Abstract
By employing transcranial magnetic stimulation (TMS) in combination with high-density electroencephalography (EEG), we recently reported that cortical effective connectivity is disrupted during early non-rapid eye movement (NREM) sleep. This is a time when subjects, if awakened, may report little or no conscious content. We hypothesized that a similar breakdown of cortical effective connectivity may underlie loss of consciousness (LOC) induced by pharmacologic agents. Here, we tested this hypothesis by comparing EEG responses to TMS during wakefulness and LOC induced by the benzodiazepine midazolam. Unlike spontaneous sleep states, a subject’s level of vigilance can be monitored repeatedly during pharmacological LOC. We found that, unlike during wakefulness, wherein TMS triggered responses in multiple cortical areas lasting for >300 ms, during midazolam-induced LOC, TMS-evoked activity was local and of shorter duration. Furthermore, a measure of the propagation of evoked cortical currents (significant current scattering, SCS) could reliably discriminate between consciousness and LOC. These results resemble those observed in early NREM sleep and suggest that a breakdown of cortical effective connectivity may be a common feature of conditions characterized by LOC. Moreover, these results suggest that it might be possible to use TMS-EEG to assess consciousness during anesthesia and in pathological conditions, such as coma, vegetative state, and minimally conscious state.
Keywords: anesthesia, high-density electroencephalography, transcranial magnetic stimulation
Theoretical considerations suggest that consciousness depends on the brain’s ability to integrate information, and that if information integration is impaired within a complex of cortical areas, consciousness should fade (1). The most common situation in which the level of consciousness changes is early non-rapid eye movement (NREM) sleep, when subjects, if awakened, report no or little conscious experience (2), despite the fact that their brain remains highly active (3). To establish whether the brain’s ability to integrate information is higher in wakefulness than in early NREM sleep, in a previous study we perturbed one brain area using transcranial magnetic stimulation (TMS) and recorded the responses of other cortical areas using TMS-compatible high-density electroencephalography (hd-EEG) (4). We found that during wakefulness, TMS initially evoked a local cortical activation, which then moved to a series of distant cortical areas, whereas during NREM sleep, the initial local response to TMS did not propagate beyond the stimulation site, thus indicating a breakdown of cortical effective connectivity and a loss of cortical integration (5). Moreover, in subsequent TMS/hd-EEG studies, we established that during wakefulness, TMS of different brain regions evoked different, specific EEG patterns (6), whereas during early NREM sleep, TMS yielded EEG responses characterized by a positive wave followed by a negative deflection, suggesting a loss of information capacity (7).
Besides sleep, the most common condition in which consciousness can be lost is general anesthesia, which is characterized by behavioral loss of consciousness (LOC) (8). Although several anesthetics can induce states with behavioral and electrophysiological features not unlike those of deep NREM sleep, pharmacological anesthesia and sleep are not identical and differ in terms of both neurophysiology and neurochemistry (9). Moreover, general anesthesia offers several advantages for investigating the neural correlates of LOC (10). Specifically, in sleep studies, it is not feasible to evaluate an individual’s level of alertness repeatedly and reliably, because the depth of sleep varies unpredictably and subjects awakened to assess consciousness cannot rapidly return to sleep. By contrast, during “general anesthesia,” a subject’s level of alertness may be assessed repeatedly without reversing the pharmacologically induced LOC. For these reasons, in this study, we asked whether cortical effective connectivity measured by using TMS and hd-EEG would be reduced during LOC induced by a pharmacological agent, midazolam, at anesthetic concentrations.
Results
Physiological and Behavioral Effects of Midazolam.
In six subjects who received i.v. midazolam at doses up to 0.2 mg/kg, OAA/S scores of “1” (unresponsive to verbal and mild physical stimulus) were reached for a sufficient period that hd-EEG responses to TMS could be measured. No subjects experienced respiratory depression (18 ± 1.4 min−1 preinduction, 16 ± 4 min−1 postinduction), desaturation (minimum SaO2 95%), or physiologically meaningful changes in heart rate (59 ± 7 min−1 preinduction, 69 ± 12 min−1 postinduction) or blood pressure (systolic, 127 ± 15 mmHg preinduction, 113 ± 10 mmHg postinduction; diastolic, 74 ± 9 mmHg preinduction, 62 ± 5 mmHg postinduction). Subjects also displayed no spontaneous/voluntary behavior during TMS/hd-EEG sessions performed at level 1 OAA/S and regained full consciousness (level 5 OAA/S) within 100 min on average (range 42–158 min). In debriefing sessions during the recovery period, subjects reported no subjective experience during LOC. By contrast, subjects interrogated while transitioning into LOC (level 3 OAA/S) reported a sensation of light-headedness, mild euphoria, and well being.
