Abstract
GABA, the principal inhibitory neurotransmitter in the adult brain, has a parallel inhibitory role in the immune system. We demonstrate that immune cells synthesize GABA and have the machinery for GABA catabolism. Antigen-presenting cells (APCs) express functional GABA receptors and respond electrophysiologically to GABA. Thus, the immune system harbors all of the necessary constituents for GABA signaling, and GABA itself may function as a paracrine or autocrine factor. These observations led us to ask further whether manipulation of the GABA pathway influences an animal model of multiple sclerosis, experimental autoimmune encephalomyelitis (EAE). Increasing GABAergic activity ameliorates ongoing paralysis in EAE via inhibition of inflammation. GABAergic agents act directly on APCs, decreasing MAPK signals and diminishing subsequent adaptive inflammatory responses to myelin proteins.
Keywords: experimental autoimmune encephalomyelitis, multiple sclerosis, neurotransmitter, antigen-presenting cells
GABA is the principal inhibitory neurotransmitter in the central nervous system (CNS) (1). GABA inhibitory neurotransmission is essential in normal brain function, in neuronal activity, information processing and plasticity, and network synchronization, and in disease. GABAergic medications are used to treat anxiety, alcohol withdrawal, epilepsy, and to induce sedation, and anesthesia (2, 3). GABA is neuroprotective in animal models of stroke (4).
In addition to its well-known CNS roles, GABA also modulates inflammation. GABA receptor transcripts are present in immune cells (5 –7). GABA treatment decreases inflammatory cytokine production in peripheral macrophages (5). GABA and GABA type A receptor (GABA-A-R) agonists decrease cytotoxic immune responses and cutaneous delayed-type hypersensitivity reactions (8, 9). Treatment with GABA decreased T cell autoimmunity and the development of inflammatory responses in the nonobese diabetic mouse model of type 1 diabetes (6). The site of action of GABA in the adaptive immune response, however, remains obscure.
Multiple sclerosis (MS) is an inflammatory, demyelinating neurodegenerative disease of the CNS, the most common neurologic disease of young adults. Although its etiology is unknown, the primary insult in MS is widely attributed to autoimmune attack against myelin, leading to axon and neuron dysfunction in the CNS (10). Inflammation is prominent early in the disease, lasting a decade or two, and accumulating neuronal injury and neurodegeneration appears later, in the chronic phase. A peripheral adaptive immune response is key in MS pathophysiology (11). MS may be associated with diminished serum levels of GABA and its synthetic enzyme glutamic acid decarboxylase (GAD) (12). Taken together, these clues prompted us to explore the use of GABAergic agents in a mouse model of MS, experimental autoimmune encephalomyelitis (EAE). We determine the site of action of these agents and clarify the possible physiologic role of GABA in the immune system.
Results
Molecular Components of GABA Metabolism and Signaling in the Immune System.
GABA is present at submicromolar levels in serum (13). Because actions of exogenous GABA on inflammation and of endogenous GABA on phasic synaptic inhibition both occur at millimolar concentrations (5, 8, 9), we hypothesized that local mechanisms may also operate in the peripheral immune system to enhance GABA levels near the inflammatory focus. We first asked whether immune cells have synthetic machinery to produce GABA by Western blotting for GAD, the principal synthetic enzyme. We found significant amounts of a 65-kDa subtype of GAD (GAD-65) in dendritic cells (DCs) and lower levels in macrophages (Fig. 1A). GAD-65 increased when these cells were stimulated (Fig. 1A, DR vs. DS, and MR vs. MS). Assays of GABA in conditioned media from purified cultures of DCs, macrophages, and T cells indicated GABA secretion by these cell types (Fig. 1B). In contrast to the alteration in GAD-65 with stimulation, the amount of GABA collected in the conditioned media did not change (Fig. 1B, MR/DR/TR vs. MS/ DS/TS, respectively). This could reflect an inability to detect local changes in the amount of secreted GABA, especially if secreted locally in quanta or if bulk GABA were influenced via other mechanisms of GABA production, such as GAD-67, or via alternative pathways involving GABA reuptake, storage, and secretion.
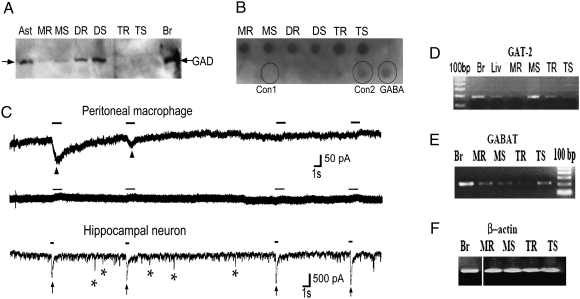
Fig. 1.
