Abstract
In the medial prefrontal cortex, the prelimbic area is emerging as a major modulator of fear behavior, but the mechanisms remain unclear. Using a selective neocortical knockout mouse, virally mediated prelimbic cortical-specific gene deletion, and pharmacological rescue with a TrkB agonist, we examined the role of a primary candidate mechanism, BDNF, in conditioned fear. We found consistently robust deficits in consolidation of cued fear but no effects on acquisition, expression of unlearned fear, sensorimotor function, and spatial learning. This deficit in learned fear in the BDNF knockout mice was rescued with systemic administration of a TrkB receptor agonist, 7,8-dihydroxyflavone. These data indicate that prelimbic BDNF is critical for consolidation of learned fear memories, but it is not required for innate fear or extinction of fear. Moreover, use of site-specific, inducible BDNF deletions shows a powerful mechanism that may further our understanding of the pathophysiology of fear-related disorders.
Keywords: learning, plasticity, prefrontal cortex, Cre/LoxP, inducible knockout
In healthy individuals, the prefrontal cortex and amygdala are critical for processing fearful and other emotional stimuli and for learning to extinguish fears in situations that are no longer threatening (1, 2). In contrast, patients suffering from posttraumatic stress disorder (PTSD) or anxiety disorders describe persistent anxiety-provoking memories that are severely debilitating and cannot be extinguished (3 –6). Therefore, the experimental analysis of fear modulation and extinction is critical for an understanding of the neurobiology of fear inhibition. The medial prefrontal cortex (mPFC) is suggested to be an important region for the regulation of fear (7 –13). Although it is established that the infralimbic cortex (IL) region of the mPFC is required for fear extinction (9, 11, 14), the role of the prelimbic cortex (PL) in the regulation of fear learning and extinction are yet to be fully understood. Although previous studies have shown that lesions of the PL do not affect acquisition or expression of fear (7, 9, 15), inactivation reduces freezing behavior in previously fear-conditioned rats (16). Additionally, activation of PL neurons are required for the expression of previously learned fears (17, 18), and microstimulation of the PL potentiates expression of conditioned fear (19). Moreover, these neurons have also shown plasticity after fear conditioning (18, 20, 21) and have sustained activity to conditioned tones (22). Overall, these data suggest that the PL is necessary for the expression of previously learned fear, but the mechanisms remain unclear.
One potential candidate may be BDNF and its receptor tyrosine kinase receptor B (TrkB); they are known to regulate neuronal structure and function and are important for synaptic plasticity (23 –26). Additionally, in vivo studies have shown a role for BDNF in learning and memory, including fear conditioning (27 –31). More specifically, we have previously shown that disruption of TrkB activation using lentiviral expression of a dominant-negative form of TrkB (TrkB.T1) into the basolateral amygdala blocked the acquisition of fear (27) and the consolidation of extinction (29), suggesting that BDNF-dependent activation of TrkB within the amygdala regulates the learning of fear and extinction memories. This role of BDNF has yet to be investigated in the subregions of the mPFC and may play an important role in the plasticity of fear-learning circuitry. Because BDNF is highly expressed in the PL, which projects heavily to basolateral amygdala (32), we hypothesized that prelimbic BDNF-dependent plasticity is necessary for the expression and possibly extinction of learned fear memories. To test our hypothesis, we used different approaches with (i) a neocortical BDNF knockout mouse, (ii) reversal of the BDNF knockout phenotype with a newly identified TrkB receptor agonist, and (iii) viral-mediated BDNF deletions limited to the prelimbic cortex.
Results
Neocortical BDNF Knockout Mice Have Impaired Learned Fear.