Changes in TMS-Evoked Brain Responses Associated with Loss of Consciousness.
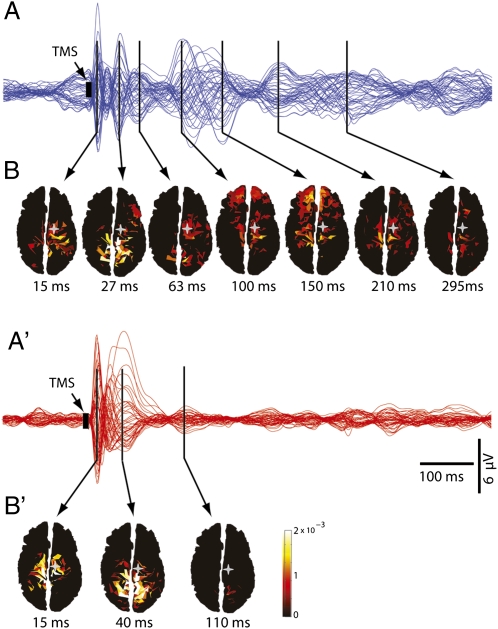
Compared with wakefulness, we found a marked change in TMS-evoked brain responses during midazolam-induced LOC. Before the injection of the anesthetic (level 5 alertness, OAA/S), TMS pulses to premotor cortex evoked a complex spatiotemporal pattern of low-amplitude, fast-frequency scalp waves, as shown by the average TMS-evoked EEG potentials recorded at all electrodes and superimposed in a butterfly plot (blue traces, Fig. 1A; data from one subject). Conversely, following midazolam-induced LOC (level 1 OAA/S), TMS pulses gave rise to high-amplitude, low-frequency EEG voltages, which faded shortly after the stimulation (red traces, Fig. 1A′). To further explore the neural events underlying these EEG patterns, we calculated the currents evoked by TMS in the cortex before and after LOC. Before LOC, TMS-evoked cortical currents lasted for at least 300 ms following the stimulation and shifted among cortical areas distant from the TMS-targeted brain area (Fig. 1B). By contrast, TMS-evoked cortical currents after LOC faded within 150 ms and remained more localized to the stimulated site (Fig. 1B′).
Fig. 1.
Spatiotemporal dynamics of TMS-evoked activity change markedly during LOC. (A and A′) Averaged TMS-evoked potentials at all electrodes, superimposed in butterfly plots (blue traces for waking, red traces for anesthesia). (B and B′) Cortical currents calculated on individual cortical meshes are shown from minimal (dark red) to maximal (white) values. During wakefulness, TMS of premotor cortex determined low-amplitude, complex scalp waves corresponding to cortical currents that lasted >300 ms and shifted among distant cortical areas. Conversely, during anesthesia, TMS gave rise to high-amplitude, short-lasting scalp voltages reflecting cortical currents that remained local, and faded within 150 ms. Gray stars, TMS target (premotor cortex); black arrows, local maxima in periods of significant TMS-evoked activation.
During LOC, TMS Evokes a Large Positive–Negative Wave in the Stimulated Area and Little Activation in Distant Areas.
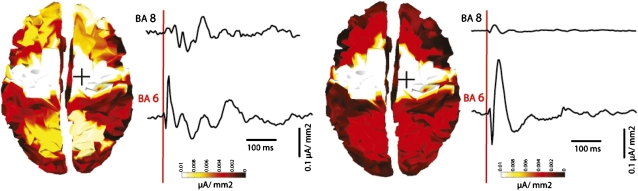
To capture the pattern of spatial activation in the two conditions, TMS-evoked cortical currents were cumulated for the entire poststimulus interval and displayed topographically for both conditions (Fig. 2). Although the activity evoked by TMS at the stimulation site, the premotor cortex (BA 6), was similar across conditions, its time course was markedly different. During wakefulness, the immediate response to TMS consisted of fast-frequency oscillations (6, 11). By contrast, during anesthesia, TMS-evoked responses were slower in frequency, although the initial component was of higher amplitude, consisting of a large positive wave followed by a negative deflection. This local response was found in all subjects during LOC. In one subject, in which TMS-evoked cortical activity was recorded at an intermediate level of sedation (level 3 OAA/S; Fig. S1), single trial analysis showed that this large positive–negative wave took shape gradually while transitioning from wakefulness (level 5) to deep sedation (level 1). Specifically, although the positive peak of the TMS-evoked wave first appeared at level 3 of sedation, the positive–negative sequence was fully established only when reaching level 1. This initial, stronger response during LOC remained largely restricted to the premotor cortices and affected only marginally the activity of other cortical areas (Fig. 2). This breakdown of cortico-cortical effective connectivity was also evident when inspecting the time courses of the TMS-evoked cortical currents. For instance, the time course of cortical currents in BA 8 (prefrontal cortex), which is anatomically connected to BA 6 (premotor cortex), was not affected by the strong activation evoked by TMS in the premotor cortex during LOC.
