Abstract
Tau is a microtubule-associated protein, which is widely expressed in the central nervous system, predominantly in neurons, where it regulates microtubule dynamics, axonal transport, and neurite outgrowth. The aberrant assembly of Tau is the hallmark of several human neurodegenerative diseases, collectively known as tauopathies. They include Alzheimer’s disease, Pick’s disease, progressive supranuclear palsy, and frontotemporal dementia and parkinsonism linked to chromosome 17. Several abnormalities in Tau, such as hyperphosphorylation and aggregation, alter its function and are central to the pathogenic process. Here, we describe biochemical and functional interactions between FKBP52 and Tau. FKBP52 is a member of the FKBP (FK506-binding protein) family that comprises intracellular protein effectors of immunosuppressive drugs (such as FK506 and rapamycin). We found that FKBP52, which is abundant in brain, binds directly and specifically to Tau, especially in its hyperphosphorylated form. The relevance of this observation was confirmed by the colocalization of both proteins in the distal part of the axons of cortical neurons and by the antagonistic effect of FKBP52 on the ability of Tau to promote microtubule assembly. Overexpression of FKBP52 in differentiated PC12 cells prevented the accumulation of Tau and resulted in reduced neurite length. Taken together, these findings indicate a role for FKBP52 in Tau function and may help to decipher and modulate the events involved in Tau-induced neurodegeneration.
Keywords: immunophilins, microtubules, tauopathies, FK506-Binding Protein
FKBPs belong to the group of proteins known as immunophilins. They are the intracellular receptors for immunosuppressive drugs such as FK506 and rapamycin (1). Interestingly, FKBPs are particularly abundant in the nervous system, suggesting other functions distinct from their immunomodulatory effects (2). An involvement of FKBPs in neurological disorders has been suggested (3), and neuroprotective effects of immunosuppressant drugs have already been described as evoking a possible mechanism via immunophilins (4, 5). Another remarkable feature of FKBPs is their rotamase (peptidyl prolyl cis-trans isomerase) activity (6), which links them to the parvulin family of isomerases such as Pin1 (7).
Among the FKBP family members, FKBP52 (FKBP of molecular mass of 52 kDa) was originally discovered as a component of steroid hormone receptor heterooligomeric complexes (8). Recently, we have reported that FKBP52 binds to tubulin and prevents microtubule formation (9). Results obtained so far suggest that the inhibition of tubulin polymerization by FKBP52 may not only result from the sequestration of tubulin or from a modification of its structure such as bending, but may also require additional factor(s). We hypothesized that the involvement of one or more microtubule-stabilizing factor(s), such as microtubule-associated proteins (MAPs), could explain the inhibition of tubulin polymerization by FKBP52, and among other MAPs, Tau protein was considered.
Tau was initially identified as a protein that copurifies with tubulin in vitro, stimulates tubulin polymerization, and stabilizes microtubules (10 –12). It is expressed in the adult human brain as 6 different isoforms varying from 352 to 441 amino acids in length (13, 14). Growing evidences underline the role attributed to abnormal forms of Tau in several neurodegenerative diseases, including Alzheimer’s disease (15, 16). Neurodegeneration is characterized by the accumulation of filamentous Tau inclusions in the central nervous system. In Alzheimer’s disease, these inclusions contain all six adult brain Tau isoforms in a hyperphosphorylated state (17). The mechanisms leading to fibrillar formation in neurons are still unclear. Deciphering the molecular mechanism(s) that control(s) Tau structure/function is therefore of great interest and may lead to the development of unique therapeutic approaches for these diseases. Here, we describe the biochemical and functional effects of FKBP52 on Tau. We show that FKBP52 interacts with Tau and inhibits its ability to promote microtubule assembly. Our findings suggest a role for FKBP52 in Tau function.
Results
Tau FKBP52 Association.
