Abstract
Phosphotidylinositol-3-kinase (PI3K) signaling is altered in the majority of human cancers. To gain insight into the roles of members of this pathway in growth regulation, we inactivated AKT1, AKT2, or PDPK1 genes by targeted homologous recombination in human colon cancer cell lines. Knockout of either AKT1 or AKT2 had minimum effects on cell growth or downstream signaling. In contrast, knockout of both AKT1 and AKT2 resulted in markedly reduced proliferation in vitro when growth factors were limiting and severely affected experimental metastasis in mice. Unexpectedly, AKT1 and AKT2 appeared to regulate growth through FOXO proteins, but not through either GSK3β or mTOR. In contrast, inactivation of PDPK1 affected GSK3β and mTOR activation. These findings show that the PI3K signaling pathway is wired differently in human cancer cells than in other cell types or organisms, which has important implications for the design and testing of drugs that target this pathway.
Keywords: PI3K, PIK3CA, metastasis, isogenic lines, microenvironment
Phosphatidylinositol 3,4,5-triphosphate (PIP3) is a ubiquitous signaling molecule whose levels are controlled by many different growth factors (1). The amounts of PIP3 and phosphatidylinositol 4,5-bisphosphate (PIP2) are tightly regulated at the inner cellular membrane by phosphatidylinositol 3-kinase (PI3K) and phosphatase and tensin homolog deleted from chromosome 10 (PTEN) (2 –5). PI3K enzymes phosphorylate PIP2 at the 3-position of the inositol ring, converting it to PIP3, whereas PTEN has the reverse function. The serine and threonine kinase AKT is the most well-characterized downstream mediator of the PIP3 signal. AKT was first identified as the cellular homolog of v-AKT, an oncogene that is transduced by the murine retrovirus AKT8 and produces thymic lymphomas (6). In mammalian cells, three human genes (AKT1, AKT2, and AKT3), located on chromosomes 14, 19, and 1, respectively, encode distinct AKT proteins (7). These AKT proteins reside in the cytosol, are activated by growth factor receptor–mediated signaling cascades, and play a central role in the physiology of both normal and abnormal cells (7, 8).
PIP3 acts by forming a docking site for pleckstrin homology (PH)-containing proteins, such as the AKTs and 3-phosphoinositide dependent protein kinase-1 (PDPK1) (7). Upon growth factor stimulation, AKT and PDPK1 proteins are translocated to the inner plasma membrane where their PH domain docks with PIP3, leading to phosphorylation of AKT proteins by PDPK1 at Thr308 (9). This Thr308 phosphorylation represents a major step in the activation of AKTs, although phosphorylation at Ser473 by mTOR is required for full enzymatic activity (10).
As might be expected given its central role in growth control, the PI3K signaling pathway is genetically altered in numerous cancer types. For example, activating mutations of PIK3CA or inactivating mutations of PTEN are found in tumors of the colon, breast, brain, prostate, stomach, uterus, and many other organs (11). Although less common, genetic alterations in AKTs, PDPK1, and other genes in this pathway also have been identified in various tumor types (9, 12 –15).
Once AKT is activated by phosphorylation at the inner membrane, it is capable of regulating cell proliferation, survival, and motility. A host of downstream mediators of these functions have been identified, including GSK3β, BAD, MDM2, p21, caspase 9, FOXO1A, FOXO3A, and mTOR (1, 7 –9). Although previous studies carried out in various species and cell types have provided the foundation for our current understanding of the PIK3 pathway, the functions and mediators that actually act to control growth in specific cancers are not yet well defined. Indeed, recent studies have shown that many regulatory pathways have cell type–specific and species-specific elements, precluding generalizations (16). In an effort to better understand the PIK3 pathway in human colorectal cancer cells, we disrupted AKT1, AKT2, or PDPK1 through targeted homologous recombination to analyze the physiological and biochemical effects in vitro and in vivo.
Results
Gene Inactivation Through Targeted Homologous Recombination.
