Abstract
Hemoproteins, hemoglobin and myoglobin, once released from cells can cause severe oxidative damage as a consequence of heme redox cycling between ferric and ferryl states that generates radical species that induce lipid peroxidation. We demonstrate in vitro that acetaminophen inhibits hemoprotein-induced lipid peroxidation by reducing ferryl heme to its ferric state and quenching globin radicals. Severe muscle injury (rhabdomyolysis) is accompanied by the release of myoglobin that becomes deposited in the kidney, causing renal injury. We previously showed in a rat model of rhabdomyolysis that redox cycling between ferric and ferryl myoglobin yields radical species that cause severe oxidative damage to the kidney. In this model, acetaminophen at therapeutic plasma concentrations significantly decreased oxidant injury in the kidney, improved renal function, and reduced renal damage. These findings also provide a hypothesis for potential therapeutic applications for acetaminophen in diseases involving hemoprotein-mediated oxidative injury.
Keywords: isoprostanes, oxidative damage, hemoglobin, myoglobin
The normal function of myoglobin (Mb) and hemoglobin (Hb) is to transport oxygen and carbon dioxide. In pathologic conditions in which these hemoproteins are released from the reducing environment of cells, the ferrous heme can be oxidized to the ferric state (FeIII), conferring peroxidase activity to the hemoproteins. Thus, hemoproteins can reduce hydroperoxides, such as hydrogen peroxide (H2O2) and lipid hydroperoxides (1 –4), in a process that further oxidizes the hemoprotein to the ferryl state (FeIV = O). Both the protein radical and ferryl heme can generate lipid-based radical species through abstraction of a lipid hydrogen atom (5). In the absence of antioxidants, these reactions can initiate oxidation of free and phospholipid-esterified unsaturated fatty acids.
An accumulating body of evidence suggests that lipid peroxidation catalyzed by these hemoproteins is responsible for the oxidative injuries associated with rhabdomyolysis, subarachnoid hemorrhage, and disorders involving hemolysis. These pathologies are associated with accumulation of hemoproteins in the kidney or in the cerebral spinal fluid (CSF) followed by intense vasoconstriction (6 –8). It is postulated that the molecules responsible for vasoconstriction are generated by the hemoprotein-catalyzed oxidation of lipids (9).
The coupling of a peroxidase-generated radical to lipid oxidation also occurs in the prostaglandin H2 synthases (PGHS). In the PGHS-peroxidase site, reduction of a hydroperoxide yields a ferryloxo protoporphyrin radical cation. Through intramolecular electron transfer, the radical generates the tyrosine radical in the PGHS-cyclooxygenase site that catalyzes oxygenation of arachidonic acid (AA). Acetaminophen (ApAP) inhibits the PGHS by reducing the protoporphyrin radical cation, thereby blocking formation of the catalytic tyrosyl radical (10 –12). As hydroperoxides oxidize and ApAP reduces the porphyrin, it may be appreciated that inhibition of the PGHS-cyclooxygenase by ApAP is an inverse function of hydroperoxide concentration.
The analogy between the peroxidase catalytic cycle of PGHS and the pseudoperoxidase activity of Mb and Hb led us to hypothesize that ApAP would prevent the peroxide-driven lipid peroxidation catalyzed by these hemoproteins. In this study we demonstrate that ApAP inhibits the oxidation of free AA catalyzed by Mb incubated with H2O2 in vitro. This action of ApAP results from reduction of the high ferryl oxidation state of Mb to the ferric state and prevention of the formation of the protein radicals.
Rhabdomyolysis is associated with extensive muscle injury that is accompanied by the release of Mb into the circulation and secondary renal failure (13 –15). We have previously obtained compelling evidence both in animals and in humans that rhabdomyolysis-induced renal injury is caused by redox cycling of the heme moiety of Mb (6, 8, 9, 16). We therefore also explored the ability of ApAP to inhibit hemoprotein-induced oxidative damage in vivo, using a rat model of rhabdomyolysis-induced renal injury.
Results
ApAP Inhibits AA Oxidation Catalyzed by Mb and Hemoglobin.
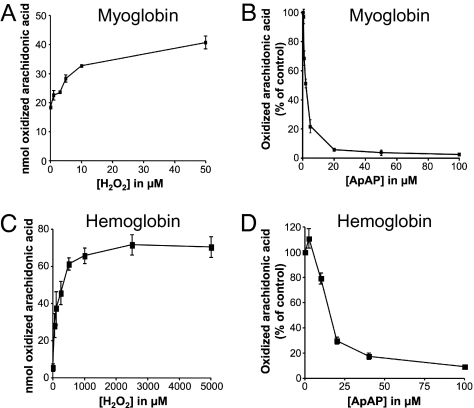
We studied oxidation of unsaturated fatty acids by Mb and Hb in presence of H2O2 in vitro as a model for the in vivo hemoprotein-catalyzed oxidation of lipids that causes oxidant injury. We determined that the increase in the oxidation of AA by Mb and Hb was dependent on H2O2 concentration (Fig. 1 A and C). Accordingly, we performed the oxidation experiments in the presence of 5 μM H2O2, unless stated otherwise. ApAP inhibits oxidation of AA catalyzed by Mb with an IC50 of 2.25 ± 0.2 μM (n = 8) (Fig. 1B) and by Hb with an IC50 of 17.7 ± 2.5 μM (Fig. 1D). The IC50 of ApAP in these experimental conditions is in the therapeutic range for humans (10–30 μg/mL; 67–200 μM). Also, we tested the commonly used soluble antioxidants ascorbic acid (vitamin C) and Trolox, a soluble analog of vitamin E (Fig. S1). They inhibited the Mb-catalyzed oxidation reaction less effectively with IC50 4-fold and 7-fold higher than ApAP, respectively (vitamin C, 8.95 ± 1.23 μM, n = 8; Trolox, 15.95 ± 1.31 μM, n = 6). The differences between each IC50 are statistically significant (Student’s t test; P < 0.005). ApAP is 6-fold less potent as an inhibitor of the oxidation of AA when Mb is incubated in presence of 50 μM of H2O2 (IC50 = 13.5 ± 1.2 μM, n = 4), thus exhibiting the same inverse relationship of inhibitor potency to peroxide concentration observed with the PGHS (10).