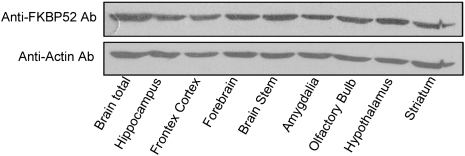
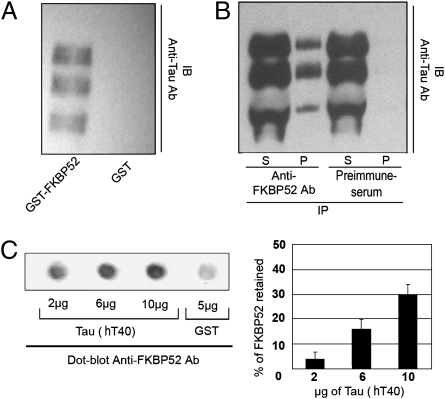
FKBP52 is widely distributed in the brain as shown by Western blots of cytosolic proteins from several brain areas (Fig. 1). To investigate whether MAPs may be involved in the effect of FKBP52 on microtubule stability (9), GST pull-down assays were carried out incubating GST-FKBP52 bound to Sepharose beads with cytosol microtubules prepared from adult rat brain. Specifically bound proteins were analyzed by immunoblotting using antibodies directed against MAP1b, MAP2, and Tau. Under these experimental conditions, no immunoreactivity was observed for MAP1b or MAP2, but Tau immunoreactivity was present (Fig. 2A). In rat brain homogenates, Tau appears as multiple bands representing different splice isoforms with various degrees of phosphorylation. Several Tau species were also found in pull-down experiments of rat brain cytosol microtubules using GST-FKBP52. Tau immunoreactivity was not detected in controls using purified GST (Fig. 2A). To confirm the specificity of this association, microtubules of adult rat brain cytosol were immunoprecipitated with a polyclonal antibody against FKBP52. Immunoprecipitates were analyzed by Western blotting with a monoclonal Tau antibody. Tau coimmunoprecipitated with FKBP52 but not with a preimmune serum (Fig. 2B). Thus, Tau and FKBP52 form a complex in rat brain. These experiments do not address whether the binding of Tau to FKBP52 is direct or whether it involves additional factors. To investigate this, recombinant Tau (hT40, the longest isoform, expressed in Escherichia coli and purified) was spotted onto nitrocellulose and incubated with purified recombinant FKBP52. Then, proteins sequestered by Tau were detected with a polyclonal antibody against FKBP52. As shown on Fig. 2C, FKBP52 was retained in a dose-dependent manner by Tau, but not by GST. These findings indicate a direct interaction between FKBP52 and Tau.
Fig. 1.
FKBP52 in the brain. Twenty micrograms of cytosol proteins from different adult rat brain regions were analyzed by Western blotting using anti-FKBP52 antibody 761. Actin served as the loading control.
Fig. 2.
Association between FKBP52 and Tau proteins. (A) GST pull-down assay: immunoblot (IB) for Tau showing the binding of soluble microtubule extract proteins incubated with GST-tagged FKBP52 or GST alone as control. (B) Coimmunoprecipitation assay: a soluble microtubule extract was subjected to immunoprecipitation (IP) with immunopurified anti-FKBP52 antibody or preimmune serum used as control. The supernatants (S) and the precipitates (P) were immunoblotted with anti-Tau antibody (clone DC25). (C) The ability of Tau proteins to bind FKBP52 directly was monitored by dot blot assay. Different amounts of recombinant Tau (hT40) were spotted onto nitrocellulose membranes and then assayed for bound FKBP52 (0.5 μg) using anti-FKBP52 antibody. Five micrograms of GST spotted onto nitrocellulose membrane was used as the control. Quantitation: 100% corresponds to 0.5 μg of FKBP52 loading before milk saturation, and 0% corresponds to 0.5 μg of FKBP52 loading after milk saturation. The background is defined as the signal when GST instead of hT40 was loaded. The level of FKBP52 captured by hT40 was calculated after substraction of background.
Effect of Tau Phosphorylation on Tau’s Interaction with FKBP52.