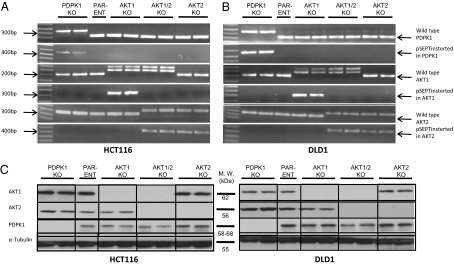
Recombinant adeno-associated virus (rAAV) vectors provide an efficient means of permanently inactivating genes in human cancer cells (17, 18). In this work, we generated targeting vectors with a promoter-trap strategy so that infected cells would express the drug resistance gene only when integrated in frame with the endogenous promoter (19). Targeting was directed to exon 4 of the AKT1 gene (Fig. S1A), to exon 2 of AKT2 (Fig. S1B), and toward exon 3 of PDPK1 (Fig. S1C). Targeting was performed in two near-diploid colon cancer cell lines, HCT116 and DLD1, with a constitutively activated PIK3 pathway by virtue of mutations. HCT116 contains the H1047R mutation and DLD1 contains the E545K mutation of the PIK3CA gene (20). Targeting was initially evaluated via PCR of genomic DNA. Following disruption of the first allele, Cre recombinase was used to remove the drug-resistance cassette integrated at the targeted locus, permitting another round of targeting to disrupt the second allele. This process of targeting by rAAV infection followed by Cre recombinase–mediated excision was performed four times sequentially to disrupt both alleles of AKT1 and AKT2. RT-PCR of mRNA from each clone was performed to verify the nature of the targeting events (Fig. 1 A and B, and Table S2). Finally, Western blot analysis was used to confirm the absence of protein expression (Fig. 1C). Clones with the identical genotype behaved similarly in the assays described below.
Fig. 1.
Confirmation of successful targeting. RNA from the indicated cells was used as a template for RT-PCR. Two independent clones for each KO are shown. Results with clones derived from HCT116 and DLD1 cells are illustrated in A and B, respectively. The first row, with the band designated “Wild type-PDPK1,” shows that wild-type cDNA is not expressed in the PDPK1 KO clone. The second row, with the band designated “pSEPT inserted into PDPK1 gene,” shows that the PDPK1 gene contains the integrated pSEPT vector in the PDPK1 KO clone. This vector was removed with Cre recombinase before functional assays were performed on the clones (Fig. S1). The same labeling scheme was applied to the AKT1 (rows 3 and 4) and AKT2 (rows 5 and 6) KO clones. (C) Western blot analysis of the targeted clones shows the expected absence of proteins. α-Tubulin is included as a loading control.
Effects of Gene Disruption on Clonal Growth in Vitro.
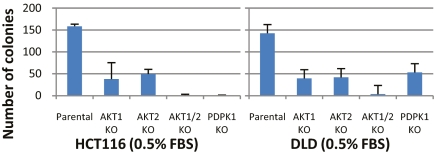
Colony-formation assays were used to assess clonal growth, because this assay was found to be more reproducible than short-term proliferation assays. Under normal culture conditions (10% FBS), cells in which both AKT1 and AKT2 genes were disrupted grew more slowly than cells in which only one of the two genes was disrupted (Fig. S2). The genotype-specific differences were magnified considerably when growth factors were limiting; there was barely any growth of cells in which both AKT1 and AKT2 genes had been disrupted, whereas cells with disruption of only one of the two genes were affected less severely (Fig. 2). Importantly, the disruption of both AKT1 and AKT2 genes produced similar, dramatic reductions in growth in both of the parental lines used in these experiments.
Fig. 2.
Colony formation in media with reduced serum concentrations. Approximately 1,500 cells of the indicated cell lines were seeded in T25 flasks, cultured for 11–12 days in medium containing 0.5% FBS, and then stained and counted.
The growth of cells in which PDPK1 was disrupted was decreased to approximately the same extent as that observed in cells with disruption of both AKT1 and AKT2 when they were grown in 10% FBS (Fig. S2). When grown in 0.5% FBS, the responses were cell line–dependent; HCT116 cells with PDPK1 KO did not grow at all, whereas DLD1 cells grew about as well as single knockouts (KOs) of AKT1 or AKT2 (Fig. 2).
