Abstract
Symplekin is a ubiquitously expressed protein involved in cytoplasmic RNA polyadenylation and transcriptional regulation and is localized at tight junctions (TJs) in epithelial cells. Nuclear symplekin cooperates with the Y-box transcription factor zonula occludens 1-associated nucleic acid-binding protein (ZONAB) to increase the transcription of cell cycle-related genes and also inhibits differentiation of intestinal cells. We detected high levels of nuclear symplekin in 8 of 12 human colorectal cancer (CRC) samples. shRNA-mediated reduction of symplekin expression was sufficient to decrease significantly the anchorage-independent growth and proliferation of HT-29 CRC cells as well as their tumorigenicity when injected into immunodeficient animals. Symplekin down-regulation also was found to alter ion transport through TJs, to promote the localization of ZONAB in the membrane rather than the nucleus, and strongly to enhance cell polarization in a 3D matrix, leading to the formation of spheroids organized around a central lumen. Claudin-2 expression was reduced following symplekin down-regulation, an effect mimicked when ZONAB expression was down-regulated using selective siRNA. Virus-mediated restoration of claudin-2 expression was found to restore nuclear expression of ZONAB in HT29ΔSym cells and to rescue the phenotypic alterations induced by symplekin down-regulation of cell polarity, paracellular transport, ZONAB localization, cyclin D1 expression, proliferation, and anchorage-independent growth. Finally, siRNA-mediated claudin-2 down-regulation increased the transepithelial resistance and decreased cyclin D1 expression and ZONAB nuclear localization, similar to observations in symplekin-depleted cells. Our results suggest that nuclear overexpression of symplekin promotes tumorigenesis in the human colon and that the regulation of claudin-2 expression is instrumental in this effect.
Keywords: polarity, transcription, tumorigenesis, colorectal, tight junctions
The role of β-catenin in maintaining the stem/proliferative cell compartment at the bottom of the intestinal crypts of Lieberkühn has been well described (1). Similarly, tight-junction (TJ) complexes are emerging as active players in the regulation of cellular proliferation, differentiation, and apoptosis (2), and recent results suggest that alteration of their function is also involved in tumorigenesis (3, 4).
Among the proteins detected at the TJ, the zonula occludens 1-associated nucleic acid-binding protein/zonula occludens protein 1 (ZONAB/ZO-1) complex was identified as a regulator of cell proliferation. In highly confluent epithelial cells, ZO-1 is located exclusively at the TJ and sequesters the Y-box transcriptional regulator ZONAB/decorin-binding protein A (DbpA) at the cytoplasmic plaque. However, under various physiological or pathological conditions, this complex can be dissociated, resulting in the accumulation of ZONAB/DbpA in the nucleus, where it promotes cell proliferation (5). In kidney and intestinal cells this function relies in part on the capacity of ZONAB/DbpA to interact in the nucleus with symplekin (6).
Like ZONAB/DbpA, symplekin belongs to a family of proteins that localize in two subcellular compartments, the nucleus and the cytoskeletal plaques of TJ (7). It is involved in the nuclear pre-mRNA cleavage and polyadenylation machinery (8 –11), and we and others recently reported a direct interaction of symplekin with two transcription factors, ZONAB/DbpA and heat shock factor protein 1 (HSF1) (6, 12). The functional interaction between symplekin and ZONAB/DbpA promotes proliferation (6) and acts as a negative modulator of goblet-cell differentiation in the intestine (13). However, despite its partial TJ localization, nothing currently is known about a potential role of symplekin in cell polarization. In addition, although ZONAB/DbpA overexpression has been reported in various carcinomas (14, 15), the expression pattern of symplekin in these tumors is not documented.
The objectives of the present study were to analyze the expression pattern of symplekin in human colorectal tumors, to assess whether it is involved in regulating tumorigenicity in intestinal cells, and to investigate the mechanisms underlying these effects.
Results
Nuclear Symplekin Is Strongly Expressed in Human Colorectal Carcinoma.
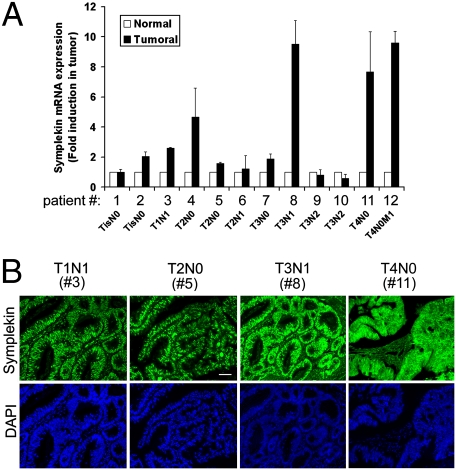
Symplekin expression in human colorectal cancer (CRC) was analyzed in the SAGE Genie database (16), which indicated that symplekin mRNA identification tags are strongly overexpressed in human tumors from the colon, lung, muscle, and prostate, compared with healthy tissue (http://cgap.nci.nih.gov/SAGE). Using RT-qPCR, we found that the symplekin mRNA was overexpressed in ∼67% (8/12) CRC tumor samples compared with matching healthy tissue, without any obvious correlation with tumor stage (Fig. 1A). Immunostaining performed in eight of these samples showed a strong nuclear expression of symplekin in all of them (Fig. 1B). Concomitant cytoplasmic staining was detected in stage IV samples, whereas membrane staining was not found in any of the tumors tested. In contrast, nuclear staining for symplekin was restricted largely to the bottom half of colonic crypts in the macroscopically healthy epithelium, as described in ref. 13 and shown in Fig. S1.