Fig. 1.
Inhibition by ApAP of Mb- and Hb-induced oxidation of AA. (A) Ferric Mb (10 μM) was incubated with 10 μM AA. The reaction was initiated by adding H2O2 and proceeded for 3 h at 37 °C. The residual AA and the products of oxidation were extracted and analyzed as described in Methods. The oxidation is represented as nanomoles of AA oxidized by Mb in 3 h. Each data point represents the average of six different values. (B) Ferric Mb (10 μM) was incubated with 10 μM AA and ApAP. The reaction was initiated by adding 5 μM of H2O2 and proceeded for 3 h at 37 °C. The oxidation is represented as the percentage of AA oxidized by Mb in 3 h compared to the control in which no ApAP is present. Each data point represents the average of eight different values. (C) Ferric Hb (45 μM) was incubated with 10 μM AA. The reaction was initiated by adding H2O2 and proceeded for 3 h at 37 °C. The oxidation was analyzed as described above. Each data point represents the average of six different values. (D) Ferric Hb (45 μM) was incubated with 10 μM AA and ApAP. The reaction was initiated by adding 30 μM of H2O2 and proceeded for 3 h at 37 °C. Each data point represents the average of six different values.
ApAP Reduces Ferryl Mb to Ferric Mb.
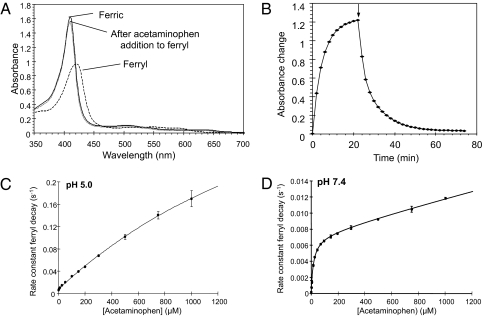
Addition of H2O2 to ferric Mb generates ferryl Mb as shown by a shift in the maximum absorption wavelength from 408 nm (ferric) to 425 nm (ferryl) (Fig. 2A). Addition of ApAP to the solution of ferryl Mb leads to reduction of the heme from its ferryl to its ferric oxidation state in a pseudofirst order time-dependent manner (Fig. 2 A and B). The final spectrum is a close match to the original high spin ferric spectrum (Fig. 2A), with a small difference in peak height typical of a subpopulation of the hemoprotein undergoing irreversible radical-induced damage. The spectral shift is not due to ApAP binding to the ferric heme, as adding ApAP to ferric Mb does not change the spectrum.
Fig. 2.
Effect of ApAP on the state of oxidation of Mb. (A) Transition from ferric to ferryl Mb and its reduction by ApAP were monitored by recording visible spectra (350–650 nm). Ferryl Mb was generated by incubating ferric Mb (10 μM) with 17.5 μM H2O2 until there was no more change at 425 nm (B). At this time, ApAP (174 μM) was added and spectra were recorded every 2 min. The arrow indicates the time of addition of ApAP. (C and D) Effect of ApAP on the rate constant of ferryl decay. Ferryl Mb (10 μM) was reacted with ApAP in sodium acetate (pH 5.0) (C) or sodium phosphate (pH 7.4) (D). The pseudofirst order rate constants for reduction of ferryl to ferric Mb were measured from the time course (425–408 nm) and plotted as a function of ApAP concentration. With no ApAP, the rate constant for ferryl reduction is not zero due to autoreduction, which is more apparent at pH 5 than at pH 7.4. The data are fitted to a double rectangular hyperbola function (n = 3).
Reduction of Ferryl Mb by ApAP Is Enhanced at Low pH.
The pseudoperoxidase activity of Mb is increased in an acidic milieu (16). As the pH may be reduced in pathophysiologic states associated with Mb released from cells, the effect of pH on the reduction of ferryl Mb by ApAP was evaluated. The pseudofirst order rate constant for ApAP-induced ferryl reduction is plotted in Fig. 2C (pH 5) and D (pH 7.4) as a function of ApAP concentration. At pH 5 the rate constant for reduction of ferryl Mb by 1 mM ApAP (1.7 × 10−1 s−1) is 14-fold greater than at pH 7.4 (1.2 × 10−2 s−1).
ApAP Quenches the Mb Protein Radical.
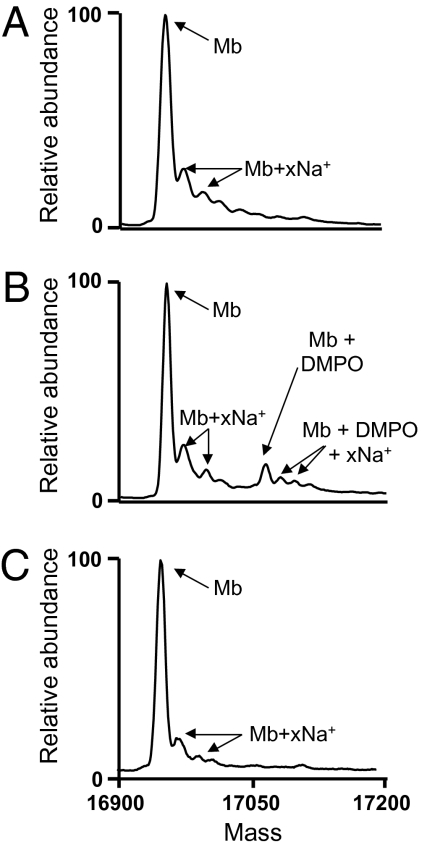
Previous work has demonstrated the effective use of 5,5-dimethyl-1-pyrroline N-oxide (DMPO) immuno-spin trapping EPR and mass spectrometry to identify the site of protein nitrone adduct formation at tyrosine-103 of sperm whale Mb (17, 18). Similar experiments were performed to examine the possible effects of ApAP on the formation of the tyrosyl radical on horse heart Mb following addition of H2O2. In the deconvoluted mass spectrum of Mb (Fig. 3A), the average molecular weight (M r) of 16,952.0 (M r calculated = 16,951.5) is observed in addition to multiple sodium adducts (e.g., M r = 16,971.0 and 16,996.0 vs. M r calculated = 16,973.5 and 16,995.5). The deconvoluted electrospray ionization (ESI) mass spectrum of the reaction mixture containing Mb plus H2O2 and DMPO (Fig. 3B) reveals another peak (M r = 17,063.0; M r calculated = 17,062.5) corresponding to a single stable DMPO nitrone adduct on the protein. In the presence of ApAP (Fig. 3C), the DMPO peak is not observed, suggesting that the ApAP quenches the radical formation faster than the DMPO adduct can be formed. Control experiments in which Mb was reacted with H2O2, DMPO, or ApAP did not show any evidence of adduct formation. These controls demonstrate that adduct formation is dependent on the presence of both H2O2 and DMPO.