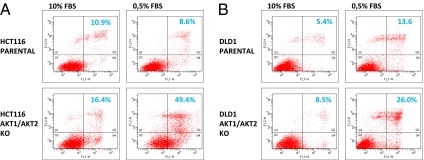
To determine whether the decreased clonal growth of the cells with both AKT1 and AKT2 KOs was due to increased apoptosis, we performed an assay combining Alexa 488–labeled annexin V and propidium iodide. Annexin V binds to phosphatidylserine translocated from the inner to the outer leaflet of the plasma membrane during the early phases of apoptosis, whereas propidium iodide stains the nuclei of dead cells at later stage of apoptosis (21). As assessed by flow cytometry, the fraction of apoptotic HCT116 cells was somewhat higher in cells with both AKT1 and AKT2 KOs compared with parental cells when grown in normal culture conditions (10% FBS; Fig. 3A Left). However, when grown in reduced serum (0.5% FBS), there was a dramatic rise in the apoptotic fraction of cells in which both AKT1 and AKT2 were disrupted (Fig. 3A Right). Similar results were obtained in the DLD1 cell line (Fig. 3B). In contrast, there was little difference among parental cells and cells with single KOs of AKT1, AKT2, or PDPK1 regardless of whether the cells were grown in 10% FBS or 0.5% FBS (data not shown).
Fig. 3.
Apoptosis following growth in medium with reduced serum concentrations. The indicated cell lines were seeded in medium containing 10% FBS and, after 24 h, were washed and incubated with medium containing 0.5% FBS. Four days later, cells were stained with Alexa Fluor 488–labeled annexin V and propidium iodide, and then analyzed with flow cytometry. Cells grown in 10% FBS for 1 day were used as controls. The upper right quadrant contains the apoptotic cells.
Effects of Gene Disruption on Downstream Signaling.
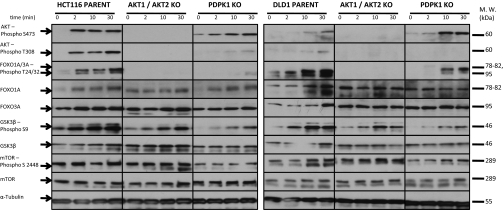
To evaluate the effects of gene disruption on known PIK3-signaling pathways, we starved cells of serum for 20 h and then stimulated them with epidermal growth factor (EGF), insulin-like growth factor (IGF), and insulin for very short periods. These three polypeptide growth factors are known to stimulate growth after binding to their receptors and activating PIK3 enzymes, producing PIP3. Proteins from the stimulated cells were assessed by Western blot analysis, using antibodies specific to known components of the PIK3 pathway. Striking changes in the levels of phosphorylated AKT, FOXO1, and FOXO3A were observed as early as 2 minutes after growth factors were added to parental cells (Fig. 4). In cells without intact AKT1 and AKT2 genes, no phosphorylated AKT proteins were observed, as expected. Importantly, no phosphorylated FOXO1A and FOXO3A could be detected in these double-KO cells in either HCT116 or DLD1 (Fig. 4). Other known constituents of the PIK3 pathway were not affected to the same degree. Although mTOR clearly is an important downstream component of PIK3 in many cell types (22, 23), few differences in mTOR activation, as assessed by phosphorylation at Ser2448 following growth factor stimulation, were seen in parental and double-KO cells (Fig. 4). The phosphorylation of GSK3β at ser9 was increased somewhat after the addition of growth factor in parental cells and less so in cells without intact AKT1 and AKT2 (Fig. 4). Protein phosphorylation patterns in cells with single KOs of either AKT1 or AKT2 were very similar to those of parental cells (data not shown).
Fig. 4.
Effect of growth factor stimulation on downstream signaling. Cells were incubated in medium containing 0.5% FBS for ∼16 h and then stimulated with insulin, EGF, and IGF for 2, 10, or 30 min as indicated. Cells were lysed, and proteins were separated by electrophoresis under denaturing conditions, then immunoblotted with antibodies to the indicated proteins. The antibody detecting phospho-FOXO Thr-24/32 gives a double band because it reacts with the phosphorylated form of FOXO1A and FOXO3A. The antibody against phospho-GSK3β gives a double band because it recognizes phosphorylated GSK3α because of sequence homology.