A GABAergic system is present in the immune system. (A) Macrophages and DCs were purified and stimulated with LPS, and CD4+ T cells were stimulated with α-CD3 and α-CD28 for 24–48 h. GAD enzyme was detected by immunoblotting in resting (MR or DR or TR) or stimulated (MS or DS or TS) peritoneal macrophages, dendritic cells, and T cells, respectively. Astrocytes (Ast) and brain extract (Br) were used as positive controls. (B) GABA secreted into conditioned media supernatant over purified immune cells, stimulated as above, was measured by dot blot. Controls are commercial pure GABA (2 μM) and same volume growth media used without cells: serum-free RPMI used for growth of DCs and macrophages (Con1) or X-Vivo 20 used for growing T cells (Con2). (C) Representative trace of a voltage-clamp recording showing functional GABA receptors in peritoneal macrophages during the first 10 s of recording (Top) and showing lack of response to GABA application 20 min after the initial responses (Middle). Bottom: Hippocampal neurons cultured 14 days in vitro are used as control. Arrowheads point to currents induced by focal application of 100 μM GABA for 1-s duration (marked by solid bars) in peritoneal macrophages. Arrows point to GABA currents induced by focal application of 100 μM GABA for 0.5-s duration in hippocampal neurons. In addition, neurons show spontaneous IPSCs, some denoted by asterisks. N = 11, n = 7 for macrophages and N = 3, n = 3 for neurons, where N is the total number of cells and n is the number of cells showing responses to GABA application. (D–F) mRNA was measured by RT-PCR in immune cells stimulated as in A. GABAT is the enzyme that degrades GABA, and GAT-2, a GABA transporter. Brain (Br), liver (Liv), and β-actin are used as controls.
We next asked whether immune cells have functional receptors for GABA. GABA-A-Rs are heteropentamers, composed of two subunits of type α (α1−6), two of type β (β1−3), and one of a third type (usually ε/γ/δ/π), that form a chloride channel in the cell membrane (14). We first sought to confirm the presence of GABA-A-R transcripts seen by others (5 –7) using RT-PCR analysis and found two of the common subunits in macrophages (Fig. S1). To determine whether these transcripts form functional GABA-A-R chloride channels, we performed whole-cell patch-clamp recordings (Fig. 1C). Focal application of 100 μM GABA on macrophages under whole-cell voltage clamp evoked inward currents similar to those in hippocampal neurons, which are known to express functional GABA-A-Rs and were used as a positive control. GABA-evoked currents were observed in 7 of the 11 macrophages recorded and were smaller in amplitude (−48 pA, n = 6) and slower to rise and decay than neuronal responses. These currents diminished in amplitude with repeated application of GABA, possibly owing to desensitization or endocytosis of GABA receptors (15, 16). Focal application of GABA did not yield any currents when the bath solution contained picrotoxin (n = 4). The macrophages were devoid of synapses and did not display spontaneous inhibitory postsynaptic currents (IPSCs) associated with spontaneous release of presynaptic vesicles, evident in neuronal recordings (Fig. 1C, asterisks). The contrasts with neurons could represent differences in receptor subunit composition, surface expression, or nonsynaptic characteristics.
We performed RT-PCR experiments to determine whether immune cells contain components of the GABA catabolic system. We found specific high-affinity GABA transporters (GATs) for reuptake of GABA from the extracellular space into the cytosol, and GABA transaminase (GABAT), the main degradation enzyme that converts GABA into intermediates to be recycled into the Krebs cycle. These components are present in both macrophages and T cells (Fig. 1 D and E). Of the four GATs described to date (17), we find GAT-2 to be present in immune cells (Fig. 1D).
GABAergic Agents Directly Affect Antigen-Presenting Cells.
To further characterize the immune effect of GABA, we used several mechanisms of manipulating this endogenous GABA system in the immune system. GABAergic agents included muscimol, a GABA structural analog; topiramate, a drug with GABA-A-R agonist activity; and irreversible inhibitors of GABAT, vigabatrin and gabaculine, which decrease GABA degradation, thereby causing an effective increase in GABA concentrations (18, 19). We also used the GABA-A-R inhibitor picrotoxin, which blocks the chloride channel (20, 21).