Fig. 3.
ApAP prevents the H2O2-dependent formation of DMPO adducts on Mb residues. Ferric Mb (100 μM) was incubated with, without, or with different combinations of H2O2 (250 μM), DMPO (100 mM), and ApAP (1 mM). After 2 h at 37 °C, the protein was analyzed by ESI mass spectrometry. The resulting deconvoluted mass spectra are shown for Mb (A), Mb plus DMPO and H2O2 (B), and Mb plus DMPO, H2O2, and ApAP (C). Mb + xNa+: peaks corresponding to salt adducts resulting from addition of 1 or 2 sodium ions per molecule of Mb.
ApAP Prevents Formation of Heme-to-Protein Cross-Links.
Heme-to-protein cross-linking (Mb-X) is a more cytotoxic form of Mb (19 –21) that is generated at low pH when Mb reacts with peroxide. This form of Mb is found in the urine of patients with rhabdomyolysis (6 –8). We analyzed the Mb and heme species by HPLC before and after addition of H2O2 and after addition of ApAP followed by H2O2. ApAP prevents both oxidative modifications and Mb-X formation in vitro at concentrations of ApAP within the therapeutic range in humans (Fig. S2). Mb, as analyzed by HPLC at 400 nm, shows only one major peak due to heme (Fe-protopophyrin-IX ∼15 min elution time). The chromatogram following the reaction of Mb with peroxide in the presence of ApAP is virtually identical to the lower trace, showing no significant oxidative damage to the heme moiety. However, in the absence of ApAP there is a decrease of the undamaged heme peak of 35%, accompanied by the appearance of a series of oxidatively damaged hemes (5–12 min) and the conversion of 21% of the heme to Mb-X (19–26 min) (Fig. S3).
Visual Appearance of Kidneys.
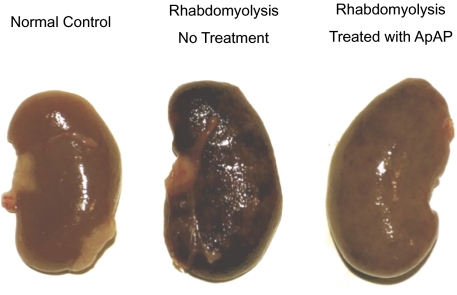
The appearance of the kidneys from rats with rhabdomyolysis was markedly altered by treatment with ApAP (Fig. 4). Rhabdomyolysis without ApAP treatment produced a generalized deep red to black discoloration of the kidneys. In rats treated with ApAP, rhabdomyolysis produced only slight mottled discoloration of the kidneys.
Fig. 4.
Visual examination of a representative kidney from a normal rat (Left), a rat with rhabdomyolysis (Center), and a rat with rhabdomyolysis treated with ApAP (Right).
Pretreatment With ApAP Inhibits Rhabdomyolysis-Induced Lipid Peroxidation.
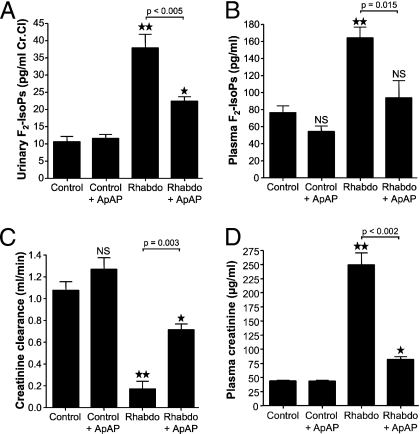
The plasma concentration of ApAP achieved in rats was 16 ± 5 μg/mL, which is within the therapeutic range in humans (10–30 μg/mL). F2-isoprostanes (F2-IsoPs) are a well substantiated biomarker for lipid peroxidation (22). The urinary excretion of F2-IsoPs was markedly increased (mean 3.5-fold) in the rhabdomyolysis group at 37.8 pg/mL Cr.Cl (pg/mL of creatinine clearance) compared to the control group at 10.6 pg/mL Cr.Cl (P < 0.001). Treatment with ApAP suppressed the mean urinary excretion of F2-IsoPs in the rhabdomyolysis group (41% reduction) from 37.8 to 22.4 pg/mL Cr.Cl (P < 0.005) (rhabdo vs. rhabdo + ApAP, Fig. 5A). The rhabdomyolysis-induced increase in plasma levels of F2-IsoPs is also significantly attenuated by ApAP from 163 pg/mL (rhabdomyolysis alone) to 93 pg/mL (rhabdo + ApAP) (P = 0.015) (Fig. 5B).
Fig. 5.
Effect of treatment with ApAP on oxidative injury (A and B) and on kidney function (C and D) in normal rats (controls), in normal rats treated with ApAP (control + ApAP), in rats with rhabdomyolysis (Rhabdo), and in rats with rhabdomyolysis treated with ApAP (Rhabdo + ApAP). (A) Urinary excretion of F2-IsoPs. **, P < 0.0001 for Rhabdo vs. controls; *, P < 0.005 for Rhabdo + ApAP vs. controls. The bracket indicates that ApAP significantly reduced urinary F2-IsoPs compared with Rhabdo alone (P = 0.015) (n ≥ 6 in each group). (B) Plasma levels of F2-IsoPs. **, P = 0.0002 for Rhabdo vs. controls. The bracket denotes a significant difference (P = 0.01) between Rhabdo + ApAP and Rhabdo, and NS denotes no significant difference from controls (n ≥ 6 in each group). (C) Creatinine clearance. **, P < 0.0001 for Rhabdo vs. controls; *, P = 0.012 for Rhabdo + ApAP vs. controls; NS denotes no significant difference from controls (n ≥ 6 in each group). (D) Plasma levels of creatinine. **, P < 0.0001 for Rhabdo vs. controls; *, P = 0.0002 for Rhabdo + ApAP vs. controls (n ≥ 6 in each group).
