watzl C, Stebbins CC, Long EO. NK cell inhibitory receptors prevent tyrosine phosphorylation of the activation receptor 2B4 (CD244) J Immunol. 2000;165:3545–3548. doi: 10.4049/jimmunol.165.7.3545. [DOI] [PubMed] [Google Scholar]
- 27.Nieto M, et al. Signaling through CD43 induces natural killer cell activation, chemokine release, and PYK-2 activation. Blood. 1999;94:2767–2777. [PubMed] [Google Scholar]
- 28.Takeda K, et al. CD27-mediated activation of murine NK cells. J Immunol. 2000;164:1741–1745. doi: 10.4049/jimmunol.164.4.1741. [DOI] [PubMed] [Google Scholar]
- 29.Kim S, et al. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3:523–528. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 30.Huntington ND, et al. NK cell maturation and peripheral homeostasis is associated with KLRG1 up-regulation. J Immunol. 2007;178:4764–4770. doi: 10.4049/jimmunol.178.8.4764. [DOI] [PubMed] [Google Scholar]
- 31.Bryceson YT, Ljunggren HG, Long EO. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood. 2009;114:2657–2666. doi: 10.1182/blood-2009-01-201632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grewal IS, et al. CD62L is required on effector cells for local interactions in the CNS to cause myelin damage in experimental allergic encephalomyelitis. Immunity. 2001;14:291–302. doi: 10.1016/s1074-7613(01)00110-8. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan LC, et al. The heterodimeric assembly of the CD94-NKG2 receptor family and implications for human leukocyte antigen-E recognition. Immunity. 2007;27:900–911. doi: 10.1016/j.immuni.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Bettelli E, et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.