Fig. 2.
TMS during anesthesia evokes a large positive–negative wave in the stimulation site but little activation in distant areas. Cortical currents evoked by TMS of premotor cortex, cumulated in a 0–500 ms post-TMS interval and displayed on the corresponding Broadmann areas (BA) in wakefulness (Left) and anesthesia (Right). To the right of each topographic plot are time courses of currents recorded from the stimulated area, premotor cortex (BA 6), and from a more anterior cortical area (BA 8). During anesthesia, TMS-evoked SCD in BA6 was similar to the SCD recorded in wakefulness, as reflective of an initial stronger but shorter-lived response during anesthesia compared to wakefulness. Conversely, SCD from BA 8, which is anatomically connected to BA 6, were markedly reduced in anesthesia compared to wakefulness, suggesting a marked decrease in cortical effective connectivity.
Indices of Cortical Connectivity Discriminate Between Consciousness and LOC.
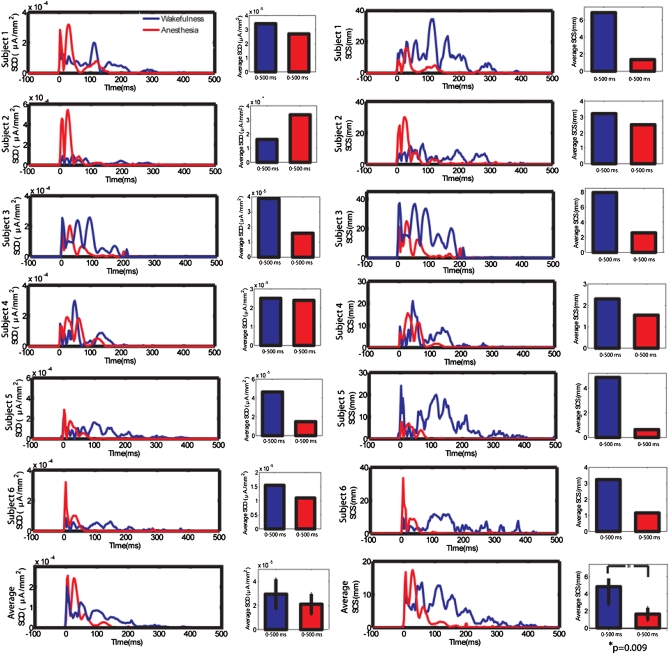
To quantify changes in strength (activity) and propagation (connectivity) of TMS-evoked cortical responses during LOC, two recently developed indexes (12), significant current density (SCD) and significant current scattering (SCS), were calculated for each subject in wakefulness and anesthesia. SCD was computed by cumulating in space the statistically significant cortical currents evoked by TMS (Fig. 3 Left). The time course of SCD revealed that, in each subject, the initial TMS-evoked cortical activity, related to the large positive–negative wave, was higher in the anesthesia condition, whereas subsequent cortical activity was stronger during wakefulness. When cumulating SCD in two post-TMS time ranges, respectively, 0–50 and 50–500 ms, we found that in the 0–50 ms interval, SCD was significantly higher during anesthesia (P = 0.016, Mann–Whitney), whereas in the 50–500 ms range, SCD was significantly higher during wakefulness (P = 0.016, Mann–Whitney). The average SCD in the entire poststimulus interval was reduced during anesthesia, indicating a diminished response to the TMS, although the two conditions differed only at trend level (P = 0.1, Mann–Whitney). By contrast, SCS, computed by measuring the geodesic distance between any significant current source and the site of stimulation, discriminated effectively between the two conditions (Fig. 3 Right). Specifically, in each subject, mean SCS was significantly higher in wakefulness compared to anesthesia (P = 0.009, Mann–Whitney). Furthermore, the time course of SCS showed that, in each subject, SCS during wakefulness was significantly increased compared to baseline for >200 ms, whereas during anesthesia, SCS returned to baseline within 100 ms of TMS.
Fig. 3.