Pretreatment With ApAP Reduces Rhabdomyolysis-Induced Renal Failure.
Following induction of rhabdomyolysis, there was a profound reduction of creatinine clearance from 1.07 mL/min in control animals to 0.17 mL/min in the rhabdomyolysis group that did not receive ApAP (P < 0.0001). Treatment with ApAP significantly (P = 0.003) attenuated the decrease in creatinine clearance compared to the untreated rhabdomyolysis group (0.71 mL/min vs. 0.17 mL/min) (Fig. 5C). Treatment with ApAP had no effect on creatinine clearance in control animals (1.26 vs. 1.07 mL/min, P value not significant). Rhabdomyolysis produced the expected rise in plasma creatinine (P < 0.0001). The increase in plasma creatinine produced by rhabdomyolysis was significantly attenuated by treatment with ApAP (P < 0.002), with creatinine rising in the ApAP-treated rhabdomyolysis group to only 18.6% of the increase seen with rhabdomyolysis plus vehicle (Fig. 5D).
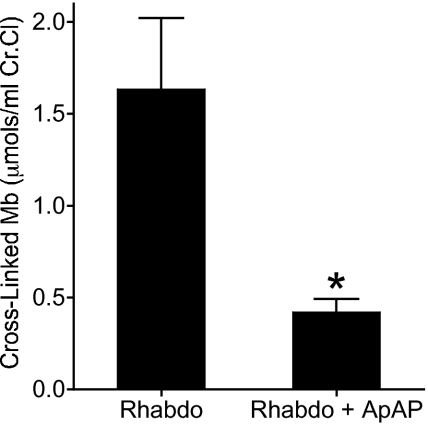
Fig. 6.
ApAP inhibits the formation of the hemoprotein cross-links in urine from rats with rhabdomyolysis. The amount of cross-linked hemoprotein in urine from rats with rhabdomyolysis (Rhabdo) and with rhabdomyolysis treated with ApAP (Rhabdo + ApAP) was determined by HPLC analysis as described in Methods. Chromatograms were measured at 400 nm as shown in Fig. S3. The peak corresponding to the cross-linked Mb at 19 min was integrated and the concentration was determined. The values represent the means ± SEM (n ≥ 5 in each group). An unpaired double-tailed Student’s t test was performed after having verified the normal distribution using the D’Agostino and Pearson omnibus normality test.
Renal Changes Associated With Rhabdomyolysis and Effects of Pretreatment With ApAP.
Periodic acid-Schiff (PAS) staining showed that compared with normal kidneys in control animals, kidneys in the Rhabdo group had widespread abnormalities of various types in proximal tubules, with necrosis, flattening of epithelium, cloudy swelling of cells, loss of brush border, and cellular debris and casts in the lumen, including yellowish pigmented casts (Fig. S4f). Kidneys in the Rhabdo + ApAP group had similar changes but these were less marked, and there were fewer pigmented casts (Fig. S4g). Measurement of one of the representative changes, the proportion of tubules with an intact brush border, showed that this proportion was 76 and 79% in two control rats, 17 and 17% in two rats in the Rhabdo group, and 27 and 31% in two rats in the Rhabdo + ApAP group. Immunohistologic study showed no Mb in the kidneys of rats in the control + ApAP group (Fig. S4a). Mb was detected in necrotic tubular epithelium and in pigmented casts in the Rhabdo kidneys (Fig. S4b). In the Rhabdo + ApAP group, there was still Mb in necrotic epithelium, but this was a little less widespread, and fewer casts contained Mb (Fig. S4c).
Posttreatment With ApAP After Induction of Rhabdomyolysis Improves Renal Function.
Treatment with ApAP 2 and 22 h after glycerol injection to induce rhabdomyolysis also was effective in improving renal function (Fig. S5), with tubular changes comparable to those in the Rhabdo + ApAP group on both PAS staining and immunoperoxidase study for Mb and a comparable proportion of tubules with brush border, 20 and 39% (Fig. S4 d and h).
Evidence That the Mechanism by Which ApAP Reduces Rhabdomyolysis-Induced Renal Failure in Vivo Is by Inhibiting Mb Redox Cycling.
Ferryl Mb is required for the formation of Mb-X. Therefore, the amount of Mb-X formed reflects the extent of redox cycling between ferric and ferryl Mb. Accordingly, inhibition of Mb redox cycling is accompanied by reduction in the formation of Mb-X. We have shown that ApAP inhibits the formation of Mb-X in vitro (Fig. S3). To firmly establish that the mechanism by which ApAP’s ability to reduce rhabdomyolysis-induced renal failure in vivo is by inhibiting Mb redox cycling we measured the urinary levels of Mb-X in untreated rats with rhabdomyolysis and rats with rhabdomyolysis treated with ApAP. In rats treated with ApAP the urinary levels of Mb-X were reduced by ∼75% compared to untreated rats (P < 0.004) (Fig. 6).
The possibility that muscle injury is decreased by acetaminophen was not directly examined by measurement of creatine kinase levels, but the observation that levels of myoglobin deposited in the kidney showed little difference between the treatment groups (Fig. S4) suggests that muscle injury was not significantly affected by acetaminophen. Moreover, the fact that acetaminophen was protective, despite being given 2 h after the initiation of muscle injury (Fig. S5), strongly supports our hypothesis that acetaminophen works mainly by decreasing renal injury through inhibition of redox cycling of the heme group of myoglobin.
Discussion
We report that ApAP inhibits lipid peroxidation catalyzed by ferryl Mb and ferryl Hb in vitro. The low micromolar IC50 for this inhibition is within the range of concentrations resulting from clinical doses of ApAP in humans. Efficacy in this range of drug concentration is important as higher plasma concentrations of ApAP following overdose may lead to hepatic necrosis and renal damage in both humans and laboratory animals (for review see refs. 23 –26). When Mb and Hb are released from their cellular environments, they undergo redox cycling that engenders lipid peroxidation and its pathophysiological consequences. This evidence that hemoprotein-induced lipid peroxidation is inhibited by ApAP therefore provides a mechanistic basis for therapeutic hypotheses.