Cells with a KO of PDPK1 exhibited a different pattern of downstream signaling events. Phosphorylation of AKT at Thr308 was markedly delayed and reduced in cells without intact PDPK1 (Fig. 4). This was expected, given that PDPK1 is known to be responsible for phosphorylation of AKT proteins at this residue. This finding also definitively demonstrates that phosphorylation of AKT at S473 is not dependent on phosphorylation of T308, because substantial phosphorylation of S473 was seen in cells without PDPK1. FOXO protein phosphorylation was delayed and reduced in the absence of functional PDPK1, particularly in HCT116 cells, although not as completely as in the absence of functional AKT1 and AKT2 (Fig. 4). Interestingly, phosphorylation of both mTOR and GSK3β at serine residues 2448 and 9, respectively, was significantly reduced in the PDPK1 KO, unlike the situation in KOs of AKT1 and AKT2.
Effects on Experimental Metastasis in Vivo.
The decreased growth of the cells with disruptions in AKT or PDPK1 genes does not necessarily mean that these cells would have impaired growth in a typical tumor environment. The fact that the impaired growth of the KO cells was largely evident only when cells were starved of serum in vitro emphasizes this point. Initial experiments using standard subcutaneous (s.c.) xenografts in nude mice showed that the KO cells, even those with disruptions of both AKT1 and AKT2, generally formed s.c. tumors, although somewhat more slowly. To determine the effects of the gene disruptions on more relevant cancer-related phenotypes in vivo, we developed a murine metastasis assay. To mimic the colorectal metastatic process, we introduced cells into the spleen and measured their capacity to form liver metastases via transit through the portal circulation. NOD-SCID-IL2Rγ–deficient mice (24) were used for this purpose. These mice have a severe immune deficiency resulting from the absence of functional B cells, T cells, and natural killer cells and do not reject human cancer cells after intrasplenic injection, whereas typical nude mice do reject the same cells. Mice receiving DLD1 cells were primed with an intraperitoneal (i.p.) injection of FBS, because the resultant circulating bovine serum proteins were empirically found to enhance the metastasis of DLD1 cells. This approach was based on our observation that DLD1 cells grow very slowly in vitro when cultured in mouse serum instead of FBS.
We assessed metastasis in three different ways. First, we measured the number of visible liver foci that formed 5 weeks after intrasplenic injection (Fig. 5A, Figs. S3 and S4, and Table S1). Second, we used DNA from liver and spleen to measure human cell content by qPCR, thereby providing a quantitative index of tumor burden (Fig. 5 B and C). Third, we purifed DNA from the plasma of mice at various times following intrasplenic injection, providing a temporal record of invasive tumor growth (Fig. S5) (25, 26).
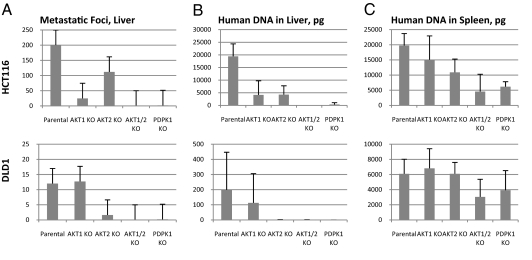
Fig. 5.
Effects of gene disruption on experimental metastasis. Cells with the indicated genotypes were injected into the spleens of immunodeficient mice, who were killed 5 weeks later. Five mice were injected with each cell line. (A) Number of metastatic foci visible on the liver surface on necropsy. There were at least 200 foci in parental HC116 cells, but the actual number was difficult to count because of adjoining lesions (Fig. S3). (B) Amount of human tumor DNA in the livers. (C) Amount of human tumor DNA in the spleens.
Our most important finding from these experiments was a striking decrease in liver metastasis in cells with disruptions of both AKT1 and AKT2. This decrease was apparent on inspection of the livers (Fig. 5A and Fig. S3), on histopathological examination of liver sections (Fig. S4), and on analysis of human DNA in the mouse liver (Fig. 5B). The amount of DNA in the spleens of these mice was reduced, but not nearly to the extent observed in the livers (Fig. 5C). Circulating tumor DNA was decreased by >99% when AKT1 and AKT2 were disrupted (Fig. S5).