Fig. 1.
Symplekin expression in human CRC. (A) Symplekin mRNA expression in tumor samples (black bars) from 12 patients with CRC, relative to the level of expression in their respective healthy epithelium (white bars), which was normalized to 1 for each patient. Values are means ± SEM (n = 3) after correction for total RNA loading and for the presence of stroma using GAPDH or vimentin and smooth muscle actin mRNA expression, respectively. The patient identification number and TNM staging of tumors are indicated below the horizontal axis. (B) Immunofluorescence (IF) detection of symplekin in four human CRC samples, representative of eight similar samples. Patient numbers correspond to samples analyzed in A. (Scale bar, 25 μm.)
Symplekin Down-Regulation Reduces Tumor Growth in Vitro and in Vivo.
To analyze the role of symplekin on CRC cell tumorigenicity, we used HT-29Cl.16E cells, which proliferate steadily, polarize when grown at high confluence (17), and generate aggressive tumors following xenotransplantation into nude mice (18). As described before (6), symplekin expression strongly decreased after tetracycline (tet) induction in clones expressing independent shRNAs directed against the symplekin mRNA (ΔSymA and ΔSymB) (Fig. S2).
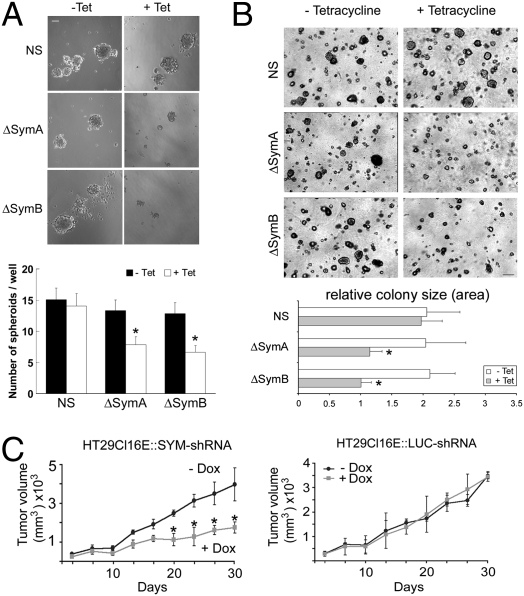
To determine whether symplekin down-regulation affects anchorage-independent growth, we quantified the capacity of ΔSym clones to grow as spheroid bodies in suspension. Both number and size of spheroid bodies were reduced in ΔSym clones as compared with control cells (Fig. 2A), indicating that symplekin down-regulation impaired the capacity of these tumor cells to grow in the absence of an extracellular matrix. Similar results were obtained in a soft agar model (Fig. 2B). The ability of symplekin-depleted cells to form xenografts was quantified following injection into BALB/c nude mice. Thirty days after injection, the volume of tumors derived from ΔSym cells was 50–55% smaller than that of tumors induced by control cells and of tumors developed in mice without doxycycline (Fig. 2C). Thus, symplekin down-regulation decreases the tumorigenicity of HT-29 cells.
Fig. 2.
Symplekin depletion reduces the tumorigenicity of HT29-Cl.16E CRC cells in vitro and in vivo. (A) (Top) Representative photograph demonstrating the formation of spheroids by symplekin-depleted (ΔSymA and ΔSymB) and control clones (NS) in suspension after 10 days with or without tet. (Scale bar, 50 μm.) (Bottom) Number of spheroids per well, expressed as the mean ± SEM obtained from 24 wells per sample. *, P < 0.05 compared with NS control cells (n = 3). ( B) (Top) Growth of ΔSymA, ΔSymB, and NS clones in soft agar in the presence or the absence of tet. (Scale bar, 300 μm.) (Bottom) Relative colony size, expressed as the mean ± SEM; *, P < 0.05 compared with NS control cells (n = 3). (C) Tumor size was checked regularly for 30 days after injection of HT29Cl16E::SYM-shRNA (Left) or of HT29Cl16E::LUC-shRNA (Right) in BALB/c nude mice. Animals were kept without (− Dox) or with (+ Dox) doxycycline in the drinking water. Results represent mean ± SEM of six mice per group. *, P < 0.05 compared with noninduced HT29Cl16E::SYM-shRNA and with HT29Cl16E::LUC-shRNA cells.
Symplekin Modulates Cell Architecture and TJ Physiology in HT-29Cl.16E Cell Monolayers.