Catalysis of lipid peroxidation by Hb and Mb is initiated by their pseudoperoxidase activity in which a hydroperoxide is reduced. This activity results in two-electron oxidation of the hemoproteins, generating a ferryl heme and, by intramolecular electron transfer, a protein radical. The ferryl heme and protein radical catalyze lipid peroxidation (5). Lipid hydroperoxides also feed the ferric/ferryl redox cycle of the hemoprotein, thus generating more ferryl heme (27, 28).
The two-electron oxidation of Mb by peroxide yields ferryl heme and, if reacting with the ferric form of the protein, a protein-based radical. We demonstrate that ApAP reduces ferryl Mb to its ferric state, a one-electron reducing step. The kinetic profile of ApAP reduction of Mb (Fig. 2 C and D) is consistent with the kinetic profiles of other reductants of ferryl Mb such as ascorbate (29). The concentration dependence of ferryl formation follows a double rectangular hyperbola function indicative of a two-site mechanism of reduction, readily seen at pH 7.4 but still observable at pH 5. One pathway is through direct electron transfer between the reductant and heme by access to the heme pocket and the second pathway is via an electron transfer pathway involving a tyrosine redox intermediate (29, 30). This protein electron transfer pathway, present in human Mb and the α-subunit of human Hb, allows rapid reduction of the ferryl heme at low concentrations of reductant (30). In addition to reduction of ferryl Hb, we show that in the presence of ApAP the peroxidase catalytic activity does not generate the globin radical that can be trapped by DMPO. In horse heart Mb, Tyr103 and Trp14 have been identified as the sites of the phenoxyl radical trapped by DMPO (17), whereas in human Hb Cys93 of the β-chain and Tyr24, Tyr42, and His of the α-chain have been identified as radical sites where DMPO forms adducts (31). Prevention of the formation of a protein–DMPO adduct is likely to arise from rapid sequestration of the protein-based radicals by ApAP, preventing propagation to other molecules such as DMPO or lipids. Therefore, the net effect of reducing both the ferryl heme and the globin radical provides a basis for the observed inhibition by ApAP of Mb-catalyzed oxygenation of AA. The consequence of reduction of the ferryl heme iron and the protein radical is the one-electron oxidation of two ApAp molecules. It has recently been reported that oxidation of ApAP by methemoglobin forms a semiquinone imine radical species that rapidly dimerizes (32). Therefore, the oxidation of ApAP provides a safe pathway for removal of Mb protein radicals and ferryl heme iron, initially through the formation of another radical species, but one that rapidly dissipates through a self-termination reaction.
We also demonstrate that ApAP inhibits formation of the oxidatively modified form of Mb in which the heme is covalently cross-linked to the apoprotein (Mb-X) (33). This finding is congruent with the evidence that ApAP sequesters the protein radical and removes the ferryl species through reduction as both ferryl heme and radical are requirements for Mb-X formation (34). Mb-X is a more potent catalyst of lipid peroxidation than is the ferryl Mb itself (19) and Mb-X also catalyzes reactions that can generate toxic oxygen species (18, 19). Thus lipid peroxidation that results from reaction of a hydroperoxide with Mb is likely the sum of that induced by the higher oxidative state of Mb itself plus that catalyzed by the smaller amounts of the more potent Mb-X. The identification of heme-to-protein cross-linked Hb in the cerebrospinal fluid of patients with subarachnoid hemorrhage and Mb-X in the urine of patients with rhabdomyolysis places the formation of this cross-linked form of respiratory hemoproteins in a pathophysiologic context (8).
Lipid peroxidation by hydroperoxide-catalyzed oxidation of Mb is accelerated by reducing the pH, likely due to the protonation of the oxoferryl heme. The protonated form of the ferryl heme (FeIV-OH−) is equivalent to the ferric species plus a radical released either as a hydroxyl radical (OH•) or as a porphyrin/protein radical (16, 35). As such, it is the instability of this species that gives enhanced peroxidatic activity (36). The formation of Mb-X also proceeds at an accelerated rate at a lower pH values (34). We have demonstrated that ApAP is even more potent in reducing the ferryl heme at lowered pH. This pH effect is important in considerations of the potential of ApAP to inhibit lipid peroxidation clinically, as some pathophysiologic states, such as myocardial ischemia, are associated with reduced pH.
Hemoprotein-induced lipid peroxidation has been implicated in a number of pathophysiologic processes. Degradation of hemoproteins by induction of heme oxygenase is known to prevent kidney failure in the rat model of rhabdomyolysis (37), and the high levels of Mb accumulating in the kidney are known to produce lipid peroxidation in that target organ with formation of the potent vasoconstrictors, F2-IsoPs (9). That the renal failure associated with rhabdomyolysis is due in part to the lipid peroxidation is further suggested by the attenuation of renal failure by alkalinization (9), by inhibitors of lipid peroxidation (38), and by a thromboxane A2 receptor antagonist that blocks F2-IsoPs vasoconstriction (39). Further support for this hypothesis is the present evidence that ApAP inhibits lipid peroxidation and improves renal failure in a rat model of rhabdomyolysis, with a reduction in the amount of structural renal damage, such as in the extent of loss of proximal tubular brush border. This effect was seen when ApAP was given before induction of rhabdomyolysis and when given only after rhabdomyolysis. These findings provide proof of the concept that the inhibition of lipid peroxidation by ApAP can alter a hemoprotein-induced pathophysiologic process in vivo.
Potentially, many compounds that are peroxidase substrates (40, 41) may exert similar effects to those of ApAP. However, ApAP is the only one available for human use, to our knowledge, which has this potency within its range of safety. Clearly, ApAP can be considered a lead compound for the development of other compounds with a higher therapeutic index that reduce the ferryl heme to its ferric state.
Mb-induced lipid peroxidation has been proposed as a contributor to the myocardial injury that results from ischemia (with its low pH) followed by reperfusion (1, 42). Indeed, lipid peroxidation has been demonstrated following coronary reperfusion in human myocardial ischemia (43). In this context, our findings provide a basis for a hypothesis that ApAP could produce therapeutic benefit in clinical myocardial ischemia/reperfusion, a hypothesis also supported by evidence that lipid peroxidation and myocardial injury are attenuated by ApAP in experimental animal models (44, 45).