Disruption of the PDPK1 gene also resulted in major decreases in liver metastasis (Fig. 5 A and B), but only small decreases in primary tumorigenesis in the spleen (Fig. 5C). In contrast, when either AKT1 or AKT2 was singly disrupted, there was a less pronounced decrease in liver metastasis (Fig. 5 A and B) and statistically insignificant decreases in primary tumorigenesis in the spleen (Fig. 5C).
Discussion
One of the most important results of the current study is that the microenvironment plays a major role in determining the effects of gene disruption. Complete inactivation of both AKT1 and AKT2, or of PDPK1, clearly was compatible with clonal growth in vitro as well as in the spleens of xenografted animals (Fig. S2 and Fig. 5C). The profound role of these genes in regulating tumor growth was evident only in more challenging microenvironments, such as in vitro with 20-fold less serum or in vivo during the metastatic process (Figs. 2 and 5 A and B). These results are consistent with those seen in cells in which a naturally occurring, mutant PIK3CA allele was disrupted by targeted homologous recombination (20). They also are consistent with genetic models for tumorigenesis (27 –29). Only mutations that can provide a growth advantage to cells or their progeny in a specific environment will be subject to selection during tumorigenesis. We speculate that cells with PIK3CA pathway mutations are selected for when tumors are starved for polypeptide growth factors, such as EGF, IGF, or insulin, due either to their distance from capillaries or to their abnormal tissue architecture.
A recent study found a low level of AKT phosphorylation in HCT116 and HCT-15 cells (derived from the same tumor as DLD1 cells) compared with other PIK3CA mutant cells (30). Although phosphorylated AKT levels are indeed low in these two lines, our results demonstrate that they are adequate to allow for robust growth, even in challenging in vitro and in vivo environments. Our results clearly show the need for this low level of AKT phosphorylation; disruption of AKT1 and AKT2 drastically affected growth under our challenging conditions. Moreover, the low level of AKT phosphorylation in these cells is fully dependent on their mutant PIK3CA genes (20). Thus, our results, in concert with those of Vasudevan et al. (30), demonstrate that the absolute level of a protein or its modification cannot be used to determine the contribution of that protein to a pathway in that particular cell, but rather, the threshold level required for function is likely to be critically dependent on the cell's genotype and environment. This point emphasizes the value of isogenic cell lines, differing only in one or a few genes, in dissecting growth-regulating mechanisms.
It is of interest to compare the results that we obtained by disrupting AKT or PDPK1 genes in human colorectal cancer cells with those obtained previously in mice. AKT1 KO mice are small and have a reduced lifespan when exposed to genotoxic stress (31 –33). AKT2 KO mice, in addition to growth retardation, also develop defects in insulin uptake and manifest a severe form of diabetes with β-cell failure (32, 34). PDPK1 null mice die on embryonic day 9 (35). Mice with combined deficiency of AKT1 and AKT2 die shortly after birth (36). Thus, disruption of a single AKT gene produces a reproducible but rather subtle phenotype compared with inactivation of both AKT1 and AKT2. These results have obvious similarities to our findings, although tumor cell phenotypes cannot be directly compared with those of the various organ systems affected in mice.
Along with the implications for understanding the neoplastic process, these data have major repercussions for drug development (37, 38). Many, if not most, drug development approaches use conventional in vitro growth assays to judge efficacy. Our data indicate that such assays may not be optimal in some instances. For example, genetic disruption of AKT1 and AKT2 had only a modest effect on growth under standard conditions (Fig. S2); the profound difference became apparent when cells were stressed through growth factor depletion in vitro or metastatic growth in vivo (Fig. 2).
Similarly, disruption of these genes had much less influence on the growth of primary tumors in the skin or spleen than on the growth of liver metastases (Fig. S6). Our data suggest that in screens for PIK3CA or AKT inhibitors, it may be prudent to use growth factor–limited in vitro environments or animal models of metastasis to assess drug efficacy.