To examine the role of cortex-specific BDNF in modulating fear, we used a newly developed cortex-specific BDNF knockout mouse model (33). Expression of Cre recombinase in these mice includes the PL and neocortex but spares the more ventral IL, along with all other paleocortical and subcortical regions, with no expression in hippocampus, thalamus, striatum, hypothalamus, or amygdala (Fig. 1A and Fig. S1). These Cre-driver mice were crossed with transgenic homozygous BDNF-floxed (fBDNF) mice (34), allowing us to selectively delete BDNF expression in select areas of the neocortex when Cre expression is present (Fig. 1 B and C). In the littermate control mice (Cre−/−), BDNF is abundantly expressed in the prefrontal cortex, including PL and IL (Fig. 1D), whereas BDNF knockout mice (Cre+/−) have loss of BDNF expression in the PL, sparing BDNF expression in the more ventrally located IL (Fig. 1C). Cre expression was also confirmed with a LacZ reporter mouse (35) (Fig. 1 A and E). Cortical BDNF deletion in Cre+/− overlays directly on the regions expressing LacZ (Fig. 1 C and F). We also generated mutant mice by crossing the Cre+/− line with a floxed-stop fluorescent reporter line (36). Membrane-bound eGFP allowed visualization of cellular patterns of the neocortical Cre expression (Fig. 1G), confirming neocortical and prelimbic-specific Cre expression.
Fig. 1.
Selective neocortical BDNF knockout mice. (A) Cortex Cre+/− mice were crossed with the floxed-stop lacZ reporter mouse line. X-gal staining is shown across anterior–posterior coronal slices. (B) In situ hybridization of Cre mRNA in cortex Cre+/− mice. PL, prelimbic cortex; IL, infralimbic cortex. (C) Cre+/−, fBDNF neocortex selective knockout mice have loss of cortical BDNF mRNA compared with (D) Cre−/−, fBDNF littermates with intact BDNF expression. (E) LacZ expression in Cre+/−, Rosa LacZ mice extends into the PL and (F) overlays with the specific knockout of BDNF in the fBDNF crosses. (G) Cortex Cre+/− mice were crossed with fluorescent reporter mice (36) with labeled Cre expression (eGFP) in the PL that spares the ventral IL. Red, Td tomato; green, eGFP; blue, DAPI.
After confirming the neocortex-specific deletion of BDNF, we examined the effects of this inducible deletion on a number of behavioral tasks. We found that Cre+/− BDNF knockout mice were similar to Cre−/− littermate controls in locomotor function, baseline startle response, shock reactivity (a control for pain sensitivity), and prepulse inhibition (a control for auditory function and sensorimotor gating) (Fig. 2). These data suggest that neocortical BDNF is not required for these sensory, pain, and startle behaviors and for normal locomotor function. Additionally, there were no differences between Cre+/− and Cre−/− mice on measures of anxiety-like behavior or unlearned/innate fear as measured by the elevated-plus or open-field mazes (Fig. 2 B and C).
Fig. 2.
Neocortical BDNF knockout mice have normal locomotor, anxiety-like, and sensory responsiveness. (A) Locomotor activity was assessed in open-field fBDNF, Cre−/−, and Cre+/− mice that showed no differences in distance traveled over 10 min. (B) Anxiety-like behavior was also assessed in the open field, where there were no differences between groups in time spent in the field center or surround. There were also no differences in (C) anxiety-like behavior assessed on an elevated-plus maze or in (D) baseline startle, (E) shock reactivity, or (F) prepulse inhibition as measured by startle responses (n = 8 per group).
The neocortical BDNF knockout mice (Cre+/−) subsequently underwent cue-dependent fear conditioning, because we hypothesized that BDNF in the PL is required for learning or expression of cued fear. The Cre+/− had normal acquisition of fear and expression of newly acquired fear memories, which was measured during cue-dependent fear training to five pairings of conditioned stimulus (CS) tones and unconditioned stimulus (US) shocks (Fig. 3A). To test their short-term memory, these mice were tested 1 h after the cessation of fear training; they were placed in a different context and measured for fear behavior (freezing) to the presentation of three CS tones. The Cre+/− had attenuated fear responses to the tone compared with the Cre−/− littermate controls (t(15) = 2.13; *P < 0.05) (Fig. 3B).
Fig. 3.