Lipid peroxidation catalyzed by Hb also has been associated with several other disease entities, including subarachnoid hemorrhage, Plasmodium falciparum malaria, and the pulmonary crisis of sickle cell disease. In subarachnoid hemorrhage, F2-IsoPs are increased in the cerebrospinal fluid with the peak levels concurrent with the time of maximum vasospasm (46). Many of the F2-IsoPs are potent cerebral vasoconstrictors (39), providing a basis for a hypothesis that formation of these vasoconstrictors by Hb-catalyzed lipid peroxidation contributes to the vasospasm that leads to delayed ischemic neurological injury in this condition. Inhibition of F2-IsoPs formation by ApAP could provide an approach to testing this hypothesis.
Methods
Detailed protocols are provided in SI Methods.
Inhibition by ApAP of Mb-Induced Oxidation of AA.
Ferric Mb was incubated at 37 °C with [14C]AA. The reaction was initiated with the addition of H2O2 and proceeded for 3 h. Radioactive products of the reaction were quantified as described in SI Methods. Control experiments for each drug concentration consisted of doing the same experiment with the exception that Mb was omitted from the tube. The radioactivity associated with products of oxidation of [14C]AA incubated without Mb (background oxidation) was subtracted from each value obtained in presence of Mb in the same conditions.
Effect of ApAP on the Visible Spectra of Ferryl Mb.
H2O2 (17.4 μM) was reacted with ferric Mb (10 μM) in 25 mM sodium phosphate buffer (pH 7.4) for 20 min. Catalase was then added (10 nM) to remove excess peroxide and ApAP (174 μM final) was added and the absorbance spectrum recorded every 2 min until the reaction was complete.
Determination of the Rate Constant of Ferryl Mb Decay.
Ferryl Mb was generated by adding a stoichiometric amount of H2O2 to ferric Mb. Following the formation of ferryl Mb, catalase was added to the reaction mixture to remove unreacted peroxide. ApAP was added in either 0.1 M sodium phosphate buffer (pH 7.4) or 0.1 M sodium acetate buffer (pH 5.0) in a 1:1 volume ratio. The decay of ferryl Mb was followed optically (425–408 nm). The rate constant of ferryl decay was calculated by fitting the time courses to a single exponential function, using the least-squares method (SI Methods).
Measurement of Heme-to-Protein Cross-Linking by Reverse Phase HPLC Analysis.
Concentrations of Mb-X were determined by analyzing samples by reverse phase HPLC (SI Methods). Mb-X amounts were determined from the integral of the peak area at 400 nm between 19 and 25 min as described in SI Methods.
Analysis of Formation of DMPO Adducts on Mb Residues.
The following samples were prepared: Mb, Mb with DMPO, Mb with H2O2, Mb with ApAP, Mb with DMPO and H2O2, and Mb with DMPO, H2O2, and ApAP. After incubation for 2 h at 37 °C, the samples were analyzed by ESI-mass spectrometry (MS) as described in SI Methods.
Inhibition by ApAP of Hb-Induced Oxidation of AA.
Human ferric Hb was incubated at 37 °C with AA (SI Methods). The reaction was carried out and analyzed as described for Mb except that the H2O2 concentration was 30 μM. When indicated, ApAP was added at the same time as AA, before adding H2O2.
Animal Experiments.
For detailed protocol see SI Methods. Animal experiments were conducted according to United Kingdom Home Office guidelines. Male Sprague–Dawley rats were maintained on a standard diet, with a light and dark cycle of 12 h, at a temperature of 19–23 °C. Rats were placed individually in polycarbonate metabolic cages with free access to food and water.
Four groups of animals were studied: control rats treated with saline (controls, n = 10), control rats treated with ApAP (control + ApAP, n = 6), rhabomyolysis-induced rats treated with saline (Rhabdo, n = 10), and rhabdomyolysis-induced rats treated with ApAP (Rhabdo + APAP, n = 6). ApAP or saline was injected at 100 mg/kg i.p. at 20 and 2 h before and at 4 and 22 h following injection of 50% glycerol or at the equivalent times for controls. Rhabdomyolysis was induced by intramuscular injection of glycerol as previously described (9).
A 24-h urine sample was collected before induction of rhabdomyolysis and at 24 h after injection. One animal in the control group was excluded from analysis of creatinine clearance because of incomplete collection of urine. Animals were killed 24 h postinduction of rhabdomyolysis. Blood samples were collected. Plasma and serum were stored at −80 °C until analysis.
Extraction and Measurement of F2-IsoPs.
F2-IsoPs in urine and plasma were extracted and quantified by stable isotope dilution mass spectrometric assay as previously described (27). Urinary excretion of F2-IsoPs was normalized to creatinine clearance (SI Methods).
Pathology.
Kidneys were stained by PAS to show structural abnormalities and by an immunoperoxidase method to detect Mb. Morphometric assessment of the amount of damage was made by a point counting method to determine the proportion of proximal tubules with an intact brush border (SI Methods).
Statistics.
Intergroup comparisons were done using the two-tailed Mann–Whitney test and were considered significant when P < 0.05. Where mean values are reported, the SEM is also indicated.
Supplementary Material
Acknowledgments
We thank Dr. David Hachey, Ms. Lisa Manier, and Mrs. Dawn Overstreet of the Mass Spectrometry Research Center of Vanderbilt University for assistance with the MS analysis. We thank Dr. Agnes Fogo for her advice about the histological data. This work was supported by grants from the National Institutes of Health (AI060827, GM15431, GM42056, and DK48831), from the Biotechnology and Biological Sciences Research Council, United Kingdom (BBF0076631), and from the Medical Research Council and the Wellcome Trust, London, United Kingdom . J.A.O. is the Thomas F. Frist, Sr., Professor of Medicine. L.J.R. is the T. Edwin Rogers Professor of Pharmacology.
Footnotes
Conflict of interest statement: J.A.O., O.B., and L.J.R. have filed a patent for the use of acetaminophen in rhabdomyolysis-induced renal failure. J.A.O. is a consultant for McNeil Pharmaceuticals.
*This Direct Submission article had a prearranged editor. G.A.F. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910174107/DCSupplemental.