In several experimental systems, mTOR and GSK3β have been shown to be central mediators of PIK3 signaling that are activated by phosphorylation (1, 7 –9). But in both the colorectal cancer cell lines used here, mTOR is not activated by growth factors in parental cells, and its phosphorylation is independent of AKT1, AKT2, FOXO1A, and FOXO3A activity (Fig. 4). GSK3β phosphorylation was reduced only slightly by inactivation of AKT1 and AKT2 and the subsequent inactivity of FOXO1A and FOXO3A. In contrast, inactivation of AKT1 and AKT2 had a dramatic effect on FOXO1A and FOXO3A phosphorylation. We speculate that FOXO1A and FOXO3A are the major proteins phosphorylated by AKT1 and AKT2, and that the consequent activation of genes resulting from the decreased nuclear activity of phosphorylated FOXO proteins is responsible for the growth-promoting effects of the AKT proteins, irrespective of mTOR and GSK3β. The genes activated by FOXO phosphorylation in colorectal cancer cells remain to be defined.
Another question raised by our data regards the nature of the kinase that phosphorylates AKT proteins at T308 in the absence of PDPK1. Although its level was low compared with that in parental cells, phosphorylation was still present (Fig. 4). The PDPK2 gene is located adjacent to PDPK1, with high homology. But sensitive PCR methods revealed that PDPK2 transcripts were not detectably expressed in either of the parental cell lines or in the KO clones developed in this study (data not shown); thus, a different kinase must be responsible for the Thr308 phosphorylation. Similarly, GSK3β and mTOR were still phosphorylated at residues S9 and S2448, respectively, following inactivation of AKT1 and AKT2, as noted above. Thus, there must be other kinases besides AKT1 and AKT2 that can phosphorylate GSK3β and mTOR at these sites. One interesting candidate for this role is PDPK1, because phosphorylation of GSK3β (and, to a lesser extent, mTOR) was reduced (but not eliminated) after KO of PDPK1. AKT3 can be excluded from consideration, because it is inactive in both HCT116 and DLD1 cells, as evidenced by the complete absence of FOXO protein phosphorylation when AKT1 and AKT2 are disrupted (Fig. 4).
From a more general perspective, our results demonstrate that the signaling pathways in colorectal cancer cells cannot be reliably inferred from the study of other organisms or cell types. The nonuniversality of such signaling pathways is an emerging theme in biomedical research (16). It was satisfying that the two parental cell lines derived from different patients’ tumors were similarly affected by gene disruption with respect to most of the assays performed in this study, despite the genetic heterogeneity among different colorectal cancers (39). This result provides hope that, at least within tumor cells derived from the same progenitor cell type, signal transduction patterns and the responses to therapeutic agents that disrupt these patterns will be broadly similar. Cells in which particular genes have been disrupted, such as those described herein, provide excellent benchmarks for assessing the potential effects of such agents.
Materials and Methods
Cell Culture and Reagents.
The colon cancer cell lines HCT116 and DLD1 (American Type Cuture Collection) were cultured in McCoy's 5A growth medium (Gibco) supplemented with 10% FBS (HyClone) and 1% penicillin/streptomycin (Gibco) at 37 °C with 5% CO2. During selection for genetic targeting events, cells were cultured in 0.4 mg/mL (HCT116) or 1 mg/mL (DLD1) of Geneticin (Invitrogen). For experiments using reduced serum concentrations, cells were cultured to 70% confluence, rinsed with PBS, and then cultured in McCoy's 5A growth medium supplemented with 0.5% FBS and 1% penicillin/streptomycin. Cells were stimulated with 100 ng/mL of insulin (Santa Cruz Biotechnology), 60 ng/mL of EGF, and 60 ng/mL IGF (Cell Signaling) for 2, 10, or 30 min.
Targeted Disruptions.
A promoter-trap targeting strategy was used, in which expression of the drug resistance cassette is obtained only when the construct is integrated in frame with an existing promoter (19). The targeting constructs were prepared using PCR products from HCT116 or DLD1 cell DNA (Fig. S1). Homology arms flanking each side of the locus to be disrupted were amplified with Phusion Hot Start High-Fidelity DNA Polymerase (Finnzymes). The PCR products were cloned and sequenced to validate their integrity.
Detailed protocols for disrupting genes using rAAV-based vectors have been reported previously (18, 19). Following transfection with rAAV vectors, PCR was used to confirm correct integrations of both the 3′ and 5′ homology arms in drug-resistant clones. Desired clones were expanded, and the drug resistance cassette was removed using an adenovirus expressing the Cre recombinase. The second allele of each gene was then targeted in an identical manner. To create “double KOs” of both AKT1 and AKT2, two totally independent clones with both alleles of AKT1 disrupted were used to sequentially disrupt both alleles of AKT2.