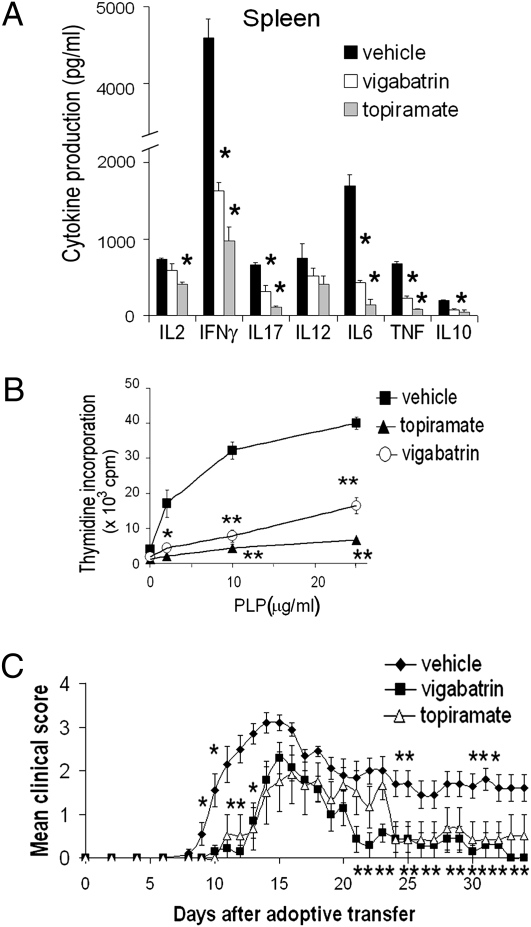
To study the adaptive immune response, we tested these GABAergic agents using C57BL/6 mice transgenic for the myelin oligodendroglial protein (MOG) T cell receptor (TCR), in which the vast majority of T cells respond to the MOG antigen. Naïve splenocytes of mice transgenic for the MOG TCR challenged in vitro with MOG 35-55 antigen proliferate and produce inflammatory cytokines. GABAergic agents added in vitro inhibit this effect in a dose-dependent manner (topiramate, Fig. 2A; vigabatrin, Fig. S2). Affected cytokines included IL17 and IFNγ, primarily produced by T cells, and TNF, IL6, and IL10, which can be produced by either T cells or antigen-presenting cells (APCs), such as DCs and macrophages (for IFNγ, see Fig. S3C, with muscimol and gabaculine). There was no change in viability of splenocytes with treatment. These responses in unfractionated splenocytes could reflect a direct effect on either T cells or APCs or both.
Fig. 2.
GABAergic agents act directly on APCs through the GABA-A-R and MAPK to suppress inflammation. (A) Naive splenocytes from C57BL/6 mice transgenic for the MOG TCR were activated in vitro with 0–10 μg/mL MOG in the presence of vehicle or various concentrations of topiramate: 2 μm, 20 μM, or 200 μM. Data are shown as mean ± SD cytokine secretion (pg/mL) of duplicate cultures measured by ELISA and are representative of the range of concentrations tested (*P < 0.05; **P < 0.005). (B and C) Purified T cell cultures were activated with 0.1–1 μg/mL plate-bound α-CD3 and α-CD28. Proliferation rates (B) and cytokine production (pg/mL) (C) were measured in the presence of vehicle, 200 μM topiramate, 100 μM gabaculine, or 100 μM muscimol. Data are shown as mean ± SD of triplicates and are representative of the range of concentrations tested. (D) Peritoneal macrophages were purified from mice treated with vehicle, vigabatrin (400 mg/kg per day), or topiramate (100 mg/kg per day) for 1 week and activated in vitro with LPS. (E) Peritoneal macrophages were stimulated with 0–800 ng/mL LPS in the presence of the GABAergic agents in vitro, 20–200 μM topiramate, 500 μM vigabatrin, 10–100 μM muscimol, or 50–500 μM gabaculine, and for each GABAergic agent with 10–100 μM picrotoxin, the GABA-A channel blocker. For D and E, data represent IL-1β production (pg/mL), mean ± SD of duplicate cultures (*P < 0.05; **P < 0.005) and are shown for a representative concentration. (F) Macrophages or T cells were purified separately from MOG TCR transgenic mice treated with vehicle (−) or topiramate (+) (100 mg/kg per day) orally for 1 week. Macrophages and T cells were mixed reciprocally and the culture stimulated in vitro with 0–20 μg/mL MOG. Cytokine production then measured shown as mean ± SD (pg/mL) of duplicate cultures is representative of the range of concentration of MOG. *P < 0.05. (G) Peritoneal macrophages purified from mice treated as in D were stimulated in vitro with LPS for various time points, as shown. Western blotting for the phosphorylated p44/42 MAPK and p38/ERK proteins was performed and compared with nonphosphorylated forms and β-actin control.
To establish the specific cell types affected by GABA, we separately tested T cells and APCs (Fig. 2 B–G). Purified T cells were directly stimulated with anti-CD3 and anti-CD28 in vitro, producing polyclonal expansion. GABAergic agents had no direct effect on T cell proliferation or production of IFNγ, TNF, IL-17, or IL6 (Fig. 2 B and C). Purified APCs were directly stimulated with LPS. In contrast to T cells, but similar to unfractionated splenocytes (Fig. 2A and Fig. S2), purified APCs responded to GABAergic drug treatment by diminished production of inflammatory cytokines, IL1β and IL6 (macrophages, Fig. 2D; DCs, Fig. S3A). The inhibitory effect of GABAergic agents was dose dependent (Fig. S3B) and reversed by picrotoxin, indicating the involvement of GABA-A-R (Fig. 2E). Similar reversal was seen with unfractionated splenocytes (Fig. S3C).