Neocortical BDNF expression is required for short-term and long-term memory of conditioned fear but not extinction. (A) Neocortical BDNF knockout mice (Cre+/−) and fBDNF littermate controls (Cre−/−) acquired fear after cued fear conditioning with five tone-shock pairings. (B) One hour later, Cre+/− expressed less overall freezing than Cre−/− during the presentation of a three CS-tone test (n = 8 per group). (C) In separate experiment, Cre+/− and Cre−/− were fear trained to a tone cue, and 24 h later, Cre+/− expressed less overall freezing than Cre−/− during the 15-tone test (n = 15 Cre−/−; n = 16 Cre+/−). (D) Cre+/− showed less freezing during the first and third blocks of CS tone presentations (n = 15 Cre−/−; n = 16 Cre+/−). (E) To investigate extinction learning, additional cortical BDNF knockout mice were fear trained to a tone cue. One day later, they were extinction trained, and 1 day later, they were tested for extinction retention. Average freezing during extinction training and extinction retention tests to 50 CS tones each day was recorded (n = 13 Cre−/−; n = 14 Cre+/−). (F) Within-session extinction occurred in both groups, and Cre+/− had less fear during (G) extinction retention (n = 13 Cre−/−; n = 14 Cre+/−). (H) Within-session extinction was normalized to percentage of initial freezing levels for each group with no differences between groups (*P < 0.05 Cre+/− versus Cre−/−).
To investigate long-term fear memory, another group of neocortical BDNF knockout mice (Cre+/−) and controls (Cre−/−) were similarly fear-conditioned and tested 24 h later for expression of previously learned fear to the presentation of 15 trials of CS cues in a different context. The Cre+/− had robustly attenuated freezing to the presentation of the CS-tones test compared with Cre−/− (t(29) = 2.44; *P < 0.05) (Fig. 3C) and diminished freezing during the first and third blocks of five CS-tone presentations (F1,92 = 5.93; *P < 0.05) (Fig. 3D).
A different set of neocortical BDNF knockout mice were fear-conditioned as above followed by extinction 24 h later with 50 CS tones in the absence of the US. The next day, these mice were exposed to another 50 CS tones in the extinction-retention test. All groups significantly extinguished fear during the extinction training period (Fig. 3 E and F). Although there were only trends in freezing during the prolonged extinction training period, the Cre+/− had significantly less average freezing during the retention test (t(25) = 2.34; *P < 0.05) (Fig. 3E) and less freezing during the first and fifth blocks of 10 tone presentations during the retention test (F 1,134 = 4.22; *P < 0.05) (Fig. 3G) compared with animals with normal cortical BDNF expression. Because the Cre+/− knockout mice consistently showed lower initial levels of fear, the rate of extinction was also examined by percent of initial freezing, which examines relative extinction rates, and there were no differences between groups (Fig. 3H). We also determined that the Cre+/− still maintained similar levels of freezing to the CS tested at least to 72 h after fear conditioning (Fig. S2), indicating that the low levels of freezing in the Cre+/− mice after extinction was not caused by decay of their fear learning over the time course of the extinction retention test (48 h postconditioning). Together, these data suggest that neocortical BDNF deletions that involve PL but not IL lead to deficits in rapid short-term and long-term learning and memory to the conditioned cue but do not impair extinction.
7,8-Dihydroxyflavone Rescues Impairment of Fear Conditioning Seen in Cortical BDNF Knockout Mice.
To investigate BDNF activation of TrkB, use of potential new TrkB-acting therapeutic agents have been limited because of the lack of any identified TrkB agonists that fully mimic the actions of BDNF at brain TrkB receptors in vivo. Most recently, Jang et al. (37) successfully screened a chemical library for compounds that activate TrkB in vitro, revealing a number of flavone derivatives; the most potent of these, 7,8-dihydroxyflavone (7,8-DHF), binds with high affinity to the TrkB receptor and provokes its dimerization and autophosphorylation, leading to downstream-signaling cascade activation (37). Systemic administration of this compound in mice substantially activates TrkB in the brain, inhibits neuronal cell death, decreases infarct volumes in stroke in a TrkB-dependent manner, and is neuroprotective in an animal model of Parkinson’s disease. We, therefore, examined whether or not systemic administration of 7,8-DHF would rescue the fear-learning deficit found in these neocortical BDNF knockout mice. Fig. 4A shows that neocortical tissue from these mice expressed significantly less BDNF protein in Western blots and that systemic 7,8-DHF increased the phosphorylation of TrkB in this tissue. These data reveal that despite the lack of cortical BDNF gene expression, endogenous TrkB receptors within the brain are activated with systemic 7,8-DHF.
Fig. 4.