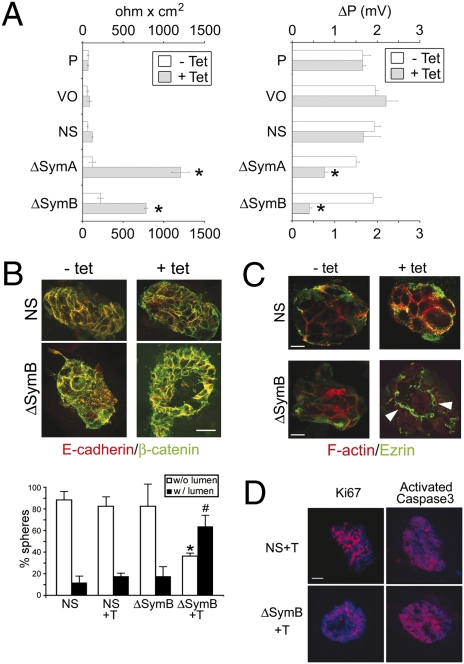
We then asked whether phenotypic modifications other than proliferation were responsible for the reduced tumorigenicity of HT-29Cl.16E/ΔSym cells. Using transmission electron microscopy, we found that confluent monolayers of ΔSym cells were much more compact and displayed smaller intercellular spaces than control cells. In addition, although superposition or cytoplasmic projections of cells above one other were often seen in controls, these occurrences were very rare after symplekin down-regulation, suggesting an increased polarization of symplekin-depleted cells (Fig. S2). Because HT29-Cl.16E cells are known to polarize spontaneously (19) when confluent and to form adherens junctions and TJs (6), transepithelial resistance (TER) measurements were performed to corroborate this result. Maximal TER values, obtained 14 days after the cells reached confluence, were strongly elevated following symplekin down-regulation (Fig. 3A), and a significant decrease in the dilution potential also was observed after symplekin depletion, reflecting a defective passage of positively charged ions (Fig. 3A).
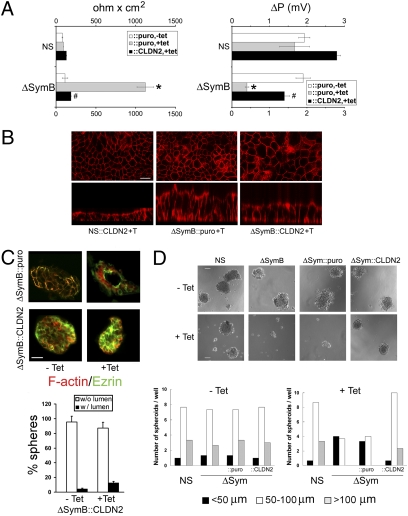
Fig. 3.
Symplekin down-regulation alters TJ physiology and increases CRC cell polarization. (A) (Left) Mean ± SEM of maximal TER values in control [parental (P), empty vector (VO), and NS (control shRNA)], ΔSymA, and ΔSymB clones grown on Transwell filters for 14 days at confluence with (gray bars) or without (white bars) tet (n = 4). Right, dilution potential (ΔP) in P, VO, NS, ΔSymA, and ΔSymB clones was measured once they reached maximal TER. Values represent mean ± SEM; n = 3. *, P < 0.05 with control cells, Student t test. (B) (Top) IF staining for E-cadherin (red) and β-catenin (green) on confocal sections generated from 3D cultures of ΔSymB and control cells grown in Matrigel for 14 days with or without tet. (Scale bar, 50 μm.) (Bottom) Pictures were taken from 25 different areas, and the number of spherical structures with (w/lumen) or without a lumen (w/o lumen) was quantified. P < 0.05 compared with percentages of spheres without (*) or with (#) a lumen (n = 3, Student t test). Similar results were obtained for clone ΔSymA. (C) Cells grown as in A were stained for F-actin (red) or ezrin (green). White arrowheads indicate the apical membrane localization of ezrin in ΔSymB + tet cells. (D) Proliferation and apoptosis were analyzed in control and ΔSymB cells grown as in A, using Ki67 or activated caspase 3 staining. Nuclei were stained with DAPI. (Scale bars, 10 μm.)
These results indicate that symplekin down-regulation induces morphological changes that promote the formation of cohesive and organized cell–cell contacts, inducing significant alterations of TJ charge-selectivity in HT29-Cl.16E cells.
Symplekin Down-Regulation Increases the Polarization and Decreases the Proliferation of CRC Cells in 3D Cultures.
To determine whether the regulation of TJ function by symplekin is concomitant with an effect on polarization, 3D cultures were prepared by growing HT29-Cl.16E cells into a Matrigel layer. Symplekin-depleted cells preferentially grew into organized spheroids containing a lumen, whereas control cells almost exclusively formed cell masses without a lumen (Fig. 3B). In addition, although ezrin localization was diffuse in controls, it was restricted to the apical membrane around the lumen of spheres grown from symplekin-depleted cells, reflecting their increased polarization (Fig. 3C). Finally, proliferative cells were distributed evenly throughout control spheroids, whereas proliferation was restricted to a smaller fraction of cells following symplekin down-regulation (Fig. 3D and Fig. S2). Apoptosis was low in all samples but increased slightly following symplekin down-regulation, as assessed via activated caspase 3 detection (Fig. 3D and Fig. S2).
Thus, symplekin down-regulation, induced by an increased cellular polarization combined with a reduction in proliferation, leads to a reorganization of CRC cancer cells.
CLDN2 Gene Expression Is Decreased in Symplekin-Depleted Cells.