A synthetic index of cortical connectivity (SCS), but not reactivity (SCD), captures cortical changes during LOC. SCD and SCS were computed for each subject in wakefulness (blue line) and anesthesia (red line) following TMS of the premotor cortex. (Left) Time course of SCD for individual and average data, and mean SCD over the entire post-TMS time interval (0–500 ms). In each subject, SCD values were initially higher (first 50 ms after TMS) during anesthesia but tended to dissipate shortly thereafter, consistent with a TMS-evoked larger initial response during anesthesia that was, however, short-lived (Fig. 1 A′ and B′). Mean SCD over the entire post-TMS period were not significantly different between wakefulness and anesthesia. (Right) Time course of SCS for individual and average data, and mean SCS over the entire post-TMS interval. In each subject, during wakefulness, SCS was present for >200 ms, whereas during anesthesia it faded after 100 ms. Notably, mean SCS values in the 0–500 ms post-TMS interval were significantly higher in wakefulness relative to anesthesia (P = 0.009, Mann–Whitney).
Recovery of Cortical Responses to TMS After Anesthesia.
In the six subjects who reached deep unconsciousness (level 1 of the OAA/S), we performed TMS blocks during recovery of consciousness (ROC). However, for most subjects, the slowness of recovery from midazolam, and the typically fluctuating level of responsiveness during the recovery period, prevented us from collecting post-LOC TMS measurements at stable levels (i.e., an unchanging OAA/S score during a complete block of 250 responses). Nevertheless, in one subject we observed a full recovery of consciousness, as assessed by two consecutive level 5 scores at the OAA/S, within 1 h of anesthesia. TMS-evoked responses in this subject were similar to the EEG potentials observed in the waking pre-anesthesia and were clearly different from the TMS-evoked activity during anesthesia. Specifically, at Cz, the electrode closer to the stimulation site, and at Fz, a prefrontal electrode distant from Cz, several low-amplitude, fast EEG oscillations were recorded during the initial 50–100 ms, and slower oscillations above baseline noise continued for >300 ms after TMS in both pre-LOC and ROC sessions (Fig. S2 Upper). Conversely, during midazolam-induced LOC, TMS evoked larger amplitude, slower EEG responses, which faded within 100 ms (at Fz) and 150 ms (at Cz) post-TMS (Fig. S2 Upper). Furthermore, mean SCS calculated in the 0–500 ms post-TMS interval for the three conditions (pre-LOC, post-LOC, and ROC) decreased between pre-LOC (SCS = 2.35) and post-LOC (SCS = 1.5), and increased to pre-LOC levels during ROC (SCS = 2.30) (Fig. S2 Lower).
Discussion
We employed TMS/hd-EEG to probe cortical effective connectivity during anesthesia-induced LOC. The methodological approach paralleled previous work in which TMS/EEG responses were evaluated during early NREM sleep, the most common condition during which consciousness fades in healthy subjects (5). The results show that, as with LOC during early sleep, anesthesia-induced LOC is associated with a breakdown of cortical effective connectivity.
Choice of Anesthetic Agent.
Here, we used the benzodiazepine midazolam to induce LOC based on several factors. First, although it is exceedingly rare, TMS can induce seizures in epileptic patients. Therefore, for this first study of anesthesia and TMS, we chose to use an agent that has a marked anticonvulsant effect. Second, the clinical tool used to evaluate the subjects’ alertness throughout the experimental procedure, the OAA/S scale, was initially tested in 18 healthy subjects receiving titrated doses of midazolam (13). This study established a correlation between OAA/S scores and drug levels, with higher doses of midazolam yielding OAA/S scores that were consistently low, and placebo yielding the highest OAA/S scores. Finally, unlike most anesthetic agents midazolam targets GABAA receptors exclusively, leading to increased inhibitory postsynaptic currents that presumably underlie its behavioral/cognitive effects (14). Other general anesthetics, such as volatile and i.v. drugs, have effects that are more difficult to interpret because of multiple interactions with several proteins, such as voltage-gated and leak channels (15). One potential drawback of midazolam is its pharmacokinetic profile, which leads to slower recovery compared with shorter-acting induction agents (16). Because of this slow recovery, we were able to measure TMS-evoked EEG responses after full recovery of vigilance (Level 5 OAA/S) in just one subject within the limited time frame of the TMS/EEG recordings.
Breakdown of Effective Connectivity During Midazolam-Induced LOC and Comparison with Slow Wave Sleep.
We found a breakdown of cortical effective connectivity during midazolam-induced LOC. Specifically, before midazolam injection the TMS-evoked EEG responses consisted of small-amplitude, long-lasting oscillations involving several brain areas beyond the premotor cortex. Conversely, following midazolam-induced LOC, TMS-evoked EEG activity consisted of an initially larger local response that, however, was short-lived and remained largely restricted to the stimulation site. These changes in EEG responses following LOC were consistent across subjects and could be quantified with synthetic indexes (SCD, SCS).