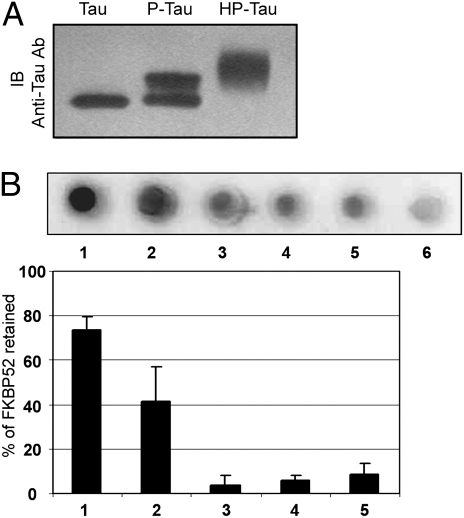
To define whether the phosphorylation of Tau modulates its association with FKBP52, dot blot experiments were performed using recombinant phosphorylated Tau (P-Tau) and hyperphosphorylated Tau (HP-Tau) (18) (Fig. 3A). The specificity of the interaction between FKBP52 and P-Tau or HP-Tau was determined by experiments using as the bait either phosphorylated hT40, nonphosphorylated hT40, or hT40 to which had been added, just before spotting, the same amount of cytosol protein as used to obtain phosphorylated or hyperphosphorylated hT40. As shown in Fig. 3B, the amount of FKBP52 recruited by Tau depends on its phosphorylation state. FKBP52 [73% (±7)] was retained by 2.2 μg HP-Tau, whereas only 3.5% and 6.6%, respectively, were retained by the same amount of hT40 and by hT40 in the presence of cytosolic proteins used as controls. When P-Tau was used as bait, only 41% (±15) of FKBP52 was captured. This difference in binding between HP-Tau and P-Tau to FKBP52 may be explained by the different degree of Tau phosphorylation at specific sites, by the global amount of Tau phosphorylation, or by a combination of both mechanisms (Fig. 3A). In any case, these results underline the importance of Tau phosphorylation for its binding to FKBP52.
Fig. 3.
Relevance of Tau phosphorylation for its interaction with FKBP52. (A) Recombinant Tau (hT40), P-Tau, and HP-Tau were analyzed by SDS–Page. Phosphorylation and hyperphosphorylation of hT40 resulted in a marked reduction in the gel mobility of recombinant Tau as shown on an immunoblot (IB) with anti-Tau antibody (clone DC25). (B) Dot blot assay with 2.2 μg of HP-Tau (1), P-Tau (2), and pure recombinant hT40 (3) to which had been added, just before spotting, the same amount of cytosol as used to generate P-Tau (4) or HP-Tau (5). Lane 6 shows the GST (5 μg) load.
Colocalization of Tau and FKBP52 in Primary Cortical Neurons and PC12 Cells.
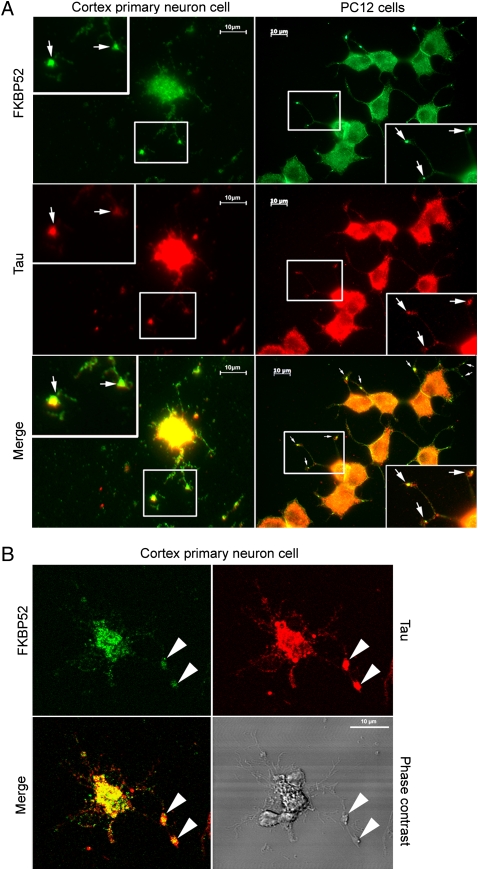
The subcellular localization of Tau and FKBP52 was examined by immunofluorescence experiments with rat primary cortical neurons. After 6 days of culture—and mild extraction selectively removing unbound cytosolic proteins while retaining proteins associated with the cytoskeleton—double staining was performed on neurons with monoclonal antibody Tau5 and affinity-purified polyclonal anti-FKBP52 antibody. In agreement with an earlier report (19), Tau was concentrated in the distal portion of axons and at the growth cone neck, where a strong accumulation of FKBP52 was also observed (Fig. 4). Colocalization and accumulation of FKBP52 and Tau were also found at the growth cones of PC12 cells (Fig. 4). Very recently it has been reported that Tau is selectively enriched at axonal tips and that this may be due to its specific anchoring (20). Our results suggest that FKBP52 may be involved in the trapping of Tau and thereby able to influence its subcellular distribution.