Colony-Formation Assays.
Approximately 1,500 cells were seeded in a 25-cm2 flask in medium containing 10% FBS. After 1 day, cells were rinsed in PBS, and growth medium containing either 10% or 0.5% FBS was added to the cells. Between 11 and 12 days later, colonies were stained with HEMA-3 (Fisher Scientific).
Apoptosis Assays.
After 0 or 4 days of growth in 0.5% FBS, cells were collected on ice, washed, and resuspended in annexin V binding buffer. The cells were resuspended at ≈1 × 106 cells/mL, and 100 μL of cell suspension was incubated at room temperature for 15 min with 5 μL of Alexa Fluor 488–labeled annexin V and 1 μL of 100 μg/mL propidium iodide. The cells were then mixed with an additional 400 μL of annexin V binding buffer and incubated on ice. The fraction of apoptotic or dead cells was determined using flow cytometry as soon as possible after staining with Alexa Fluor 488–labeled annexin V and propidium iodide (Invitrogen).
Western Blot Analysis.
Cells were allowed to reach 65%–70% confluence, after which they were rinsed in PBS and then exposed to growth medium with 0.5% FBS for 20 h. Cells were then rinsed with ice-cold PBS and lysed in a solution containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, 1 μg/mL leupeptin, and 1 mM PMSF. Lysates were immediately frozen and stored at −80 °C. They were later thawed, sonicated to shear the DNA, and clarified by a 1-min centrifugation at 12,000 × g. Cell lysates were kept on ice at all times. A bicinchoninic acid assay was performed to estimate the protein concentration. Lysates containing 5–20 μg of protein (depending on the experiment) were mixed with sample buffer (125 mM Tris, 10% vol/vol SDS, 0.01% bromophenol blue, and 50% glycerol), boiled for 5 min, chilled on ice, and loaded on a SDS/PAGE gel for size separation. The proteins were then transferred to a PVDF membrane; blocked at room temperature for 1 h with either 5% milk-TBST or protein-free blocking buffer (Pierce), depending on the primary antibody; incubated with primary antibody overnight at 4 °C; washed; and then incubated with a HRP-labeled secondary antibody at room temperature for 1 h. Chemiluminescence was detected with an ECL kit (Amersham). Antibodies were purchased from Santa Cruz Biotechnology (AKT1 and AKT2), Sigma-Aldrich (α-Tubulin), Upstate (FOXO3A), and Cell Signaling Technologies (all other proteins).
Metastasis Assays.
Approximately 2 × 105 HCT116 cells and 3 × 106 DLD1 cells were injected per mouse. Mice injected with the DLD1 cell line also were injected intraperitoneally with 500 μL of filtered FBS twice a week, starting 1 day before surgery. Five mice were included in each group.
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice were purchased from Jackson Laboratories. Before surgery, the mice were anesthetized with 2.5% isoflurane (Abbott Laboratories) using an induction chamber, with anesthetic maintainance provided by a nose cone. A 10-mm sagittal incision was made on the left side through the skin and through the abdominal muscle layer to allow access to the mouse spleen. Then 50 μL of cells were injected slowly into the spleen using a 31G needle on a Threaded Plunger Syringe (Hamilton) to achieve a steadily controlled injection rate. The wound was closed with two sutures in the abdominal muscle layer and two metal clips in the skin. The mice recovered quickly after the procedure. All mice were euthanized with an overdose of halothane (Sigma-Aldrich) 5 weeks after surgery. Livers were photographed, and the total number of visible metastasis foci on the liver surface was recorded. The instruments used for organ collection were thoroughly washed in detergent and ethanol, and residual DNA was destroyed by sterilization at 250 °C in a glass bead sterilizer (Fine Science Instruments). Livers and spleens were snap- frozen and stored at −80 °C until analysis. Samples for pathology were fixed in 10% buffered formalin and H&E-stained sections were prepared from five lobes of the liver using standard techniques. Human DNA in livers and spleens, as well as in plasma samples, was quantified using a qPCR assay with primers for hLINE-1, as described previously (26). Animal protocols were designed in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and were approved by Johns Hopkins University's Institutional Animal Care and Use Committee.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908133107/DCSupplemental.