References
- 1.Grisham MB. Myoglobin-catalyzed hydrogen peroxide dependent arachidonic acid peroxidation. J Free Radic Biol Med. 1985;1:227–232. doi: 10.1016/0748-5514(85)90122-9. [DOI] [PubMed] [Google Scholar]
- 2.Harel S, Kanner J. The generation of ferryl or hydroxyl radicals during interaction of haemproteins with hydrogen peroxide. Free Radic Res Commun. 1988;5:21–33. doi: 10.3109/10715768809068555. [DOI] [PubMed] [Google Scholar]
- 3.Hogg N, et al. The role of lipid hydroperoxides in the myoglobin-dependent oxidation of LDL. Arch Biochem Biophys. 1994;314:39–44. doi: 10.1006/abbi.1994.1409. [DOI] [PubMed] [Google Scholar]
- 4.Patel RP, Svistunenko DA, Darley-Usmar VM, Symons MC, Wilson MT. Redox cycling of human methaemoglobin by H2O2 yields persistent ferryl iron and protein based radicals. Free Radic Res. 1996;25:117–123. doi: 10.3109/10715769609149916. [DOI] [PubMed] [Google Scholar]
- 5.Ortiz de Montellano PR, Catalano CE. Epoxidation of styrene by hemoglobin and myoglobin. Transfer of oxidizing equivalents to the protein surface. J Biol Chem. 1985;260:9265–9271. [PubMed] [Google Scholar]
- 6.Holt S, Moore K. Pathogenesis of renal failure in rhabdomyolysis: The role of myoglobin. Exp Nephrol. 2000;8:72–76. doi: 10.1159/000020651. [DOI] [PubMed] [Google Scholar]
- 7.Holt S, et al. Increased lipid peroxidation in patients with rhabdomyolysis. Lancet. 1999;353:1241. doi: 10.1016/S0140-6736(98)05768-7. [DOI] [PubMed] [Google Scholar]
- 8.Reeder BJ, et al. Toxicity of myoglobin and haemoglobin: Oxidative stress in patients with rhabdomyolysis and subarachnoid haemorrhage. Biochem Soc Trans. 2002;30:745–748. doi: 10.1042/bst0300745. [DOI] [PubMed] [Google Scholar]
- 9.Moore KP, et al. A causative role for redox cycling of myoglobin and its inhibition by alkalinization in the pathogenesis and treatment of rhabdomyolysis-induced renal failure. J Biol Chem. 1998;273:31731–31737. doi: 10.1074/jbc.273.48.31731. [DOI] [PubMed] [Google Scholar]
- 10.Boutaud O, Aronoff DM, Richardson JH, Marnett LJ, Oates JA. Determinants of the cellular specificity of acetaminophen as an inhibitor of prostaglandin H(2) synthases. Proc Natl Acad Sci USA. 2002;99:7130–7135. doi: 10.1073/pnas.102588199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanel AM, Lands WE. Modification of anti-inflammatory drug effectiveness by ambient lipid peroxides. Biochem Pharmacol. 1982;31:3307–3311. doi: 10.1016/0006-2952(82)90565-2. [DOI] [PubMed] [Google Scholar]
- 12.Ouellet M, Percival MD. Mechanism of acetaminophen inhibition of cyclooxygenase isoforms. Arch Biochem Biophys. 2001;387:273–280. doi: 10.1006/abbi.2000.2232. [DOI] [PubMed] [Google Scholar]
- 13.Better OS, Stein JH. Early management of shock and prophylaxis of acute renal failure in traumatic rhabdomyolysis. N Engl J Med. 1990;322:825–829. doi: 10.1056/NEJM199003223221207. [DOI] [PubMed] [Google Scholar]
- 14.Bywaters EG, Beall D. Crush injuries with impairment of renal function. BMJ. 1941;1:427–432. doi: 10.1136/bmj.1.4185.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grossman RA, Hamilton RW, Morse BM, Penn AS, Goldberg M. Nontraumatic rhabdomyolysis and acute renal failure. N Engl J Med. 1974;291:807–811. doi: 10.1056/NEJM197410172911601. [DOI] [PubMed] [Google Scholar]
- 16.Reeder BJ, Wilson MT. Hemoglobin and myoglobin associated oxidative stress: from molecular mechanisms to disease states. Curr Med Chem. 2005;12:2741–2751. doi: 10.2174/092986705774463021. [DOI] [PubMed] [Google Scholar]
- 17.Detweiler CD, et al. Identification of the myoglobin tyrosyl radical by immuno-spin trapping and its dimerization. Free Radic Biol Med. 2005;38:969–976. doi: 10.1016/j.freeradbiomed.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 18.Gunther MR, Sturgeon BE, Mason RP. A long-lived tyrosyl radical from the reaction between horse metmyoglobin and hydrogen peroxide. Free Radic Biol Med. 2000;28:709–719. doi: 10.1016/s0891-5849(00)00164-7. [DOI] [PubMed] [Google Scholar]
- 19.Osawa Y, Korzekwa K. Oxidative modification by low levels of HOOH can transform myoglobin to an oxidase. Proc Natl Acad Sci USA. 1991;88:7081–7085. doi: 10.1073/pnas.88.16.7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osawa Y, Nakatsuka K, Williams MS, Kindt JT, Nakatsuka M. Reactions of reactive metabolites with hemoproteins—toxicological implications: Covalent alteration of hemo-proteins. Adv Exp Med Biol. 1996;387:37–45. [PubMed] [Google Scholar]
- 21.Vuletich JL, Osawa Y, Aviram M. Enhanced lipid oxidation by oxidatively modified myoglobin: Role of protein-bound heme. Biochem Biophys Res Commun. 2000;269:647–651. doi: 10.1006/bbrc.2000.2349. [DOI] [PubMed] [Google Scholar]
- 22.Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoc. 2007;2:221–226. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- 23.Carpenter HM, Mudge GH. Acetaminophen nephrotoxicity: Studies on renal acetylation and deacetylation. J Pharmacol Exp Ther. 1981;218:161–167. [PubMed] [Google Scholar]
- 24.Schiødt FV, Rochling FA, Casey DL, Lee WM. Acetaminophen toxicity in an urban county hospital. N Engl J Med. 1997;337:1112–1117. doi: 10.1056/NEJM199710163371602. [DOI] [PubMed] [Google Scholar]