Fig. 4.
Colocalization of FKBP52 and Tau in primary cortical neurons and PC12 cells. (A) Immunofluorescence staining of primary cortical neurons and PC12 cells treated with 50 nM NGF for 5 days. Double staining for Tau and FKBP52 was performed after cytosol extraction to reveal cytoskeletal association. Arrows indicate preferential colocalization of both proteins in the distal part of the nerve cell axon and at the extremity of PC12 cell neurites. (B) Confocal images of primary cortical neurons. Double staining was performed as in A. Analysis of 0.5-μm slices confirms the preferential colocalization in the distal part of the axon (arrowheads).
FKBP52 Inhibits Tubulin Polymerization Induced by Tau in vitro.
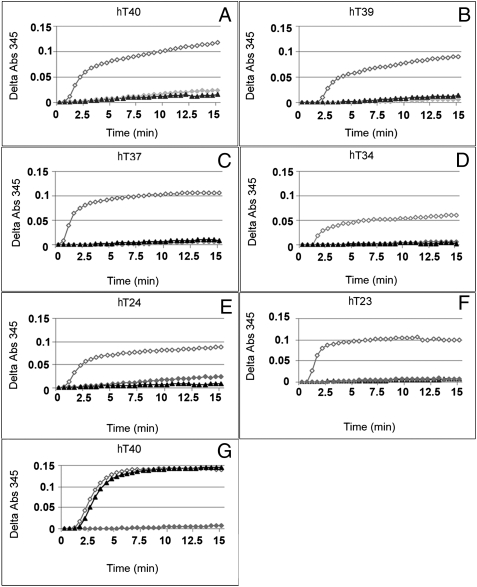
To demonstrate a functional interaction between Tau and FKBP52, a microtubule kinetic assay was set up. In experiments with purified rat brain tubulin alone, no microtubule was formed, whereas in experiments with recombinant human Tau isoforms, an increased absorbance reflecting microtubule assembly was observed (Fig. 5). However, when Tau was added to the tubulin in the presence of purified recombinant FKBP52, formation of microtubules was prevented, whereas GST was ineffective. Similar results were obtained with the six isoforms of human Tau (Fig. 5). We concluded that FKBP52 inhibits the promotion of microtubule assembly by Tau.
Fig. 5.
Effect of FKBP52 on tubulin polymerization induced by recombinant Tau isoforms. Tubulin polymerization was performed by switching the samples from 4 °C to 37 °C, and the change in turbidity was monitored at 345 nm for 15 min. Tubulin (1 mg/mL) purified from rat brain was incubated in the absence ( ) or presence of 1.7 μM (23 μg for HT40) different human Tau isoforms (as indicated in panels A‒F) without FKBP52 (◇) or with 3.5 μM (55 μg) FKBP52 (▲). Tau isoforms differ from each other by the number of repeats in the microtubule-binding domain and insertions in the N terminus. The labeling of Tau isoforms uses the published nomenclature (21). (G) This control experiment was carried out as in A, except that 3.5 μM GST (▲) was used instead of FKBP52.
) or presence of 1.7 μM (23 μg for HT40) different human Tau isoforms (as indicated in panels A‒F) without FKBP52 (◇) or with 3.5 μM (55 μg) FKBP52 (▲). Tau isoforms differ from each other by the number of repeats in the microtubule-binding domain and insertions in the N terminus. The labeling of Tau isoforms uses the published nomenclature (21). (G) This control experiment was carried out as in A, except that 3.5 μM GST (▲) was used instead of FKBP52.
FKBP52 Prevents Tau Accumulation and Neurite Outgrowth in PC12 Cells.