7,8-DHF rescues a fear-expression deficit in neocortical BDNF knockouts. (A) Immunoblotting revealed that there was TrkB activation in cortical tissue after systemic 7,8-DHF administration but not with vehicle, in cortical BDNF knockout mice, or in controls. Systemic 7,8-DHF lead to phosphorylation of TrkB in cortex independently of the presence of BDNF, which is shown to be minimal in the BDNF knockout mice (anti-BDNF). (B) Average freezing to tone CS 24 h after cued fear conditioning (predrug) and again after reconditioning to a different tone (postdrug) is shown. Cre+/− had less freezing than Cre−/− (predrug). These mice were then injected (i.p.) with either 7,8-DHF or vehicle followed by reconditioning to a novel tone. Cre+/− injected with vehicle had less freezing to the new tone than did Cre−/− (*Student's t test; P < 0.05). However, Cre+/− injected with 7,8-DHF showed equivalent levels of freezing compared with 7,8-DHF–injected controls. (C) Within-training freezing during reconditioning to a new CS tone after injections of 7,8-DHF or vehicle is similar across groups. (D) When analyzing freezing 24 h after conditioning by blocks of three CS tones, Cre+/− showed diminished levels compared with Cre−/− in the absence of drug. After reconditioning with 7,8-DHF administration, Cre+/− had greater freezing compared with vehicle-injected Cre+/− but no differences compared with 7,8-DHF or vehicle-injected Cre−/− controls (*P < 0.05 versus respective Cre−/−; #P < 0.05 versus respective vehicle; n = 12 Cre-vehicle; n = 7 Cre+/− vehicle; n = 8 Cre+/− DHF; n = 7 Cre−/− DHF).
Neocortical BDNF knockout mice (Cre+/−) and littermate controls (Cre−/−) were cue fear-conditioned by administering five pairings of tone (6 kHz) coterminating with shock followed 24 h later by fear testing. Cre+/− showed significantly less total freezing compared with Cre−/− after the initial predrug fear-conditioning session [t(32) = 3.073; P < 0.05] (Fig. 4B). This was supported by an overall significant genotype effect (F 1,169 = 9.445; P < 0.05) with freezing deficits in the Cre+/− during predrug testing sessions in the first three blocks of CS trials (Fig. 4 B and D, predrug). These mice were then retrained 30 min after the administration of either 7,8-DHF (5 mg/kg, i.p.) or vehicle using a new, different CS tone (12 kHz) and training context. There were no differences in freezing between groups during fear acquisition to the new CS (Fig. 4C). These mice were then tested to the new CS 24 h later in the absence of drug (Fig. 4 B and D, postdrug). The Cre+/− injected with vehicle still had freezing deficits compared with their littermate controls. In contrast, Cre+/− injected with 7,8-DHF now had equivalently robust fear expression as the control mice (genotype effect F 1,174 = 15.483; P < 0.05; drug by genotype interaction F 1,174 = 15.314; P < 0.05) (Fig. 4D, postdrug). Notably, the agonist was only present and active during the fear-conditioning period (Fig. 4 B and C), but it was no longer present during the fear test. This provides additional evidence that the mPFC BDNF deletion does not affect expression of conditioned fear but is required for learning or consolidating of fear memories. Also of note was that the additional TrkB activation did not increase the fear learning in the Cre−/− control mice beyond their drug-free level. Together, these data suggest that systemic 7,8-DHF can rescue the learning deficit in mice with a selective neocortical BDNF deletion by activating TrkB receptors in the absence of endogenous neocortical BDNF.
Targeted Lentivirus-Mediated Inducible BDNF Deletion Within PL.