- 25.Bessems JG, Vermeulen NP. Paracetamol (acetaminophen)-induced toxicity: Molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol. 2001;31:55–138. doi: 10.1080/20014091111677. [DOI] [PubMed] [Google Scholar]
- 26.Lee WM. Acute liver failure in the United States. Semin Liver Dis. 2003;23:217–226. doi: 10.1055/s-2003-42641. [DOI] [PubMed] [Google Scholar]
- 27.Pace-Asciak CR. Hemoglobin- and hemin-catalyzed transformation of 12L-hydroperoxy-5,8,10,14-eicosatetraenoic acid. Biochim Biophys Acta. 1984;793:485–488. doi: 10.1016/0005-2760(84)90267-4. [DOI] [PubMed] [Google Scholar]
- 28.Wilcox AL, Marnett LJ. Polyunsaturated fatty acid alkoxyl radicals exist as carbon-centered epoxyallylic radicals: A key step in hydroperoxide-amplified lipid peroxidation. Chem Res Toxicol. 1993;6:413–416. doi: 10.1021/tx00034a003. [DOI] [PubMed] [Google Scholar]
- 29.Reeder BJ, Cutruzzola F, Bigotti MG, Hider RC, Wilson MT. Tyrosine as a redox-active center in electron transfer to ferryl heme in globins. Free Radic Biol Med. 2008;44:274–283. doi: 10.1016/j.freeradbiomed.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 30.Reeder BJ, et al. Tyrosine residues as redox cofactors in human hemoglobin: Implications for engineering nontoxic blood substitutes. J Biol Chem. 2008;283:30780–30787. doi: 10.1074/jbc.M804709200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deterding LJ, Ramirez DC, Dubin JR, Mason RP, Tomer KB. Identification of free radicals on hemoglobin from its self-peroxidation using mass spectrometry and immuno-spin trapping: Observation of a histidinyl radical. J Biol Chem. 2004;279:11600–11607. doi: 10.1074/jbc.M310704200. [DOI] [PubMed] [Google Scholar]
- 32.González-Sánchez MI, Manjabacas MC, García-Carmona F, Valero E. Mechanism of acetaminophen oxidation by the peroxidase-like activity of methemoglobin. Chem Res Toxicol. 2009;22:1841–1850. doi: 10.1021/tx9002512. [DOI] [PubMed] [Google Scholar]
- 33.Catalano CE, Choe YS, Ortiz de Montellano PR. Reactions of the protein radical in peroxide-treated myoglobin. Formation of a heme-protein cross-link. J Biol Chem. 1989;264:10534–10541. [PubMed] [Google Scholar]
- 34.Reeder BJ, Svistunenko DA, Sharpe MA, Wilson MT. Characteristics and mechanism of formation of peroxide-induced heme to protein cross-linking in myoglobin. Biochemistry. 2002;41:367–375. doi: 10.1021/bi011335b. [DOI] [PubMed] [Google Scholar]
- 35.Reeder BJ, Wilson MT. The effects of pH on the mechanism of hydrogen peroxide and lipid hydroperoxide consumption by myoglobin: A role for the protonated ferryl species. Free Radic Biol Med. 2001;30:1311–1318. doi: 10.1016/s0891-5849(01)00534-2. [DOI] [PubMed] [Google Scholar]
- 36.Silaghi-Dumitrescu R, Reeder BJ, Nicholls P, Cooper CE, Wilson MT. Ferryl haem protonation gates peroxidatic reactivity in globins. Biochem J. 2007;403:391–395. doi: 10.1042/BJ20061421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nath KA, et al. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992;90:267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nath KA, Balla J, Croatt AJ, Vercellotti GM. Heme protein-mediated renal injury: A protective role for 21-aminosteroids in vitro and in vivo. Kidney Int. 1995;47:592–602. doi: 10.1038/ki.1995.75. [DOI] [PubMed] [Google Scholar]
- 39.Hou X, et al. Isomer-specific contractile effects of a series of synthetic f2-isoprostanes on retinal and cerebral microvasculature. Free Radic Biol Med. 2004;36:163–172. doi: 10.1016/j.freeradbiomed.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 40.Koshkin V, Dunford HB. Reactions of prostaglandin endoperoxide synthase with hydroperoxide and reducing substrates under single turnover conditions. Biochim Biophys Acta. 1999;1431:47–52. doi: 10.1016/s0167-4838(99)00041-2. [DOI] [PubMed] [Google Scholar]
- 41.Markey CM, Alward A, Weller PE, Marnett LJ. Quantitative studies of hydroperoxide reduction by prostaglandin H synthase. Reducing substrate specificity and the relationship of peroxidase to cyclooxygenase activities. J Biol Chem. 1987;262:6266–6279. [PubMed] [Google Scholar]
- 42.Gunther MR, Sampath V, Caughey WS. Potential roles of myoglobin autoxidation in myocardial ischemia-reperfusion injury. Free Radic Biol Med. 1999;26:1388–1395. doi: 10.1016/s0891-5849(98)00338-4. [DOI] [PubMed] [Google Scholar]
- 43.Delanty N, et al. 8-epi PGF2 alpha generation during coronary reperfusion. A potential quantitative marker of oxidant stress in vivo. Circulation. 1997;95:2492–2499. doi: 10.1161/01.cir.95.11.2492. [DOI] [PubMed] [Google Scholar]
- 44.Golfetti R, Rork T, Merrill G. Chronically administered acetaminophen and the ischemia/reperfused myocardium. Exp Biol Med. 2003;228:674–682. doi: 10.1177/153537020322800605. [DOI] [PubMed] [Google Scholar]
- 45.Rork TH, Van Dyke K, Spiler NM, Merrill GF. Acetaminophen in the hypoxic and reoxygenated guinea pig myocardium. Exp Biol Med. 2004;229:1154–1161. doi: 10.1177/153537020422901110. [DOI] [PubMed] [Google Scholar]
- 46.Lin CL, et al. Increased levels of F2-isoprostanes following aneurysmal subarachnoid hemorrhage in humans. Free Radic Biol Med. 2006;40:1466–1473. doi: 10.1016/j.freeradbiomed.2005.12.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.