These studies imply that the GABA system of the immune system can be modulated by GABAergic agents. The effect of vigabatrin and gabaculine could occur by increasing local GABA concentrations in the inflammatory focus to levels that cause GABA-A-R activation in APCs through blockage of GABAT present in immune cells (Fig. 1E). Local concentrations of GABA increase by a secondary effect of blocking GABAT, causing the reversal of reuptake via the GATs, which exist in immune cells (Fig. 1C). GABA is secreted in this manner via GAT-1 (22, 23). Although picrotoxin can block chloride channels other than GABA-A-Rs, some of which are immunomodulatory (24), we only saw significant effects of picrotoxin on cytokine production with manipulation of GABA signaling, not in vehicle-treated cultures (Fig. 2E), suggesting that picrotoxin acted specifically to reverse GABA effect of these agents. Although topiramate, muscimol, gabaculine, and vigabatrin each have different sets of off-site and nonspecific effects, a common function is their GABAergic activity. The similar effect of these agents on APCs and reversal with picrotoxin in vitro support the conclusion that these drugs are working through the GABA pathway.
To determine whether the GABAergic effect on APCs is sufficient for the change in adaptive response seen in unfractionated splenocytes, we examined MOG-specific T cell activation when only macrophages or T cells were exposed to topiramate. Macrophages were purified from topiramate- or vehicle-treated mice. In a reciprocal manner, they were cocultured in vitro with T cells expressing the MOG TCR purified from topiramate- or vehicle-treated mice and challenged with MOG. Treatment of macrophages with topiramate diminished production of IFNγ by T cells, and TNF (which can be produced by both macrophages and T cells) in co-cultures (Fig. 2F). Treatment of T cells alone did not have this effect. Furthermore, in cultures in which both macrophages and T cells were taken from topiramate-treated animals, treating the T cells produced no additional effect.
MAPKs are involved in later steps of GABA-A-R signaling and are critical in the immune response (25 –28). MAPKs interact with immunomodulators such as signal transducer and activator of transcription molecules that up-regulate cytokine genes in APCs, and specifically, p38 MAPK can increase transcription of IL6 and IL1β, and p44/42 MAPK affects IL6 (29). Because GABAergic agents decreased the production of these cytokines in APCs (Fig. 2E and Fig. S3 A and B), we hypothesized that they modulate MAPK phosphorylation. We stimulated vigabatrin- and topiramate-treated macrophages with LPS in vitro and compared them with vehicle-treated macrophages. Indeed, vigabatrin decreased the phosphorylation of p38 MAPK and p44/42 ERKs during LPS stimulation by 30% and 12%, respectively, and topiramate by 53% and 45%, respectively (Fig. 2E). Thus, GABAergic agents modulate the function of APCs through GABA-A-R and MAPK phosphorylation but do not directly act on T cells, and this effect is sufficient to inhibit inflammatory cytokine production in T cell responses during inflammation.
GABAergic Agents Ameliorate EAE.
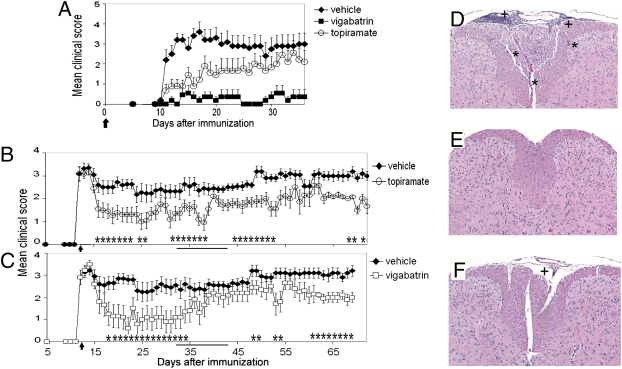
We next tested whether increasing GABAergic activity could affect EAE. We administered topiramate and vigabatrin in daily oral doses similar to equivalent human doses in current use, scaled for the mouse using accepted guidelines (www.fda.gov/downloads/Drugs/.../Guidances/UCM078932.pdf). SJL/J mice were immunized with the myelin peptide proteolipid protein (PLP) 139-151 to induce EAE. In this EAE model, immunization engenders a characteristic syndrome of ascending paralysis, starting with tail paralysis and progressively involving the hind and fore limbs and then the brain. When GABAergic agents were started at the time of immunization, they prevented and delayed EAE onset and decreased severity of symptoms in a dose-dependent manner (Fig. 3A and Fig. S4; quantified in Table S1). Control and treatment groups were killed at the end of the experiment (day 37) and brains and spinal cords examined histologically. There was a significant decrease in number of parenchymal inflammatory foci (Fig. 3 D–F and Fig. S5; quantified in Table S1). Recently, T helper cell Th1 and Th17 responses were shown to be important in EAE development. We therefore examined spleen and lymph node cells of treated mice for Th1 cytokines, IL12 and IFNγ, and the Th17 cytokines, IL6 and IL17, in memory T cell responses to restimulation with PLP 139-151 ex vivo at 10 days after immunization, and found a significant reduction in these cytokines and in proliferation in the treatment groups (Fig. 4 A and B and Fig. S6). This is consistent with results of our in vitro studies in unprimed naïve splenocytes (Fig. 2 A and F and Figs. S2, and S3C). Both agents also reversed paralysis when treatment was initiated after the establishment of EAE (Fig. 3 B and C) and decreased the number of relapses (Table S1, relapse rate being determined for each animal).