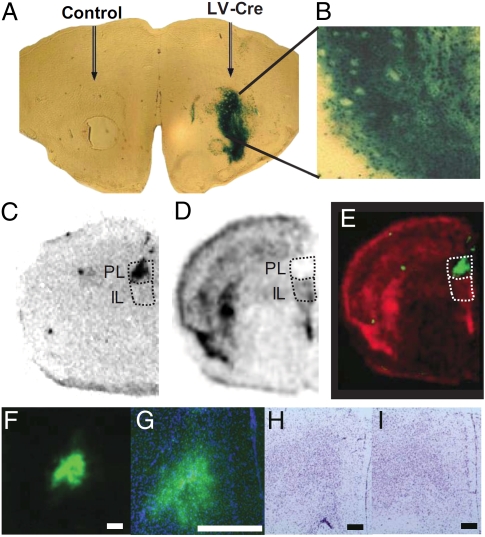
Based on prior studies suggesting a specific role for the PL in fear expression (16), we hypothesized that the PL is the likely neocortical subregion requiring BDNF within our transgenic mice. To test this, we used the fBDNF mice in combination with Cre-mediated gene deletion in a localized fashion through bilateral injections of Cre-expressing (LV-Cre) or control eGFP-expressing (LV-GFP) lentivirus vectors (31, 38) targeting the PL. First, we showed that robust Cre expression was observed when this lentivirus was injected into LacZ reporter mice (35) (Fig. 5 A and B). Using in situ hybridization for Cre and BDNF mRNA, we then showed that lentivirus-mediated Cre expression (Fig. 5C) overlays with the loss of BDNF expression (Fig. 5 D and E) in the PL with only minor dorsal spread in the LV-Cre infected mice. In the LV-GFP control mice, GFP was strongly expressed in the PL (Figs. 5 F and G). Nissl stain illustrated minimal cell damage by lentivirus infection into PL in LV-GFP (Fig. 5H) and LV-Cre mice (Fig. 5I).
Fig. 5.
Inducible BDNF deletion in PL with Cre lentivirus. (A and B) Robust lentiviral Cre expression was labeled by LacZ when injected into Rosa LacZ reporter mice in striatum. (C) Cre recombinase mRNA expression showed the infection site targeting the PL. (D) BDNF mRNA was specifically deleted in the PL and (E) overlays with the infection of Cre recombinase lentivirus. Red, BDNF mRNA; green, Cre mRNA. LV-GFP control infections in the PL of fBDNF mice at (F) 40× magnification and (G) 100× magnification. Nissl stain with cresyl violet showed no appreciable cell damage after lentiviral infection of (H) LV-GFP or (I) LV-Cre. (Scale bars: 500 μm.)
To examine whether or not specific deletions of prelimbic BDNF would attenuate fear expression, mice were trained with cue-dependent fear conditioning (as described above) 10 days after lentiviral infections. The LV-Cre mice with prelimbic BDNF deletions had no differences in acquisition and expression of newly acquired fear compared with LV-GFP controls (Fig. 6A). As hypothesized, 24 h later, when tested for fear to the cue, the LV-Cre mice had a massive decrease in average freezing compared with the controls [t(12) = 3.25; *P < 0.05] (Fig. 6B) and throughout the test (F 1,141 = 11.66; *P < 0.05) (Fig. 6C and Fig. S3). These findings replicate the deficit of fear observed in the neocortex BDNF knockout mice with an even more dramatic loss of fear expression.
Fig. 6.
Prelimbic BDNF is critical for consolidation and expression of learned fear. (A) Mice infected with LV-GFP or LV-Cre acquired and expressed fear during cued fear conditioning. (B) One day after fear conditioning, mice were tested with CS tones. LV-Cre mice had attenuated average freezing compared with LV-GFP mice. (C) During fear testing, CS presentations across 15 tones showed less freezing in LV-Cre mice. (D) There were no differences in locomotor activity in the open field. (E) Anxiety-like (innate fear) behavior was assessed in the open field, showing no differences in time spent in the center or surround. (F) Anxiety-like behavior was similar on the elevated-plus maze. (G) All mice learned equally well on an object recognition test where all mice spent significantly more time exploring a novel object during short-term (4 h) and long-term (24 h) testing of memory (# P < 0.05 versus familiar object). For all figures, *P < 0.05 versus LV-GFP (n = 7 per group).
Additionally, the LV-Cre mice showed no differences in locomotor activity in the open-field maze (Fig. 6D). They also showed no difference in baseline anxiety level in terms of time spent in the center of the open-field maze (Fig. 6E), time spent in the open arms of the elevated-plus maze (Fig. 6F), or novelty seeking in the novel-object recognition test, which serves as a measure of both novelty seeking and non-emotional learning (Fig. 6G). These data with virus-mediated, inducible BDNF prelimbic deletions replicate our similar findings in the neocortical BDNF knockout mice, indicating that the deficits in freezing are not caused by altered locomotion or sensory function, and innate fear remained intact.