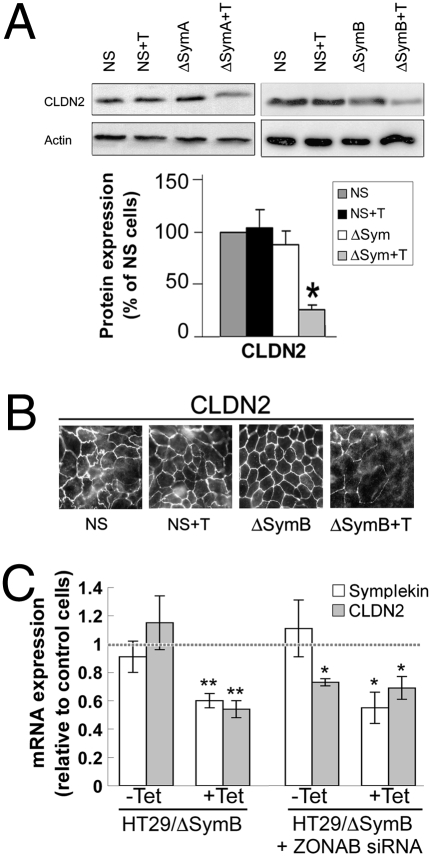
The claudin family of TJ proteins is essential to regulate paracellular ion transport (20, 21) and epithelial cell polarity (22). Because both events are altered following symplekin down-regulation, we analyzed whether the expression levels or localization of claudin isoforms are altered in ΔSym cells. Among the isoforms expressed by HT-29Cl.16E cells, symplekin depletion strongly down-regulates the expression of claudin-2 (Fig. 4 and Fig. S3), while increasing claudin-15 mRNA expression (Fig. S3). mRNA levels for claudin-1, -3, -4, and -7, as well as for weakly expressed claudin-9, -14, -17, and -22, are not significantly affected by symplekin down-regulation. In addition, claudin-2 mRNA expression displays a strong correlation trend with symplekin expression in a panel of 12 CRC samples (Spearman’s correlation coefficient r = 0.52, P = 0.08) (Fig. S3).
Fig. 4.
Symplekin down-regulation modifies the pattern of claudin expression in CRC cells. (A) (Top) Claudin-2 and actin expression in ΔSymA, ΔSymB, and NS clones grown with or without tet for 10 days at confluence. (Bottom) Quantification of claudin-2 expression in clones expressing control or symplekin-specific shRNA. Results are a percentage of the average expression in two untreated NS clones (dark gray bars). Results are mean ± SEM, n = 3. *, P < 0.05 compared with control cells, Student t test. (B) Confocal images of control (NS) or HT29-Cl.16E ΔSymB cells treated or not treated with tet and stained for CLDN2. (Scale bar, 20 μm.) (C) CLDN2 mRNA expression in HT29-Cl.16E ΔSymB clones grown with or without tet and transfected or not with ZONAB-selective siRNA. Results are expressed relative to the expression levels in NS and NS + tet cells, with or without luciferase siRNA, as represented by the dashed line. (*, P < 0.05; **, P < 0.01 compared with control cells; n = 3).
This transcriptional down-regulation of claudin-2 expression in ΔSym cells (Fig. 4C and Fig. S3) was confirmed at protein level using Western blotting (Fig. 4A) and immunofluorescent staining (Fig. 4B).
We then determined whether the previously described cooperation between symplekin and ZONAB/DbpA in the regulation of transcription extendsd to the regulation of claudin-2 expression. Indeed, CLDN2 mRNA levels also are decreased in cells transiently transfected with ZONAB/DbpA-selective (6) siRNA, and these siRNA do not further decrease CLDN2 mRNA expression in cells where symplekin is already down-regulated (Fig. 4C), suggesting that these two proteins cooperate to regulate CLDN2 expression.
The down-regulation of claudin-2 mRNA in symplekin-depleted cells and the increase of TER and the decrease of the symplekin/ZONAB target cyclin D1 (6) are reversed following transfection with a large amount of human symplekin construct, corroborating the specificity of these effects (Fig. S4).
Claudin-2 Is Instrumental for Symplekin’s Effects on TJ Function, Cell Polarity, and Anchorage-Independent Growth.
Our results show that symplekin drives, at least in part, claudin-2 expression in HT29 CRC cells. The strong nuclear expression of symplekin in CRC cells thus could be involved in claudin-2 overexpression in CRC (23), and this overexpression recently was shown to increase the invasive potential of cancer cells (24). We therefore analyzed whether claudin-2 is involved in the biological effects of symplekin on tumorigenicity. We used infection with claudin-2-expressing retroviral constructs to restore claudin-2 expression in symplekin-depleted cells (ΔSymB::CLDN2) and compared these cells with ΔSym cells transduced with control retrovirus (ΔSymB::puro) (Fig. S5).