Fig. 3.
GABAergic agents ameliorate EAE. (A–C) SJL/J mice were immunized with 100 μg of PLP 139-151 in CFA. Graphs represent prevention of EAE (A) by oral treatment with topiramate (100 mg/kg per day) or vigabatrin (400 mg/kg per day) starting at the time of immunization, as indicated by the arrow; and treatment of established EAE (B and C) by oral topiramate (100 mg/kg per day) or vigabatrin (400 mg/kg per day) starting at the peak of disease, as indicated by the arrow. Data represent clinical scores, as described in Materials and Methods, mean ± SEM, representative of two independent experiments, n = 10 per group. *P < 0.05, Mann-Whitney analysis. In B and C, mice were treated daily except during days 32–42, as indicated by the solid bar, when they were treated every 3 days. (D–F) H&E-stained transverse sections of spinal cord of mice killed at the end of the experiment (day 37; A). Representative control (D), topiramate- (E), and vigabatrin-treated animals (F) are shown. *Parenchymal inflammatory foci; +meningeal inflammatory foci. 125× magnification.
Fig. 4.
GABAergic agents ameliorate EAE through an effect on the immune system. (A and B) SJL/J mice were immunized with PLP 139-151 (PLP) and treated with vehicle (PBS), topiramate (100 mg/kg per day), or vigabatrin (400 mg/kg per day) for 10 days after immunization. Splenocytes were then taken and restimulated ex vivo with 0–25 μg/mL PLP. Cytokine responses (A) and proliferation rates (B) of cells to rechallenge with 10 μg/mL of PLP are shown as mean ± SD of triplicate culture wells. *P < 0.05; **P < 0.005. Results are representative of three independent experiments and the range of PLP concentration. (C) Splenocytes were taken from PLP-immunized SJL/J donor mice treated with topiramate, vigabatrin, or vehicle for 10 days as in A and B and adoptively transferred into untreated naïve recipient SJL/J mice. EAE induced adoptively in recipients is shown as mean clinical scores ± SEM, n = 7–10 per group. *P < 0.05, Mann-Whitney analysis.
GABAergic Effect on the Immune System Is Sufficient to Ameliorate EAE.
These GABAergic agents cross the blood–brain barrier. Therefore, they might be ameliorating EAE via an effect on the immune compartment (which includes the peripheral immune system and immune cells in the CNS) or the neuronal compartment or both. To further clarify this, we immunized donor SJL/J mice and treated them with topiramate or vigabatrin for 10 days. We then collected spleen and lymph node cells and restimulated with PLP 139-151 ex vivo. We injected these cells i.v. into naïve recipient mice to induce EAE, not treating recipient mice with any GABAergic agents. In recipient mice injected with topiramate- or vigabatrin-treated immune cells, EAE onset was delayed and severity decreased significantly (Fig. 4C). This indicates that a direct effect of these agents on the immune cell compartment in vivo is sufficient to ameliorate EAE.
Discussion
Intricate and reciprocal regulatory relationships exist between the nervous system and the immune system, mediated in part by shared chemical messengers (30). This has been clearly demonstrated for the CNS through the hypothalamic–pituitary–adrenal axis and for the autonomic nervous system via an anatomic connection where nerve terminals end in peripheral immune organs (31, 32). Immune cells have receptors for various neurotransmitters, neuropeptides, and hormones, and in turn, neural cells have receptors for cytokines (33, 34). What has been much less clear is that molecules and signaling mechanisms used within the nervous system can be recapitulated in the immune system (35, 36). Our experiments provide evidence for an endogenous inhibitory GABAergic system within the immune system.
Here we demonstrate endogenous GABA secretion by both APCs and T cells, functional GABA channels on macrophages, and a direct effect of GABAergic agents on APCs. A previous study showed functional GABA channels and the action of exogenous GABA on proliferation in an encephalitogenic T cell line (37). Although we did not see a direct effect of GABAergic agents on T cells, this could represent a difference in mouse strain, cell lines used, or the subtype of receptor expressed. GAT-1 is expressed on antigen-activated T cells, and GAT-1 knockout mice have exacerbated and atypical EAE (38). Taken together with our results, these studies are consistent with the involvement of GABA in crosstalk between immune cells at the inflammatory focus.