These results bear a striking resemblance with those obtained in a previous study where we employed TMS/EEG to evaluate effective connectivity during early NREM sleep, the phase of sleep most likely associated with loss of consciousness (5). However, in sleep studies, it was not feasible to evaluate repeatedly and reliably the subjects’ level of alertness under stable conditions. The depth of sleep can vary un-predictably, and if awakened to assess consciousness, subjects cannot rapidly return to sleep. In this study, subjects could be repeatedly assessed for LOC. Additionally, in one subject, we could evaluate the effects of progressively reduced arousal, from level 3 (sedation) to level 1 (LOC). The results show that at sedation level, the TMS-evoked initial activity becomes stronger than in wakefulness (Fig. S1) but is followed by smaller and shorter-lived oscillations. This initial response to TMS is even larger during LOC, with a positive–negative wave similar to the spontaneous sleep slow oscillation, while subsequent activity is obliterated; this demonstrates that brain responses to TMS become progressively shorter and sleep-like while transitioning into pharmacologic LOC.
We also employed novel indices of cortical activation (SCD) and connectivity (SCS) (12), which enabled us to better quantify the effects of anesthesia. Of these measures, SCS was the more sensitive to the effects of midazolam. In a recent study, SCS was introduced as a measure of the propagation of activity evoked by TMS of the visual cortex (12). The authors found that TMS-evoked activity traveled from the visual cortex to ipsilateral frontal areas in the first 100 ms, and that this propagation was captured by SCS values, which peaked 70–100 ms after stimulation. Similarly, in the present study, we found that in wakefulness SCS peaked at ∼100 ms post-TMS and lasted for >200 ms; by contrast, during midazolam-induced LOC, the propagation of the TMS-evoked activity faded within the first 100 ms. The advantage of a global index such as SCS is that it captures a decrease in connectivity in a single number, which is easy to evaluate and compare statistically.
The decrease in cortical effective connectivity demonstrated using TMS/EEG is consistent with reported changes in functional connectivity (covariation in the activity of multiple regions) in anesthesia or in disorders of consciousness. A PET study performed before and after general anesthetic-induced LOC found impairments in thalamo-cortical and cortico-cortical functional connectivity, especially between frontal cortical areas (17). Similarly, PET studies in vegetative state showed a marked reduction of connectivity between premotor, prefrontal, and posterior parietal areas both at rest (18) and following auditory or somatosensory stimulation (19). Thus, LOC seems to be associated with a decrease in both effective and functional connectivity in the cortico-thalamic system, consistent with theoretical and experimental evidence concerning the neural substrates necessary for consciousness (20). Compared to functional connectivity studies with PET/fMRI, probing effective connectivity with TMS/EEG allows establishing the effectiveness of causal interactions among brain regions and not just their correlation patterns (21). Moreover, TMS/EEG can be used to probe corticothalamic interactions at the time scale at which conscious experience changes, tens to hundreds of milliseconds (22). Finally, using TMS as opposed to peripheral stimuli has the additional advantage of probing cortical circuits directly, bypassing sensory pathways, thalamic gates (23), and primary cortical areas, and of recording cortical responses over multiple areas. For instance, studies evaluating auditory and somatosensory evoked potentials with traditional EEG after midazolam injections (24, 25) found only minor effects of anesthesia, such as a slight decrease in latency and in amplitude of late res-ponses, respectively.
Mechanisms Underlying Breakdown of Effective Connectivity.
Re-garding the mechanisms responsible for the breakdown of cortical effective connectivity during midazolam-induced anesthesia, two considerations deserve notice: first, midazolam acts selectively on GABAA receptors to induce LOC (26); second, cortical responses to TMS during midazolam-induced LOC are strikingly similar to those observed during early NREM sleep. Recently, a large-scale modeling study has investigated the mechanisms underlying the breakdown of effective connectivity during slow wave sleep (27). Of the various mechanisms tested, it was found that a shift in the balance between synaptic excitation and inhibition toward inhibition, determined by increased GABA release, was the most likely mechanism to account for the reduction in cortico-cortical signal transmission during sleep. Hence, during midazolam-induced LOC, a similar mechanism may be engaged through enhanced GABAA-mediated inhibition, either locally within the cortex (28), or in endogenous sleep-promoting pathways (29). An alternative mechanism is the bistability between up- and down-states of thalamo-cortical neurons, which underlies the generation of spontaneous sleep slow waves (30). Because of bistability, thalamo-cortical networks are unable to sustain activity and inevitably tend to fall into a silent hyperpolarized state (down-state) after a short period of intense activation [up-state (31 –34)]. Bistability may account both for the initial, large positive–negative wave evoked by TMS in sleep and midazolam-induced anesthesia and for the subsequent block of long-range interactions among cortical areas (21). Finally, the thalamus may also be involved in blocking the spread of spontaneous and evoked cortical activity during LOC, in line with evidence that thalamic cells may be part of cortico-thalamo-cortical circuits that transmits driving input from one cortical area to a higher order one (35). Although the extent of these cortico-thalamo-cortical pathways is still controversial, it is possible that an action of midazolam on the thalamus (36) may profoundly affect cortical effective connectivity. The striking correspondence between our present finding that TMS evoked a large, slow, local response, and similar changes in cortical evoked responses in rats produced by inhaled anesthetics (37), suggests that these findings may be generalized to include other anesthetics, and offers an opportunity to explore underlying mechanisms in animal models.