Claudin-2 reexpression in ΔSym::CLDN2 cells reverses both the increase in TER and the reduction of dilution potential induced by symplekin depletion, whereas claudin-2 overexpression in control clones slightly increases the dilution potential without affecting TER, in agreement with the known positive effect of claudin-2 on TJ selectivity for positively charged ions (Fig. 5A) (25, 26). In addition, phalloidin staining of the actin cytoskeleton in confluent HT29-Cl.16E cells (Fig. 5B) shows that the distance between basal and apical membranes is increased in ΔSym clones, but claudin-2 reexpression largely prevents this increase (Fig. 5B). Furthermore, claudin-2–transduced cells loose their capacity to organize around a central lumen in 3D Matrigel cultures and to display a polarized expression of apical markers such as ezrin (Fig. 5C). These results demonstrate that modulation of claudin-2 expression is essential for the effects of symplekin on cell polarity. In addition, claudin-2 reexpression in ΔSym::CLDN2 cells restores the ability of HT-29 cells to form large spheroids when grown in suspension, as well as the proliferative phenotype within these spheres (Fig. 5D and Fig. S5).
Fig. 5.
Restoration of claudin-2 reverses the phenotype induced by symplekin down-regulation in CRC cells. (A) TER (Left) and dilution potential (Right) were measured as described in Fig. 3A in NS and ΔSymB cells transduced with a retroviral construct expressing claudin-2 (::CLDN2) or a control empty vector (::puro). Results are mean ± SEM. n = 3, P < 0.05 compared with control cells (*) or with symplekin-depleted cells (#); Student t test. (B) xy- (Top) and xz- (Bottom) confocal sections were collected from TRITC-phalloidin–stained NS::CLDN2, ΔSymB::puro, and ΔSymB::CLDN2 cells grown with tet. (Scale bar, 50 μm.) (C) (Top) IF detection of F-actin (red) and ezrin (green) in ΔSymB::CLDN2 and ΔSymB::puro cells grown in the presence or absence of tet. (Scale bar 10 μm.) (Bottom) Quantification of spheres with (w/lumen) or without (w/o lumen) a lumen, as described in Fig. 3B. (D) (Top) Growth in suspension of NS, ΔSymB, ΔSym::puro, and ΔSym::CLDN2 cells after 10 days in serum-free medium. (Scale bar, 50 μm.) (Bottom) Quantification of spheroids of increasing size in the absence (Left) or the presence (Right) of tet.
Similar to the effect of symplekin down-regulation, siRNA-mediated claudin-2 down-regulation decreases the expression of cyclin D1 mRNA and increases the transepithelial resistance in HT-29Cl.16E control cell monolayers (Fig. S6). The effects of symplekin and claudin-2 down-regulation on cyclin D1 expression and TER are not additive, arguing in favor of both proteins being part of the same pathway (Fig. S6). Accordingly, we found that claudin-2 overexpression (ΔSym::CLDN2 cells without tet) increases cyclin D1 levels and subconfluent cell proliferation in 2D, in comparison with ΔSym::puro cells. Once cells are highly confluent, claudin-2 overexpression no longer has any effect on proliferation but still reverses the inhibition induced by symplekin down-regulation (Fig. S6), thus mimicking the results obtained in 3D.
Finally, to examine the effects of symplekin on cell polarization and proliferation in the absence of claudin-2, siRNA-mediated symplekin down-regulation was performed in SW480 CRC cells, in which the expression of the CLDN2 gene appears to be turned off (Fig. S7). In these cells, cyclin D1 expression, cell polarization, proliferation, and TER are not significantly affected by symplekin down-regulation (Fig. S7).
Taken together, these results indicate that claudin-2 is an essential mediator in the effects of symplekin in promoting proliferation and anchorage-independent growth and that it is instrumental for symplekin-mediated modulation of TJ function and cell polarization.
Claudin-2 Expression Modulates ZONAB Localization in HT-29Cl.16E CRC Cells.
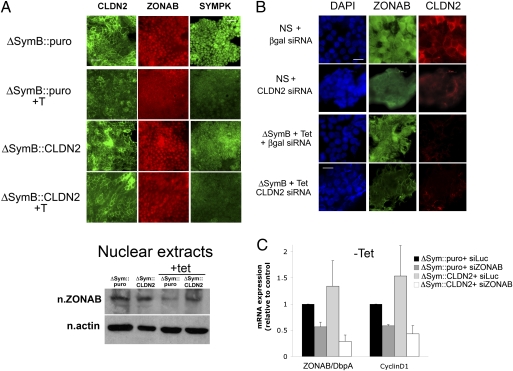
Finally, we determined that symplekin levels are similar in tet-induced ΔSym, ΔSym::puro, and ΔSym::CLDN2, indicating that the phenotypic rescue induced by CLDN2 reexpression in symplekin-depleted cells is not caused by a positive feedback on symplekin levels (Fig. S5). Because we previously had shown that symplekin and ZONAB form a transcriptionally active complex within the nucleus, we also analyzed ZONAB localization in ΔSym::CLDN2 cells and found that the nuclear localization of ZONAB is largely reinstated when normal CLDN2 expression is restored despite the down-regulation of symplekin (Fig. 6A). Similarly, transfection of claudin-2–selective siRNA in control HT-29Cl.16E cells induces a partial shift of ZONAB away from the nucleus (Fig. 6B) but does not further modify ZONAB localization in symplekin-depleted cells, strongly suggesting that the decreased claudin-2 expression induced by symplekin down-regulation is involved in the alteration of ZONAB localization. Finally, claudin-2 overexpression is not able to rescue the decrease in cyclin D1 expression induced by transient down-regulation of ZONAB (Fig. 6C), corroborating our hypothesis that the latter is essential for claudin-2-mediated regulation of cyclin D1.