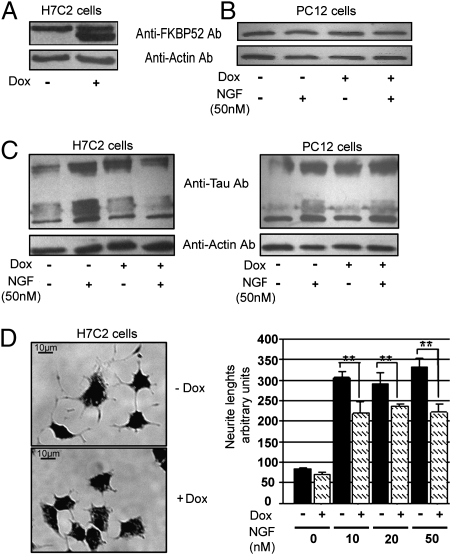
An FKBP52-inducible expression system based on a tetra-cycline-responsive element allowing the generation of a stably transformed PC12 cell line was used (22) to determine a cellular role for FKBP52. Among clones that were positively tested, one clone, so-called H7C2, was selected and used to study the effects of FKBP52 overexpression on PC12 cells and to further investigate the possible relationship between FKBP52 and Tau. Under basal conditions, H7C2 cells expressed endogenous FKBP52, and treatment with doxycycline (Dox) resulted in a marked increase of recombinant FKBP52 protein expression (Fig. 6A). FKBP52 induction in H7C2 cells was about fourfold after 5 days of Dox treatment.
Fig. 6.
Effect of FKBP52 overexpression on Tau accumulation and neurite outgrowth. (A) The FKBP52 level was determined by Western blot using anti-FKBP52 antibody 761, in H7C2 cells treated or not with Dox. The use of rabbit FKBP52 as the exogenous protein explains the small difference in gel mobility with the endogenous rat protein. (B) PC12 cells treated or not with NGF (50 nM) in the absence or presence of 1 μg/mL Dox (doxycycline). Ten micrograms of total protein extracts were analyzed for FKBP52 levels as in A. (C) H7C2 and PC12 cells were treated or not with NGF for 5 days in the presence or absence of Dox for 1 week; 50 μg of extracts was subjected to SDS–PAGE and immunoblotted with anti-Tau (antibody clone DC25). Actin was used as the loading control. (D) Representative H7C2 cells in the presence of NGF (50 nM) with or without Dox. Neurite length was quantified from random photographs (Materials and Methods). Similar results were obtained in three separate experiments. **P < 0.01 (Student–Newman–Keuls test, ANOVA).
The effect of FKBP52 on the accumulation of Tau was examined next. The amount of Tau protein was determined by Western blotting of extracts from cultures of either PC12 cells or H7C2 cells, treated or not with nerve growth factor (NGF) (50 nM) for 5 days with or without Dox. In PC12 cells, FKBP52 expression was unchanged after treatment with NGF (Fig. 6B). As expected, in both PC12 and H7C2 cells an increase in Tau was observed after NGF treatment. However, when H7C2 cells were exposed to Dox in addition to NGF, thus overexpressing FKBP52, no additional accumulation of Tau protein occurred. An increase in Tau protein was still observed in PC12 cells treated with NGF and Dox, ruling out the possibility that Dox was responsible for the lack of decrease in Tau (Fig. 6C). In conclusion, FKBP52 prevented the accumulation of Tau induced by NGF in PC12 cells.
Because one role of Tau is to stimulate neurite outgrowth (12), we investigated the consequence of FKBP52 overexpression on neurite length in both PC12 and H7C2 cells. In the absence of NGF, no neurite outgrowth was observed in H7C2 cells, whether or not they were treated with Dox for a week. However, in H7C2 cells treated with 50 nM NGF and Dox, a 40% (±7) decrease in neurite length, compared to control (H7C2 not treated with Dox) was observed (Fig. 6D). The same effect of Dox on neurite length was observed in H7C2 cells treated with 10 or 20 nM NGF. That Dox by itself was involved in the process of neurite outgrowth could be ruled out because no difference in neurite length between Dox-treated and untreated PC12 cells was observed. The inhibition of neurite outgrowth resulting from FKBP52 overexpression is in agreement with our previous report showing that the loss of FKBP52 in PC12 cells results in the formation of neurite extensions (9). The FKBP52 effect on neurite length could be explained by the binding of Tau to FKBP52, removing Tau from microtubules. In addition, the prevention of Tau accumulation by overexpression of FKBP52 is consistent with the decrease of neurite length and suggests a potential role of this immunophilin in Tau function.