Future Developments.
LOC is usually described as the inability of a subject to respond to a verbal command or, in case of deep sedation, to mild physical stimuli. This definition of unconsciousness relies on the person’s willingness to respond to meaningful probes, thus raising the issue of how to disentangle unresponsiveness from unconsciousness following the administration of an anesthetic. For example, anesthetics like ketamine can have dissociative effects, which affect the subject’s capacity to follow a command. Even more problematically, paralyzing agents are often used to prevent unwanted movements during anesthesia, but they do not remove consciousness (38). For this reason, measures such as the bispectral index (BIS; Aspect Medical Systems) and the Patient State Index (PSI; Physiometrix) have been proposed as tools for detecting “awareness” during anesthesia.
These measures are derived empirically from EEG/muscle activity by using proprietary algorithms. Although practically useful, they lack theoretical validity. Moreover, although valid on average, they can be inaccurate when evaluating the level of consciousness of individuals at specific times, as demonstrated by using the isolated forearm technique (39). By contrast, the idea that LOC should be associated with a breakdown of effective connectivity is theoretically motivated (1); indeed, both the previous NREM sleep study and the current study of midazolam-induced LOC were designed to test theoretical ideas. Moreover, multiple analytic approaches can be developed to obtain an index of unconsciousness, provided they are sensitive to reductions in effective connectivity, as is the case with SCS. Finally, TMS-EEG responses can be evaluated every few seconds in a way that is sensitive to moment by moment fluctuation of vigilance, even though the spontaneous EEG may not show appreciable changes (21). Future studies will be essential to characterize how TMS-evoked EEG responses change when a subject progresses from conscious to pharmacologically induced unconscious states. Here, we recorded the evoked EEG responses during transitional levels of alertness in just a few subjects, and our preliminary findings encouragingly suggest that they are intermediate between full alertness (level 5 of the OAA/S) and deep sedation (level 1) EEG responses (Fig. S1). It will also be essential to replicate these TMS/EEG findings with other anesthetics, including volatile and i.v. agents, which are commonly used in surgical procedures and likely have different mechanisms of action than the increase in GABAergic receptor activity induced by midazolam. This is especially important because neuroimaging, electrophysiological, and molecular studies have shown that different anesthetics have different molecular targets, cause different patterns of cortical and subcortical activation/deactivation, and differentially affect peripherally evoked EEG responses (40). Nonetheless, given the striking similarity between TMS/EEG responses during midazolam-induced LOC and during NREM sleep, when LOC occurs spontaneously and through partially distinct neurobiological mechanisms, a breakdown of effective connectivity may turn out to be a convenient indicator of unconsciousness (1, 8). In that case, indices of effective connectivity, such as SCS, could be developed to assess pharmacologically induced LOC in subjects undergoing surgical procedures as well as in patients affected by disorders of consciousness, including coma, vegetative, and minimally conscious states (21).
Methods
Participants.
Eleven male subjects (age, 21–29 years) participated in the study. All participants gave written informed consent, and the experiment was approved by the University of Wisconsin Human Subjects Committee. Before the experiment, physical examinations were performed to exclude medical conditions that were incompatible with the anesthesia and/or the TMS procedure. TMS was performed in accordance with current safety guidelines (41). TMS was administered during full consciousness (level 5 of the OAA/S) as well as during deep unresponsiveness (level 1 OAA/S). Three subjects were excluded because they did not reach level 1 of unresponsiveness for at least 10 min. Two subjects provided EEG data that were excessively contaminated by artifacts. Thus, the data presented here are from six subjects.
Experimental Procedure.