Fig. 6.
Reduction of nuclear ZONAB expression following CLDN2 reexpression in HT29-Cl.16E ΔSym cells. (A) (Top) Localization of claudin-2, ZONAB/DbpA, and symplekin in ΔSymB::CLDN2 and ΔSymB::puro cells grown without or with tet. (Scale bar, 50 μm.) (Bottom) Western blot representing the expression of ZONAB in nuclear extracts from ΔSymB::CLDN2 and ΔSymB::puro cells, induced (+ tet) or not induced with tet. Nuclear actin was used as a loading control. (B) Localization of claudin-2 and ZONAB/DbpA in NS and ΔSymB + tet cells, transfected with β-galactosidase or CLDN2-selective siRNA. Nuclei are visualized with DAPI. (Scale bar, 20 μm.) (C) RT-qPCR quantification of mRNA for ZONAB and cyclin D1 in noninduced ΔSymB::CLDN2 and ΔSymB::puro cells, transfected or not (− tet) for 48 h with ZONAB/DbpA-selective siRNA. Results are expressed relative to ΔSymB::puro cells transfected with siRNA against Luciferase.
These data suggest the existence of a positive feedback loop in which high levels of claudin-2 expression, maintained in part by the activity of the symplekin/ZONAB complex, promote in turn the localization of ZONAB in the nucleus, where it is transcriptionally active (Fig. S8).
Discussion
In this study, we detected a high level of symplekin expression in the nucleus of human CRC cells, similar to that recently detected in the proliferative compartment at the bottom of healthy colonic crypts (13). We also demonstrated that symplekin down-regulation in CRC cells reduces their tumorigenicity in vitro and in vivo. This effect probably is related to the role of symplekin as a transcriptional cofactor in the nucleus, where it modulates the expression of genes involved in proliferation and stress responses in vitro (6, 12) and inhibits intestinal differentiation in vivo (13). In addition, symplekin down-regulation promotes polarization in CRC cells, strongly suggesting that symplekin overexpression in these cells also plays an active part in the disruption of tissue homeostasis by disrupting epithelial cell organization, resulting in a promotion of tumor progression.
These effects of symplekin down-regulation on CRC cell polarization and tumorigenicity involve a transcriptional inhibition of the gene encoding the transmembrane TJ protein claudin-2. This protein is known to convert TJ from a tight to a leaky strand phenotype (20), and its expression is regulated by cingulin, another TJ cytoplasmic plaque protein that modulates proliferation in Madin-Darby canine kidney cells (27). The transcriptional regulation of CLDN2 by nuclear symplekin provides a potential explanation for the similarities of their expression pattern in human CRC, where claudin-2 also is overexpressed (23), and in healthy human and rodent colonic epithelia, where claudin-2 expression is restricted to the bottom section of the Lieberkühn crypts (28 –30). In addition, because de novo experimental expression of claudin-2 restores the proliferation and anchorage-independent potential of cells with down-regulated symplekin, we conclude that claudin-2 is an essential mediator in the tumor-promoting role of symplekin. A tumor-promoting role for claudin-2 was suggested recently in adenocarcinoma gastric carcinoma cells (24). Overexpression of this protein increases cell invasion but not proliferation in these cells, a discrepancy that could be caused by the difference in tumor type or in the density of cell populations used. Because claudin-2 overexpression prevents the relocalization of ZONAB away from the nucleus upon symplekin down-regulation, and because it cannot restore elevated cyclin D1 levels following ZONAB down-regulation, we conclude that, in CRC cells, the role of claudin-2 is related to its ability to alter the recruitment of ZONAB by TJ complexes, thus facilitating the transcriptional activity of this factor within the nucleus.
Finally, we found that siRNA-mediated down-regulation of ZONAB/DbpA triggers a regulation of CLDN2 expression similar to that induced by symplekin depletion. The effects of ZONAB siRNA and symplekin shRNA are not additive, suggesting that the regulation of CLDN2 expression by symplekin depends on its functional interaction with ZONAB. It is noteworthy that ZONAB/DbpA is strongly overexpressed in pancreatic carcinoma and hepatocellular carcinomas (14, 15), whereas symplekin expression is slightly decreased in the latter (31). In tumors such mutually exclusive alterations are found frequently for genes/proteins that are involved in a similar signaling pathway (32, 33), suggesting that overexpression of symplekin or ZONAB could have similar consequences for tumorigenesis. Although E2F1-mediated transcriptional stimulation (34), as well as the presence of mutations and polymorphism in the ZONAB/DbpA promoter (35), has been suggested as a cause for the increased transcriptional activity detected in hepatocarcinoma, the reasons underlying symplekin overexpression remain to be investigated.
In conclusion, this study suggests that nuclear overexpression of symplekin cooperates with ZONAB/Dbpa to promote the transcription of claudin-2 in CRC cells, resulting in increased proliferation, a partial loss of polarization, and in the promotion of tumorigenesis (Fig. S8).
Materials and Methods
Further information about materials and methods is provided in SI Materials and Methods.
Cell Culture.