Discussion
In summary, the transgenic neocortical BDNF knockout mice had robust deficits in freezing following cued fear conditioning at both 1 h and 24 h after fear conditioning, and extinction learning was moderately enhanced. Notably, the mice acquired fear and expressed immediate fear learning and freezing normally. Additionally, the neocortical BDNF knockout phenotype was rescued with the systemic TrkB agonist, 7,8-DHF, showing that BDNF activation is required for fear learning but not during fear testing for normal expression. Together, these data indicate that the presence of BDNF within PFC is important for learning or consolidation of both short-term and long term-memory. In the absence of BDNF, the knockout mice may potentially have dramatically altered or adapted circuitry, leading to rapid change in the output during the presentation of CS and the deficit observed even at the 1-h short-term test. Therefore, BDNF signaling may be critical for strengthening of neural connections in neocortical regions known to regulate expression of cued fear during learning.
The Western blot analyses of the cortical tissue of the neocortical BDNF knockouts revealed robust loss of BDNF protein in the cortex, whereas cortical TrkB-receptor expression remained unchanged. Moreover, systemic 7,8-DHF administration resulted in increased phosphorylated TrkB in the cortex. This indicates that systemic 7,8-DHF does cross the blood–brain barrier and targets TrkB activation, which replicates the initial findings of Jang et al. (37); however, our case was specifically within neocortical tissue of BDNF knockout mice. We then found that 7,8-DHF was able to rescue the fear learning deficit in mice that had a neocortex-specific deletion of BDNF. These behavioral data parallel the protein analyses of 7,8-DHF increasing phosphorylated-TrkB levels in the cortex, both suggesting that cortical TrkB receptor activation is important for fear learning or consolidation. Future studies remain to determine whether or not 7,8-DHF augments fear learning by targeting TrkB receptors in the prefrontal cortex or via downstream projections to TrkB in the basolateral amygdala to enhance plasticity in these mice that lack endogenous prefrontal BDNF expression.
In combination with the neocortical BDNF knockout mouse findings, the prelimbic lentivirus experiments show normal fear expression during the learning/acquisition period of training, but robust deficits in fear after consolidation; this provides further evidence that BDNF expression in the PL is likely necessary for the strengthening of fear-expression circuitry involved during the conditioned learning of fear memories. Notably, the viral-mediated PL BDNF deletions seem to have a more robust deficit in learned fear than the neocortical knockouts, suggesting greater specificity to the role of prelimbic cortical BDNF in regulating fear. Additionally, the neocortical transgenic Cre recombinase is driven by a cholecystokinin (CCK) promoter and likely preferentially targets an interneuron population within the select areas of the neocortex. Thus, the more extensive cellular (excitatory, inhibitory, and glial) BDNF deletion that is obtained with the PL-targeted lentiviral approach using a cytomegalovirus constitutive promoter may lead to more dense behavioral effects because of the enhanced regional specificity combined with a more dense BDNF deletion within this area. Overall, these data are consistent with reports that the IL is the major mPFC site involved with extinction, whereas PL is involved with driving output of learned fear and gating of fear extinction (9, 16, 19, 22). Future studies remain to determine whether or not BDNF is important for the plasticity of PL and IL circuits that may interact together synergistically to modulate expression and extinction of learned fear.
The viral-mediated PL BDNF deletions in adult mice also provide evidence that the deficits in learning or consolidation of fear in the neocortical BDNF knockout mice is unlikely caused by BDNF-induced structural changes in the cortex during development. This is further supported by our studies showing that both the neocortical BDNF knockout mice and the induced PL BDNF deletion mice have generally intact sensory and motor functions; this indicates that the deficits in freezing (fear expression) were not simply caused by alterations in motor or sensory deficits. Future experiments will determine the differential role of PL and IL BDNF in the extinction of fear memories.