All experimental procedures were performed at the Ambulatory Procedure Center (APC) of the University of Wisconsin Hospital and Clinics. After preparation for EEG recordings and calibration of the neuronavigational system, a 20-gauge i.v. catheter was placed for anesthetic drug delivery, and participants were given supplemental oxygen at 3 L/min via nasal cannula and an antacid (Bicitra) to minimize possible complications in the event of nausea and vomiting caused by the anesthetic drug (midazolam). During the TMS procedure, the participant’s EKG, noninvasive blood pressure, SaO2, exhaled CO2, and axillary skin temperature were continuously monitored by an anesthesiologist. Additionally, the subject’s level of consciousness was evaluated before and after each TMS session with the OAA/S. A first 8- to 10-min TMS-EEG session (∼250 stimuli, with a 2,000-ms period and a ±250-ms jitter) was collected in each subject before midazolam injection (level 5 responsiveness of the OAA/S). Midazolam was then given at an initial dose of 0.1 mg/kg, followed by additional doses of 0.02 mg/kg each 2–3 min until the subject was unresponsive (level 1 of the OAA/S), up to a maximal dose of 0.2 mg/kg. During midazolam administration, 3-min TMS blocks at 0.2 Hz interleaved by alertness assessments with the OAA/S were performed. Additionally, in the six subjects who reached deep unconsciousness (level 1 of the OAA/S), a longer TMS session mirroring the preinjection TMS session was performed. All stimulation parameters were set according to international safety guidelines (41). The navigation of the TMS coil over the cortical target (the premotor cortex), the acquisition and processing of the TMS-evoked EEG responses, and the source modeling analysis performed on these EEG data are presented in SI Methods. From the TMS-evoked significant cortical currents identified with the source modeling analysis two synthetic indexes of brain responsiveness were computed: significant current density (SCD), which captures the strength of the TMS-evoked cortical currents, was calculated by cumulating the absolute amplitude of all of the significant cortical currents evoked by TMS; and significant current scattering (SCS), which represents the spatial scattering of the TMS-evoked significant activations, was computed by cumulating the geodesic distance between any significant current source and the TMS cortical target.
Supplementary Material
Acknowledgments
We thank Drs. Michael Alkire and Kirk Hogan for advice on trial design and Drs. Deborah Rusy, Frank Sasse, and Ken Van Dyke for their participation in anesthetic delivery and monitoring. This work was supported by a National Institutes of Health Pioneer Award, National Institute of Mental Health Grant 5P20MH077967, National Institute of Neurological Disorders and Stroke Grant 5ROINS055185, (to G.T.), by Grant 1UL1RR025011 from the Clinical and Translational Science Award program of the National Center for Research Resources, National Institutes of Health, and by National Institutes of Health Grants P01-GM47818 and R01-NS056411 (to R.A.P.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0913008107/DCSupplemental.
References
- 1.Tononi G. An information integration theory of consciousness. BMC Neurosci. 2004;5:42–62. doi: 10.1186/1471-2202-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat Rev Neurosci. 2002;3:679–693. doi: 10.1038/nrn915. [DOI] [PubMed] [Google Scholar]
- 3.Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- 4.Ilmoniemi RJ, et al. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport. 1997;8:3537–3540. doi: 10.1097/00001756-199711100-00024. [DOI] [PubMed] [Google Scholar]
- 5.Massimini M, et al. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- 6.Rosanova M, et al. Natural frequencies of human corticothalamic circuits. J Neurosci. 2009;29:7679–7685. doi: 10.1523/JNEUROSCI.0445-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massimini M, et al. Triggering sleep slow waves by transcranial magnetic stimulation. Proc Natl Acad Sci USA. 2007;104:8496–8501. doi: 10.1073/pnas.0702495104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322:876–880. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Dort CJ, Baghdoyan HA, Lydic R. Neurochemical modulators of sleep and anesthetic states. Int Anesthesiol Clin. 2008;46:75–104. doi: 10.1097/AIA.0b013e318181a8ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 11.Ferrarelli F, et al. Reduced evoked gamma oscillations in the frontal cortex in schizophrenia patients: a TMS/EEG study. Am J Psychiatry. 2008;165:996–1005. doi: 10.1176/appi.ajp.2008.07111733. [DOI] [PubMed] [Google Scholar]
- 12.Casali AG, Casarotto S, Rosanova M, Mariotti M, Massimini M. General indices to characterize the electrical response of the cerebral cortex to TMS. Neuroimage. 2010;49:1459–1468. doi: 10.1016/j.neuroimage.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 13.Chernik DA, et al. Validity and reliability of the Observer’s Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990;10:244–251. [PubMed] [Google Scholar]