Control and ΔSym HT29-Cl.16E clones are described in ref. 6, and 3D culture in Matrigel was performed as in ref. 36. Suspension cultures were performed by seeding 5 × 103 cells/well into 96-well ultra-low attachment microplates (Corning), in DMEM (InVitrogen) + 1% FCS (Eurobio), with or without tet (1 μg/mL). For the soft agar colony assay, 1 × 105 cells were seeded into 60-mm cell culture dishes in 2 mL DMEM + 10% FBS + 0.33% agar (Sigma), on top of a 4-mL layer of 0.5% agar. Cells were grown for 2 weeks in the presence (1 μg/mL) or absence of tet . At least 20 randomly chosen fields were photographed with a CCD camera coupled to a light microscope. Maximal colony diameter was measured using the National Institutes of Health Image software (National Institute of Mental Health Research Services Branch).
Retroviral Production.
Human CLDN-2 cDNA was subcloned into pBABEpuro using BamHI and EcoRI linkers. Retroviral particles were produced in Phoenix helper cells using standard procedures. We infected 2 × 105 HT29-Cl.16E cells with 1 mL of viral supernatant containing 15 μg Polybrene (Sigma). Supernatants were removed 24 h later, and 1 μg/mL puromycin (Gibco/InVitrogen) was added the following day to select for stable integration.
Tissue Samples.
Specimens of colon tumors and histologically normal epithelium from 12 patients, as well as paraffin-embedded sections from eight patients, were obtained from the pathologist after resection, in accordance with French government regulations and with the approval of the ethical committee of the Nîmes University Hospital.
Ultrastructural Characterization.
Processing and observation of samples for transmission electron microscopy was performed as described in ref. 13.
Growth of HT29 Xenografts in Nude Mice.
In vivo experiments complied with the French guidelines for experimental animal studies (Direction des Services Vétérinaires (DSV) Agreement No. B 34–172-27) and with the U.K. Co-ordinating Committee on Cancer Research Guidelines for the welfare of animals in experimental neoplasia. BALB/cnu /nu mice were injected s.c. with 107 control HT29-Cl.16E cells in one flank and with the same amount of ΔSymA or B cells on the opposite side. The mice were divided into two groups, one provided with, and the other without, doxycycline in the drinking water. Tumor growth was measured every fourth day once tumors became visible. Tumor volume was estimated as (length × width × thickness)/2.
Supplementary Material
Acknowledgments
We are grateful to B.H. Keon for multiple helpful discussions, J. Ollier for his technical assistance, P. Corbeau for his advice on viral constructs, N. Lautredou and C. Cazevieille for their help with microscopy, J.L. Ryan for statistical advice, and Dr. C. Pignodel for providing pathology slides. F.H. received grants from the Association pour la Recherche Contre le Cancer (ARC) (3563), Institut National de la Santé et de la Recherche Médicale (CreS 4CR04G), Groupement d’Entreprises Françaises dans la Lutte contre le Cancer, and the Helen McPherson Smith Trust. M.B. and C.D. were supported by ARC and Conseil National de la Recherche Scientifique-Liban.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. V.V. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903747107/DCSupplemental.
References
- 1.Radtke F, Clevers H. Self-renewal and cancer of the gut: Two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 2.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 3.Dhawan P, et al. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest. 2005;115:1765–1776. doi: 10.1172/JCI24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matter K, Aijaz S, Tsapara A, Balda MS. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr Opin Cell Biol. 2005;17:453–458. doi: 10.1016/j.ceb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Sourisseau T, et al. Regulation of PCNA and cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA. Mol Cell Biol. 2006;26:2387–2398. doi: 10.1128/MCB.26.6.2387-2398.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kavanagh E, et al. Functional interaction between the ZO-1-interacting transcription factor ZONAB/DbpA and the RNA processing factor symplekin. J Cell Sci. 2006;119:5098–5105. doi: 10.1242/jcs.03297. [DOI] [PubMed] [Google Scholar]
- 7.Keon BH, Schäfer S, Kuhn C, Grund C, Franke WW. Symplekin, a novel type of tight junction plaque protein. J Cell Biol. 1996;134:1003–1018. doi: 10.1083/jcb.134.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnard DC, Ryan K, Manley JL, Richter JD. Symplekin and xGLD-2 are required for CPEB-mediated cytoplasmic polyadenylation. Cell. 2004;119:641–651. doi: 10.1016/j.cell.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann I, Schnölzer M, Kaufmann I, Franke WW. Symplekin, a constitutive protein of karyo- and cytoplasmic particles involved in mRNA biogenesis in Xenopus laevis oocytes. Mol Biol Cell. 2002;13:1665–1676. doi: 10.1091/mbc.01-12-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolev NG, Steitz JA. Symplekin and multiple other polyadenylation factors participate in 3′-end maturation of histone mRNAs. Genes Dev. 2005;19:2583–2592. doi: 10.1101/gad.1371105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takagaki Y, Manley JL. Complex protein interactions within the human polyadenylation machinery identify a novel component. Mol Cell Biol. 2000;20:1515–1525. doi: 10.1128/mcb.20.5.1515-1525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xing H, Mayhew CN, Cullen KE, Park-Sarge OK, Sarge KD. HSF1 modulation of Hsp70 mRNA polyadenylation via interaction with symplekin. J Biol Chem. 2004;279:10551–10555. doi: 10.1074/jbc.M311719200. [DOI] [PubMed] [Google Scholar]