In our study, increasing GABAergic activity potently ameliorated the manifestations of EAE. This effect occurred at least in part through a direct effect of the GABAergic agents on the immune system. The additional possibility is that these agents modulate neuronal and glial susceptibility to injury and their intrinsic repair mechanisms may be important in MS (39). Studies to explore these issues are underway.
Although topiramate and similar agents are currently used by some patients with established MS who also have symptoms of epilepsy or migraines, no epidemiologic data exist on the effect of these agents on underlying MS disease activity. Conducting retrospective epidemiologic studies to determine whether MS patients treated for seizures or migraine benefit from GABAergic drug treatment is problematic owing to the variability of doses, agents, and regimen. Importantly, in MS patients, these agents are generally started long after the onset and establishment of MS and in the chronic neurodegenerative phase, whereas the greatest effect of these agents on the disease process may be in the early inflammatory and relapsing phase of the disease.
Current disease-modifying treatments for MS are given parenterally, have significant adverse side effects, and are only partially effective (40). Our study focuses on agents that modulate GABA. Although the risk of visual field defect by vigabatrin may limit or preclude its use in human MS, topiramate and many other well-tolerated agents with GABAergic activity are currently available. GABA-A-Rs have a variety of allosteric modulators (41). Drugs targeting GATs are also in current use (42). Because these medications, some currently approved for other conditions like epilepsy and migraine, show significant promise in EAE, they are attractive for testing in MS patients. In addition, these drugs have different side-effect profiles than the current MS disease-modifying agents, namely the interferons and glatiramer acetate, and thus they could be useful in combination with these agents. Glatiramer acetate may also have its beneficial effect in EAE through macrophage modulation (43). Thus the combination with GABAergic agents may prove to be synergistic in MS. GABA system targets should be explored in human MS and other autoimmune diseases.
Materials and Methods
Macrophages, DCs, and CD4 T cells were purified from spleens and lymph nodes by positive selection using CD11b+, CD11c+, and CD4 microbeads, respectively (Miltenyi Biotech), and were >95% pure by FACS. For DCs, spleens were first treated with collagenase. Primary peritoneal macrophages were obtained from peritoneal exudates 72 h after injecting mice i.p. with 2 mL of 0.05% wt/vol sodium thioglycollate medium (Becton Dickinson). For in vitro assays, purified GABAergic agents and picrotoxin were purchased from Sigma-Aldrich and Tocris Bioscience and added to cultures 30–60 min before stimulation. Splenocytes or lymph node cells were activated with PLP 139-151 or MOG 35-55 peptide (0–25 μg/mL), APCs with LPS (Sigma-Aldrich), and T cells with αCD3 + αCD28 (eBioscience) for 24–120 h. Cytokines were measured using specific ELISA. RT-PCR, Western, and dot blotting were performed with primers and antibodies listed in SI Materials and Methods. For electrophysiology, whole-cell recordings were performed on peritoneal macrophages or hippocampal neurons using a MultiClamp 700B (Axon Instruments) amplifier. GABA-mediated currents were evoked by applying pulses of 100 μM GABA using a digitally controlled PV820 Pneumatic PicoPump (World Precision Instruments) with the glass micropipette positioned 20 μm from the recorded cell, voltage-clamped at −70 mV. See SI Materials and Methods for electrode solutions. EAE was induced in naïve female SJL/J mice (aged 8–12 weeks) via s.c. immunization with 100 μg of PLP 139-151 emulsified with 1:1 complete Freund’s adjuvant (CFA). For adoptive transfer, EAE was induced by injecting 5 × 107 cells i.v. Prescription formulations of topiramate (Ortho-McNeil) or vigabatrin (Aventis) were dissolved in PBS and given orally once daily by gavage. Clinical signs of EAE were scored daily: 0 = no clinical disease, 1 = tail paralysis, 2 = hindlimb weakness, 3 = complete hindlimb paralysis, 4 = forelimb paralysis, and 5 = moribund. Data are means ± SEM or SD, as indicated. For clinical EAE scores, groups were compared using a Mann-Whitney U test. All other statistics were analyzed by one-way, multirange ANOVA for multiple comparisons or t test. P < 0.05 was considered significant. See SI Materials and Methods for full methods.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health (NIH) and National Multiple Sclerosis Society (NMSS) grants to L.S., by a postdoctoral fellowship grant to R.B. from the NMSS, and by NIH Grant NS24067 (to R.W.T.). The GAD6 monoclonal antibody was obtainned from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biological Sciences.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0915139107/DCSupplemental.