Discussion
This newly discovered “anti-Tau” activity of FKBP52 leads us to re-examine the functions of this protein, which was originally identified and cloned as a modulator of hormone steroid receptors (8, 23). FKPB52 is a multimodular protein, which includes a peptidyl prolyl isomerase (“rotamase”) segment, the function of which is blocked by FK506 (24); rapamycin; and some related nonimmunosuppressive derivatives. There is a noteworthy structural similarity between FKBP52 and Pin1: both proteins have peptidyl–prolyl isomerase (PPIase) activity and a specific protein–protein interaction domain (7). Because the Pin1 PPIase activity restores the function of phosphorylated Tau protein in a model of Alzheimer’s disease (7), the interaction observed between Tau and FKBP52 may have implications for the pathogenesis of the tauopathies, including Alzheimer’s disease. It must be remembered that, unlike FKBP12 (25), FKBP52 does not bind calcineurin (26), and thus FKBP52 does not mediate the immunosuppressant capacity of FK506. Therefore, the pharmacological modulation of the rotamase activity of FKBP52 by nonimmunosuppressive FK506/rapamycin derivatives may offer a unique approach for preventing/reducing the pathogenic effects of misfolded Tau.
In addition to its peptidyl–prolyl isomerase activity, FKBP52 serves as a molecular chaperone. This activity depends on its tetratricopeptide repeat domain (27) to which the molecular chaperone HSP90 and other proteins bind. It has already been noted that chaperone–cochaperone protein complexes play a critical role in neurodegenerative diseases characterized by Tau accumulation (28). We now report that FKBP52 could decrease the function/accumulation of Tau and therefore suggest its possible involvement in these described cochaperone systems (28).
Our results establish a role of FKBP52 in Tau function. The interaction described in this report deserves to be studied further rapidly because effective pharmacological targeting of FKBP52 is likely to become a reality in the near future.
Materials and Methods
Antibodies and Reagents.
Anti-Tau mAB (clone DC25) and anti-Tau mAB (Tau5) were from Sigma and Calbiochem, respectively. Anti-FKBP52 pAB 761 was as described (9). GTP was from Sigma and doxycycline was from Clontech.
Preparation of Tubulin and Microtubule Assembly Assay.
Male adult Sprague–Dawley rats (body weight ∼250 g) were obtained from Janvier. They were killed by decapitation, according to institutional guidelines, and whole brains were used immediately to prepare tubulin as described (9). Microtubule assembly assays were performed as described (9).
Protein Purification and Overexpression of Different Tau Isoforms and FKBP52 Protein.
The six isoforms of human brain Tau were expressed in E. coli from clones hT40, hT39, hT37, hT34, hT24, hT23 and purified as described (21). Full-length FKBP52 was affinity purified as described (24). For the tubulin polymerization assay, FKBP52 bound to glutathione–Sepharose beads (GE Healthcare) was cleaved overnight at 4 °C with 2 units of thrombin (GE Healthcare) and dialyzed against buffer L (0.1 M Mes, 1 mM EGTA, 1 mM MgCl2, 0.1 mM EDTA) with 10% glycerol and complemented before use to 1 mM GTP and 1 mM DTT and 10 μM D-Phe-Pro-Arg-CMK (a potent irreversible inhibitor of thrombin) (Biomol).
Phosphorylation and Hyperphosphorylation of Recombinant Tau.
Rat brain extract was used as the source of kinase activity as described (18). Briefly, recombinant hT40 was incubated with cytosol of adult rat brain, in the presence or absence of okadaic acid, to give HP-Tau and P-Tau, respectively.
Protein-Binding Assays.
GST-pull down assay.
One hundred microliters of glutathione–Sepharose beads preloaded with 1 nmol GST-FKBP52 or 1 nmol GST were washed four times with 500 μl of buffer A (buffer L complemented with 1 mM DTT and 1 mM GTP) and then resuspended in the same buffer containing protein microtubule extract. The proteins were analyzed for the presence of Tau isoforms by SDS–PAGE Western blot analysis using antibody anti-Tau (clone DC25) diluted 1/1,000.
Coimmunoprecipitation assay.
This assay was carried out with 1 mg cytosol microtubule extract as described (12).
Dot blot assay.
One hundred microliters of buffer A containing different amounts of hT40 were applied to a nitrocellulose membrane, blocked with 5% nonfat milk in PBS containing 0.1% Tween 20 (PBS-T) at room temperature, and washed with PBS-T and buffer A, followed by 2 h incubation at room temperature with 100 μl of buffer A containing 0.5 μg recombinant FKBP52. The membranes were washed with buffer IP [50 mM Tris (pH 7.5)/150 mM NaCl/2 mM MgCl2/0.1% Brij97 (Sigma)/10% glycerol/protease inhibitors] and with PBS-T. After blocking with milk in PBS-T, the membranes were incubated with anti-FKBP52 761 antibody. The presence of FKBP52 was revealed by ECL. Quantitation was performed with Quantity-one software using Chemidoc XRS fitted with a 16-bit CCD camera (Bio-Rad).