- 13.Buchert M, et al. The symplekin/ZONAB complex inhibits intestinal cell differentiation by the repression of AML1/Runx1. Gastroenterology. 2009;137(1):156–164. doi: 10.1053/j.gastro.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 14.Nakatsura T, et al. Gene cloning of immunogenic antigens overexpressed in pancreatic cancer. Biochem Biophys Res Commun. 2001;281:936–944. doi: 10.1006/bbrc.2001.4377. [DOI] [PubMed] [Google Scholar]
- 15.Yasen M, et al. The up-regulation of Y-box binding proteins (DNA binding protein A and Y-box binding protein-1) as prognostic markers of hepatocellular carcinoma. Clin Cancer Res. 2005;11:7354–7361. doi: 10.1158/1078-0432.CCR-05-1027. [DOI] [PubMed] [Google Scholar]
- 16.Boon K, et al. An anatomy of normal and malignant gene expression. Proc Natl Acad Sci USA. 2002;99:11287–11292. doi: 10.1073/pnas.152324199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lesuffleur T, et al. Increased growth adaptability to 5-fluorouracil and methotrexate of HT-29 sub-populations selected for their commitment to differentiation. Int J Cancer. 1991;49:731–737. doi: 10.1002/ijc.2910490517. [DOI] [PubMed] [Google Scholar]
- 18.Marshall CJ, Franks LM, Carbonell AW. Markers of neoplastic transformation in epithelial cell lines derived from human carcinomas. J Natl Cancer Inst. 1977;58:1743–1751. doi: 10.1093/jnci/58.6.1743. [DOI] [PubMed] [Google Scholar]
- 19.Jay P, Berta P, Blache P. Expression of the carcinoembryonic antigen gene is inhibited by SOX9 in human colon carcinoma cells. Cancer Res. 2005;65:2193–2198. doi: 10.1158/0008-5472.CAN-04-1484. [DOI] [PubMed] [Google Scholar]
- 20.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou J, Gomes AS, Paul DL, Goodenough DA. Study of claudin function by RNA interference. J Biol Chem. 2006;281:36117–36123. doi: 10.1074/jbc.M608853200. [DOI] [PubMed] [Google Scholar]
- 22.Balkovetz DF. Claudins at the gate: Determinants of renal epithelial tight junction paracellular permeability. Am J Physiol Renal Physiol. 2006;290:F572–F579. doi: 10.1152/ajprenal.00135.2005. [DOI] [PubMed] [Google Scholar]
- 23.Kinugasa T, et al. Selective up-regulation of claudin-1 and claudin-2 in colorectal cancer. Anticancer Res. 2007;27(6A6A):3729–3734. [PubMed] [Google Scholar]
- 24.Mima S, et al. NSAIDs suppress the expression of claudin-2 to promote invasion activity of cancer cells. Carcinogenesis. 2008;29:1994–2000. doi: 10.1093/carcin/bgn134. [DOI] [PubMed] [Google Scholar]
- 25.Amasheh S, et al. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 26.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 27.Guillemot L, Citi S. Cingulin regulates claudin-2 expression and cell proliferation through the small GTPase RhoA. Mol Biol Cell. 2006;17:3569–3577. doi: 10.1091/mbc.E06-02-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Escaffit F, Boudreau F, Beaulieu JF. Differential expression of claudin-2 along the human intestine: Implication of GATA-4 in the maintenance of claudin-2 in differentiating cells. J Cell Physiol. 2005;203:15–26. doi: 10.1002/jcp.20189. [DOI] [PubMed] [Google Scholar]
- 29.Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr Patterns. 2006;6:581–588. doi: 10.1016/j.modgep.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroen-terology. 2001;120:411–422. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- 31.Cao Y, Chang H, Li L, Cheng RC, Fan XN. Alteration of adhesion molecule expression and cellular polarity in hepatocellular carcinoma. Histopathology. 2007;51:528–538. doi: 10.1111/j.1365-2559.2007.02820.x. [DOI] [PubMed] [Google Scholar]
- 32.Saal LH, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2554–2559. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- 33.Sparks AB, Morin PJ, Vogelstein B, Kinzler KW. Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998;58:1130–1134. [PubMed] [Google Scholar]
- 34.Arakawa Y, et al. Transcription of dbpA, a Y box binding protein, is positively regulated by E2F1: Implications in hepatocarcinogenesis. Biochem Biophys Res Commun. 2004;322:297–302. doi: 10.1016/j.bbrc.2004.04.208. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi J, et al. Somatic mutation and SNP in the promoter of dbpA and human hepatocarcinogenesis. Int J Oncol. 2002;21:847–850. [PubMed] [Google Scholar]
- 36.Darido C, et al. Defective claudin-7 regulation by Tcf-4 and Sox-9 disrupts the polarity and increases the tumorigenicity of colorectal cancer cells. Cancer Res. 2008;68:4258–4268. doi: 10.1158/0008-5472.CAN-07-5805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.