References
- 1.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 2.Katso R, et al. Cellular function of phosphoinositide 3-kinases: Implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- 3.Vogt PK, Gymnopoulos M, Hart JR. PI 3-kinase and cancer: Changing accents. Curr Opin Genet Dev. 2009;19:12–17. doi: 10.1016/j.gde.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keniry M, Parsons R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene. 2008;27:5477–5485. doi: 10.1038/onc.2008.248. [DOI] [PubMed] [Google Scholar]
- 5.Kok K, Geering B, Vanhaesebroeck B. Regulation of phosphoinositide 3-kinase expression in health and disease. Trends Biochem Sci. 2009;34:115–127. doi: 10.1016/j.tibs.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: Amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci USA. 1987;84:5034–5037. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT: A major therapeutic target. Biochim Biophys Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Whiteman EL, Cho H, Birnbaum MJ. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab. 2002;13:444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- 9.Franke TF. PI3K/Akt: Getting it right matters. Oncogene. 2008;27:6473–6488. doi: 10.1038/onc.2008.313. [DOI] [PubMed] [Google Scholar]
- 10.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 11.Samuels Y, Velculescu VE. Oncogenic mutations of PIK3CA in human cancers. Cell Cycle. 2004;3:1221–1224. doi: 10.4161/cc.3.10.1164. [DOI] [PubMed] [Google Scholar]
- 12.Parsons DW, et al. Colorectal cancer: Mutations in a signaling pathway. Nature. 2005;436:792. doi: 10.1038/436792a. [DOI] [PubMed] [Google Scholar]
- 13.Carpten JD, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 14.Davies MA, et al. A novel AKT3 mutation in melanoma tumours and cell lines. Br J Cancer. 2008;99:1265–1268. doi: 10.1038/sj.bjc.6604637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellacosa A, et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer. 1995;64:280–285. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- 16.Grueneberg DA, et al. Kinase requirements in human cells, I: Comparing kinase requirements across various cell types. Proc Natl Acad Sci USA. 2008;105:16472–16477. doi: 10.1073/pnas.0808019105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirata R, Chamberlain J, Dong R, Russell DW. Targeted transgene insertion into human chromosomes by adeno-associated virus vectors. Nat Biotechnol. 2002;20:735–738. doi: 10.1038/nbt0702-735. [DOI] [PubMed] [Google Scholar]
- 18.Rago C, Vogelstein B, Bunz F. Genetic knockouts and knockins in human somatic cells. Nat Protoc. 2007;2:2734–2746. doi: 10.1038/nprot.2007.408. [DOI] [PubMed] [Google Scholar]
- 19.Topaloglu O, Hurley PJ, Yildirim O, Civin CI, Bunz F. Improved methods for the generation of human gene knockout and knockin cell lines. Nucleic Acids Res. 2005;33:e158. doi: 10.1093/nar/gni160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samuels Y, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–573. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 21.van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 22.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Shultz LD, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 25.Diehl F, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985–990. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rago C, et al. Serial assessment of human tumor burdens in mice by the analysis of circulating DNA. Cancer Res. 2007;67:9364–9370. doi: 10.1158/0008-5472.CAN-07-0605. [DOI] [PubMed] [Google Scholar]
- 27.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 28.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 29.Jones S, et al. Comparative lesion sequencing provides insights into tumor evolution. Proc Natl Acad Sci USA. 2008;105:4283–4288. doi: 10.1073/pnas.0712345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vasudevan KM, et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell. 2009;16:21–32. doi: 10.1016/j.ccr.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen WS, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 33.Yang ZZ, et al. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- 34.Garofalo RS, et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest. 2003;112:197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawlor MA, et al. Essential role of PDK1 in regulating cell size and development in mice. EMBO J. 2002;21:3728–3738. doi: 10.1093/emboj/cdf387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng XD, et al. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17:1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 38.Vogt PK, Bader AG, Kang S. Phosphoinositide 3-kinase: From viral oncoprotein to drug target. Virology. 2006;344:131–138. doi: 10.1016/j.virol.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 39.Wood LD, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.