Cell Line and Stable DNA Transfection.
Generation of H7C2 cells.
The cDNA encoding rabbit FKBP52 was inserted into the HindIII and AccI restriction sites of the pTRE2 vector (Clontech) to give pTRE2-FKBP52. Transfection of 100 μg of pTR2-FKBP52 and 10 μg of pTK-hygromicin was carried out in a commercially available PC12 Tet-on cell line (Clontech) that expresses the reverse tetracycline-controlled transactivator, using lipofectamine (Invitrogen). Stably transfected cells were selected with 100 μg/mL hygromycin and screened individually.
Cell culture.
PC12 cells and H7C2 cells were grown in DMEM containing 10% (vol/vol) horse serum and 5% (vol/vol) FBS (Invitrogen) at 37 °C in 90% O2/10% CO2. The differentiated neuronal phenotype of cells grown on plastic dishes coated with 10 μg/mL poly(L)-lysine (Sigma) was induced by adding NGFs (Invitrogen) for 5 days. Primary cultures from cerebral cortex of embryonic day 17 rat fetuses were carried out. Dissociated cells were plated (50,000 cells/mL) on glass coverslips coated with poly(L-ornithine) and cultured in a defined medium in 95% O2/5% CO2 at 37 °C.
Immunocytochemistry.
Cells were grown on glass coverslips precoated in 12-well tissue culture plates. Primary cells and PC12 cells were incubated for 2.5 and 3 min, respectively, in PEM buffer (80 mM Piperazine-N,N'-bis-(ethanesulfonic acid), 1 mM MgCl2, 2 mM EGTA, pH 6.9) with 0.05% Triton, rinsed with warm Triton-free PEM, fixed for 5 min with methanol at –20 °C, and incubated with affinity-purified anti-FKBP52 761 (1/1,000) and anti-Tau5 (1/100). Anti-rabbit Alexa Fluor 488–conjugated (Invitrogen), anti-mouse FITC-conjugated, or Cy3 red-conjugated (GE Healthcare) antibodies were used at 1/500 and 1/1,000, respectively. The coverslips were examined by epifluorescence using a Zeiss axioplan 2 microscope with either a 63× objective or by confocal microscopy (Zeiss).
Quantitation of neurite outgrowth.
Random field photographs of PC12 and H7C2 cells, in each of three wells, were analyzed with Neuron J software. The average neurite length was determined by measuring the longest neurites of at least 200 randomly selected cells.
Acknowledgments
We are grateful to Dr. Krzysztof Rajkowski (Institut National de la Santé et de la Recherche Médicale U788, Kremlin-Bicêtre, France) for critical reading. We thank Dr. Martine El-Etr (Institut National de la Santé et de la Recherche Médicale U788, Kremlin-Bicêtre, France) for her contribution and P. Leclerc for confocal photography. We acknowledge the support of the foundation Vivre Longtemps (FVL) Fondation Caisses d’Epargne pour la solidarité - (FCEs-Paris), the Institut Baulieu (Paris), The Florence Gould Foundation (New York), and the Société de Secours des Amis des Sciences (Paris).
Footnotes
The authors declare no conflict of interest.