References
- 1.Somogyi P, Tamás G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- 2.Clarkson AN, Sutherland BA, Appleton I. The biology and pathology of hypoxia-ischemia: An update. Arch Immunol Ther Exp (Warsz) 2005;53:213–225. [PubMed] [Google Scholar]
- 3.Wong CG, Bottiglieri T, Snead OC., 3rd GABA, gamma-hydroxybutyric acid, and neurological disease. Ann Neurol. 2003;54(Suppl 6):S3–S12. doi: 10.1002/ana.10696. [DOI] [PubMed] [Google Scholar]
- 4.Clarkson AN, et al. Clomethiazole: Mechanisms underlying lasting neuropro-tection following hypoxia-ischemia. FASEB J. 2005;19:1036–1038. doi: 10.1096/fj.04-3367fje. [DOI] [PubMed] [Google Scholar]
- 5.Reyes-Garcia MG, Hernandez-Hernandez F, Hernandez-Tellez B, Garcia-Tamayo F. GABA (A) receptor subunits RNA expression in mice peritoneal macrophages modulate their IL-6/IL-12 production. J Neuroimmunol. 2007;188:64–68. doi: 10.1016/j.jneuroim.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Tian J, et al. Gamma-aminobutyric acid inhibits T cell autoimmunity and the development of inflammatory responses in a mouse type 1 diabetes model. J Immunol. 2004;173:5298–5304. doi: 10.4049/jimmunol.173.8.5298. [DOI] [PubMed] [Google Scholar]
- 7.Alam S, Laughton DL, Walding A, Wolstenholme AJ. Human peripheral blood mononuclear cells express GABAA receptor subunits. Mol Immunol. 2006;43:1432–1442. doi: 10.1016/j.molimm.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 8.Tian J, Chau C, Hales TG, Kaufman DL. GABA(A) receptors mediate inhibition of T cell responses. J Neuroimmunol. 1999;96:21–28. doi: 10.1016/s0165-5728(98)00264-1. [DOI] [PubMed] [Google Scholar]
- 9.Bergeret M, et al. GABA modulates cytotoxicity of immunocompetent cells expressing GABAA receptor subunits. Biomed Pharmacother. 1998;52:214–219. doi: 10.1016/S0753-3322(98)80019-X. [DOI] [PubMed] [Google Scholar]
- 10.Bennett JL, Stüve O. Update on inflammation, neurodegeneration, and immuno-regulation in multiple sclerosis: Therapeutic implications. Clin Neuropharmacol. 2009;32:121–132. doi: 10.1097/WNF.0b013e3181880359. [DOI] [PubMed] [Google Scholar]
- 11.Bhat R, Steinman L. Innate and adaptive autoimmunity directed to the central nervous system. Neuron. 2009;64:123–132. doi: 10.1016/j.neuron.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Demakova EV, Korobov VP, Lemkina LM. [Determination of gamma-aminobutyric acid concentration and activity of glutamate decarboxylase in blood serum of patients with multiple sclerosis] Klin Lab Diagn. 2003;Apr:15–17. [PubMed] [Google Scholar]
- 13.Erdö SL, Wolff JR. gamma-Aminobutyric acid outside the mammalian brain. J Neurochem. 1990;54:363–372. doi: 10.1111/j.1471-4159.1990.tb01882.x. [DOI] [PubMed] [Google Scholar]
- 14.Farrant M, Kaila K. The cellular, molecular and ionic basis of GABA(A) receptor signalling. Prog Brain Res. 2007;160:59–87. doi: 10.1016/S0079-6123(06)60005-8. [DOI] [PubMed] [Google Scholar]
- 15.Leidenheimer NJ. Regulation of excitation by GABA(A) receptor internalization. Results Probl Cell Differ. 2008;44:1–28. doi: 10.1007/400_2007_039. [DOI] [PubMed] [Google Scholar]
- 16.Kneussel M. Dynamic regulation of GABA(A) receptors at synaptic sites. Brain Res Brain Res Rev. 2002;39:74–83. doi: 10.1016/s0165-0173(02)00159-5. [DOI] [PubMed] [Google Scholar]
- 17.Conti F, Minelli A, Melone M. GABA transporters in the mammalian cerebral cortex: Localization, development and pathological implications. Brain Res Brain Res Rev. 2004;45:196–212. doi: 10.1016/j.brainresrev.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 18.White HS, Brown SD, Woodhead JH, Skeen GA, Wolf HH. Topiramate enhances GABA-mediated chloride flux and GABA-evoked chloride currents in murine brain neurons and increases seizure threshold. Epilepsy Res. 1997;28:167–179. doi: 10.1016/s0920-1211(97)00045-4. [DOI] [PubMed] [Google Scholar]
- 19.Choi S, Silverman RB. Inactivation and inhibition of gamma-aminobutyric acid aminotransferase by conformationally restricted vigabatrin analogues. J Med Chem. 2002;45:4531–4539. doi: 10.1021/jm020134i. [DOI] [PubMed] [Google Scholar]
- 20.Inoue M, Akaike N. Blockade of gamma-aminobutyric acid-gated chloride current in frog sensory neurons by picrotoxin. Neurosci Res. 1988;5:380–394. doi: 10.1016/0168-0102(88)90024-7. [DOI] [PubMed] [Google Scholar]