- 14.Tanelian DL, Kosek P, Mody I, MacIver MB. The role of the GABAA receptor/chloride channel complex in anesthesia. Anesthesiology. 1993;78:757–776. doi: 10.1097/00000542-199304000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Verbny YI, Merriam EB, Banks MI. Modulation of gamma-aminobutyric acid type A receptor-mediated spontaneous inhibitory postsynaptic currents in auditory cortex by midazolam and isoflurane. Anesthesiology. 2005;102:962–969. doi: 10.1097/00000542-200505000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gan TJ. Pharmacokinetic and pharmacodynamic characteristics of medications used for moderate sedation. Clin Pharmacokinet. 2006;45:855–869. doi: 10.2165/00003088-200645090-00001. [DOI] [PubMed] [Google Scholar]
- 17.White NS, Alkire MT. Impaired thalamocortical connectivity in humans during general-anesthetic-induced unconsciousness. Neuroimage. 2003;19:402–411. doi: 10.1016/s1053-8119(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 18.Laureys S, et al. Impaired effective cortical connectivity in vegetative state: preliminary investigation using PET. Neuroimage. 1999;9:377–382. doi: 10.1006/nimg.1998.0414. [DOI] [PubMed] [Google Scholar]
- 19.Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol. 2004;3:537–546. doi: 10.1016/S1474-4422(04)00852-X. [DOI] [PubMed] [Google Scholar]
- 20.Laureys S, Tononi G. The Neurology of Consciousness. Amsterdam: Elsevier; 2009. [Google Scholar]
- 21.Massimini M, Boly M, Casali A, Rosanova M, Tononi G. A perturbational approach for evaluating the brain’s capacity for consciousness. Prog Brain Res. 2009;177:201–214. doi: 10.1016/S0079-6123(09)17714-2. [DOI] [PubMed] [Google Scholar]
- 22.Bachmann T. Microgenetic Approach to the Conscious Mind. Amsterdam: John Benjamins; 2000. [Google Scholar]
- 23.Steriade M. Thalamic Oscillations and Signaling. New York: Wiley; 1990. [Google Scholar]
- 24.Schwender D, Klasing S, Madler C, Pöppel E, Peter K. Effects of benzodiazepines on mid-latency auditory evoked potentials. Can J Anaesth. 1993;40:1148–1154. doi: 10.1007/BF03009604. [DOI] [PubMed] [Google Scholar]
- 25.Coulthard P, Rood JP. Midazolam and somatosensory evoked potentials. Br J Oral Maxillofac Surg. 1993;31:28–31. doi: 10.1016/0266-4356(93)90093-c. [DOI] [PubMed] [Google Scholar]
- 26.Judge O, Hill S, Antognini JF. Modeling the effects of midazolam on cortical and thalamic neurons. Neurosci Lett. 2009;464:135–139. doi: 10.1016/j.neulet.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 27.Esser SK, Hill SL, Tononi G. Breakdown of effective connectivity during slow wave sleep: investigating the mechanism underlying a cortical gate using large-scale modeling. J Neurophysiol. 2009 doi: 10.1152/jn.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Florian J, Müller-Dahlhaus M, Liu Y, Ziemann U. Inhibitory circuits and the nature of their interactions in the human motor cortex a pharmacological TMS study. J Physiol. 2008;586:495–514. doi: 10.1113/jphysiol.2007.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson LE, et al. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci. 2002;5:979–984. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- 30.Tononi G, Massimini M. Why does consciousness fade in early sleep? Ann N Y Acad Sci. 2008;1129:330–334. doi: 10.1196/annals.1417.024. [DOI] [PubMed] [Google Scholar]
- 31.Llinás RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95:3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- 32.McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 33.McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 35.Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 36.Veselis RA, et al. Midazolam changes cerebral blood flow in discrete brain regions: an H2(15)O positron emission tomography study. Anesthesiology. 1997;87:1106–1117. doi: 10.1097/00000542-199711000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Hudetz AG, Vizuete JA, Imas OA. Desflurane selectively suppresses long-latency cortical neuronal response to flash in the rat. Anesthesiology. 2009;111:231–239. doi: 10.1097/ALN.0b013e3181ab671e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Topulos GP, Lansing RW, Banzett RB. The experience of complete neuromuscular blockade in awake humans. J Clin Anesth. 1993;5:369–374. doi: 10.1016/0952-8180(93)90099-z. [DOI] [PubMed] [Google Scholar]
- 39.Schneider G, Gelb AW, Schmeller B, Tschakert R, Kochs E. Detection of awareness in surgical patients with EEG-based indices—bispectral index and patient state index. Br J Anaesth. 2003;91:329–335. doi: 10.1093/bja/aeg188. [DOI] [PubMed] [Google Scholar]
- 40.Franks NP. Molecular targets underlying general anaesthesia. Br J Pharmacol. 2006;147(Suppl 1):S72–S81. doi: 10.1038/sj.bjp.0706441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108(1):1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.