References
- 1.Schreiber SL. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991;251:283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- 2.Steiner JP, et al. High brain densities of the immunophilin FKBP colocalized with calcineurin. Nature. 1992;358:584–587. doi: 10.1038/358584a0. [DOI] [PubMed] [Google Scholar]
- 3.Snyder SH, Sabatini DM. Immunophilins and the nervous system. Nat Med. 1995;1:32–37. doi: 10.1038/nm0195-32. [DOI] [PubMed] [Google Scholar]
- 4.Gold BG. FK506 and the role of immunophilins in nerve regeneration. Mol Neurobiol. 1997;15:285–306. doi: 10.1007/BF02740664. [DOI] [PubMed] [Google Scholar]
- 5.Shim S, et al. Peptidyl-prolyl isomerase FKBP52 controls chemotropic guidance of neuronal growth cones via regulation of TRPC1 channel opening. Neuron. 2009;64:471–483. doi: 10.1016/j.neuron.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiene C, Fischer G. Enzymes that catalyse the restructuring of proteins. Curr Opin Struct Biol. 2000;10:40–45. doi: 10.1016/s0959-440x(99)00046-9. [DOI] [PubMed] [Google Scholar]
- 7.Lu KP, Zhou XZ. The prolyl isomerase PIN1: A pivotal new twist in phosphorylation signalling and disease. Nat Rev Mol Cell Biol. 2007;8:904–916. doi: 10.1038/nrm2261. [DOI] [PubMed] [Google Scholar]
- 8.Lebeau MC, et al. P59, an hsp 90-binding protein. Cloning and sequencing of its cDNA and preparation of a peptide-directed polyclonal antibody. J Biol Chem. 1992;267:4281–4284. [PubMed] [Google Scholar]
- 9.Chambraud B, Belabes H, Fontaine-Lenoir V, Fellous A, Baulieu EE. The immunophilin FKBP52 specifically binds to tubulin and prevents microtubule formation. FASEB J. 2007;11:2787–2797. doi: 10.1096/fj.06-7667com. [DOI] [PubMed] [Google Scholar]
- 10.Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 11.Lee VMY, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 12.Garcia ML, Cleveland DW. Going new places using an old MAP: Tau, microtubules and human neurodegenerative disease. Curr Opin Cell Biol. 2001;13:41–48. doi: 10.1016/s0955-0674(00)00172-1. [DOI] [PubMed] [Google Scholar]
- 13.Goedert M, Wischik CM, Crowther RA, Walker JE, Klug A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: Identification as the microtubule-associated protein tau. Proc Natl Acad Sci USA. 1988;85:4051–4055. doi: 10.1073/pnas.85.11.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: Sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 15.Delacourte A, Buée L. Tau pathology: A marker of neurodegenerative disorders. Curr Opin Neurol. 2000;13:371–376. doi: 10.1097/00019052-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Mandelkow EM, Mandelkow E. Tau in Alzheimer’s disease. Trends Cell Biol. 1998;8:425–427. doi: 10.1016/s0962-8924(98)01368-3. [DOI] [PubMed] [Google Scholar]
- 17.Ballatore C, Lee VMY, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 18.Goedert M, et al. The abnormal phosphorylation of tau protein at Ser-202 in Alzheimer disease recapitulates phosphorylation during development. Proc Natl Acad Sci USA. 1993;90:5066–5070. doi: 10.1073/pnas.90.11.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kempf M, Clement A, Faissner A, Lee G, Brandt R. Tau binds to the distal axon early in development of polarity in a microtubule- and microfilament-dependent manner. J Neurosci. 1996;16:5583–5592. doi: 10.1523/JNEUROSCI.16-18-05583.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weissmann C, et al. Microtubule binding and trapping at the tip of neurites regulate tau motion in living neurons. Traffic. 2009;11:1655–1668. doi: 10.1111/j.1600-0854.2009.00977.x. [DOI] [PubMed] [Google Scholar]
- 21.Goedert M, Jakes R. Expression of separate isoforms of human tau protein: Correlation with the tau pattern in brain and effects on tubulin polymerization. EMBO J. 1990;9:4225–4230. doi: 10.1002/j.1460-2075.1990.tb07870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riggs DL, et al. The Hsp90-binding peptidyl isomerise FKBP52 potentiates glucocorticoid signalling in vivo. EMBO J. 2003;22:1158–1167. doi: 10.1093/emboj/cdg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chambraud B, et al. Overexpression of p59-HBI (FKBP59), full length and domains, and characterization of PPlase activity. Biochem Biophys Res Commun. 1993;196:160–166. doi: 10.1006/bbrc.1993.2229. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, et al. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 26.Lebeau MC, Myagkikh I, Rouvière-Fourmy N, Baulieu EE, Klee CB. Rabbit FKBP-59/HBI does not inhibit calcineurin activity in vitro . Biochem Biophys Res Commun. 1994;203:750–755. doi: 10.1006/bbrc.1994.2246. [DOI] [PubMed] [Google Scholar]
- 27.Pirkl F, Fischer E, Modrow S, Buchner J. Localization of the chaperone domain of FKBP52. J Biol Chem. 2001;276:37034–37041. doi: 10.1074/jbc.M102595200. [DOI] [PubMed] [Google Scholar]
- 28.Dickey CA, et al. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest. 2007;117:648–658. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]