- 21.Yoon KW, Covey DF, Rothman SM. Multiple mechanisms of picrotoxin block of GABA-induced currents in rat hippocampal neurons. J Physiol. 1993;464:423–439. doi: 10.1113/jphysiol.1993.sp019643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, Wang W, Richerson GB. Vigabatrin induces tonic inhibition via GABA transporter reversal without increasing vesicular GABA release. J Neurophysiol. 2003;89:2021–2034. doi: 10.1152/jn.00856.2002. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Wang W, Díez-Sampedro A, Richerson GB. Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron. 2007;56:851–865. doi: 10.1016/j.neuron.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schilling T, Eder C. A novel physiological mechanism of glycine-induced immunomodulation: Na+-coupled amino acid transporter currents in cultured brain macrophages. J Physiol. 2004;559:35–40. doi: 10.1113/jphysiol.2004.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frye CA, Walf AA. Membrane actions of progestins at dopamine type 1-like and GABA(A) receptors involve downstream signal transduction pathways. Steroids. 2008;73:906–913. doi: 10.1016/j.steroids.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simi A, Ingelman-Sundberg M, Tindberg N. Neuroprotective agent chlomethiazole attenuates c-fos, c-jun, and AP-1 activation through inhibition of p38 MAP kinase. J Cereb Blood Flow Metab. 2000;20:1077–1088. doi: 10.1097/00004647-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Obrietan K, Gao XB, Van Den Pol AN. Excitatory actions of GABA increase BDNF expression via a MAPK-CREB-dependent mechanism—a positive feedback circuit in developing neurons. J Neurophysiol. 2002;88:1005–1015. doi: 10.1152/jn.2002.88.2.1005. [DOI] [PubMed] [Google Scholar]
- 28.Shin T, et al. Activation of mitogen-activated protein kinases in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2003;140:118–125. doi: 10.1016/s0165-5728(03)00174-7. [DOI] [PubMed] [Google Scholar]
- 29.Valledor AF, et al. Selective roles of MAPKs during the macrophage response to IFN-gamma. J Immunol. 2008;180:4523–4529. doi: 10.4049/jimmunol.180.7.4523. [DOI] [PubMed] [Google Scholar]
- 30.Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol. 2004;5:575–581. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- 31.Straub RH. Complexity of the bi-directional neuroimmune junction in the spleen. Trends Pharmacol Sci. 2004;25:640–646. doi: 10.1016/j.tips.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Raber J, et al. Inflammatory cytokines: putative regulators of neuronal and neuro-endocrine function. Brain Res Brain Res Rev. 1998;26:320–326. doi: 10.1016/s0165-0173(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 33.Elenkov IJ, Kovács K, Duda E, Stark E, Vizi ES. Presynaptic inhibitory effect of TNF-alpha on the release of noradrenaline in isolated median eminence. J Neuroimmunol. 1992;41:117–120. doi: 10.1016/0165-5728(92)90203-w. [DOI] [PubMed] [Google Scholar]
- 34.Perry SW, Dewhurst S, Bellizzi MJ, Gelbard HA. Tumor necrosis factor-alpha in normal and diseased brain: Conflicting effects via intraneuronal receptor crosstalk? J Neurovirol. 2002;8:611–624. doi: 10.1080/13550280290101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connell PJ, et al. A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and T cells. Blood. 2006;107:1010–1017. doi: 10.1182/blood-2005-07-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergquist J, Tarkowski A, Ekman R, Ewing A. Discovery of endogenous catecholamines in lymphocytes and evidence for catecholamine regulation of lymphocyte function via an autocrine loop. Proc Natl Acad Sci USA. 1994;91:12912–12916. doi: 10.1073/pnas.91.26.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bjurstöm H, et al. GABA, a natural immunomodulator of T lymphocytes. J Neuroimmunol. 2008;205:44–50. doi: 10.1016/j.jneuroim.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, et al. Gamma-aminobutyric acid transporter 1 negatively regulates T cell-mediated immune responses and ameliorates autoimmune inflammation in the CNS. J Immunol. 2008;181:8226–8236. doi: 10.4049/jimmunol.181.12.8226. [DOI] [PubMed] [Google Scholar]
- 39.Stangel M. Neuroprotection and neuroregeneration in multiple sclerosis. J Neurol. 2008;255(Suppl 6):77–81. doi: 10.1007/s00415-008-6014-x. [DOI] [PubMed] [Google Scholar]
- 40.Menge T, et al. Disease-modifying agents for multiple sclerosis: Recent advances and future prospects. Drugs. 2008;68:2445–2468. doi: 10.2165/0003495-200868170-00004. [DOI] [PubMed] [Google Scholar]
- 41.Nutt D. GABAA receptors: Subtypes, regional distribution, and function. J Clin Sleep Med. 2006;2:S7–S11. [PubMed] [Google Scholar]
- 42.Schachter SC. Tiagabine. Epilepsia. 1999;40(Suppl 5):S17–S22. doi: 10.1111/j.1528-1157.1999.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 43.Weber MS, et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.