These data may seem inconsistent with recent pharmacological inactivation studies indicating that PL is not necessary for long-term fear memory but specifically for output during fear expression (16, 39). One explanation for this difference is that the cortical BDNF knockouts or PL BDNF deletions are permanent, and they may lead to structural changes and adaptations in the existing circuitry between the prefrontal cortex and targets, including the amygdala. However, our data also show that a single systemic dose of the BDNF agonist, 7,8-DHF, rescued this effect, and after this treatment, fear expression is normalized, even in the absence of the drug. Thus, a more parsimonious explanation may be that BDNF, whether acting locally within intracortical circuits or distally (e.g., in PL projections to amygdala), may be required at the time of plasticity to alter the output of cue-specific fear but not for the process of fear expression itself. Note that our data showing normal fear expression to unconditioned shocks during acquisition are consistent with this interpretation. Moreover, future studies remain to determine whether or not prefrontal BDNF may be targeting downstream regions such as the basolateral amygdala, where we have previously shown TrkB in the basolateral amygdala to be critical for fear learning (29).
In addition to animal studies that indicate the importance of mPFC interaction with the amygdala for normal fear learning and extinction, neuroimaging has shown that the human PFC is involved in fear modulation (1, 2). Clinical studies also implicate dysfunction between these two regions in many affective disorders, including anxiety (4), depression (3), and fear-related disorders like PTSD (5, 6). Most relevant to our findings are recent studies indicating that BDNF polymorphisms are correlated with prefrontal cortex anatomy (40), and they may be implicated in emotionality and anxiety disorders (41, 42). Overall, our studies provide support for BDNF-mediated neuroplasticity as a molecular mechanism underlying prelimbic-specific regulation of fear learning, likely within mPFC–amygdala circuits, which may ultimately lead to improvements in pathophysiology and treatment of uncontrollable fear in patients.
Methods
Animals.
Lentivirus experiments used homozygous BDNF-floxed mice (34) (Bdnftm3Jae/J; Jackson Labs), which possess loxP sites both upstream and downstream of exon 5 of the BDNF gene. The cortex-specific Cre mouse line, previously described as “transgenic line C” (33), was created when the coding sequence for Cre recombinase was placed downstream of a 3-kb CCK promoter. The cortex-specific Cre line was crossed to floxed BDNF mice, floxed-stop[Jackson Labs, Gt(ROSA)26Sor] lacZ reporter mice (35), and floxed-stop EGFP-tdTomato mice (36). All experiments were performed on group-housed adult (2–4 months) males. All procedures used were approved by the Institutional Animal Care and Use Committee of Emory University and were in compliance with National Institutes of Health guidelines for the care and use of laboratory animals.
Drugs.
7,8-DHF (TCI) was dosed systemically (i.p.) at 5 mg/kg in vehicle (17% DMSO in PBS).
Cre Recombinase Lentivirus Infection.
LV-Cre or a GFP-expressing control vector (LV-GFP), delta8.9, and VSV-g were cotransfected into HEK293T cells to produce replication-incompetent virus, which was concentrated by ultracentrifugation as described previously (27, 29, 31) to 1 × 109 infectious particles per milliliter. LV-GFP or LV-Cre virus was bilaterally injected using a Hamilton syringe on a microinjection pump into the PL (Bregma; AP +2.0; ML ±0.4; DV −1.2) using stereotaxic surgery under ketamine/dormitor anesthesia.
Anxiety Measures.
The elevated-plus maze is a platform with two walled, closed arms and two nonwalled, open arms connected by an open center. The mice were placed onto the center between the plus maze arms and were recorded exploring the plus maze for 5 min. The open field was an open Plexiglass box. Mice were allowed to explore for 10 min. Activity data were obtained and analyzed using the Activity Software (Med Associates Inc.).
Novel Object Recognition.
Mice explored an open-field box for 10 min each day for 3 days. On the following day, two identical objects were placed in the open-field box, and the mice explored objects for 5 min. Four hours later, one of the objects was replaced by a novel object, and mice explored the objects for 5 min. Twenty-four hours later, another novel object was introduced along with one familiar object, and mice explored the objects for 5 min. Time spent exploring objects was recorded.
Cue-Dependent Fear Conditioning.
Mice were preexposed to conditioning chambers (San Diego Instruments) 3 days before training. During cued fear training, mice received five paired CS tones (30 s, 6 or 12 kHz, 90 db) and US shock (500 ms, 1.0 mA) trials with a 5-min intertrial interval (ITI). Startle response to the shocks and percentage of time spent freezing to the tones was measured by SR-LAB software (San Diego Instruments).
Cued Fear Expression, Extinction Training, and Retention.
The expression of fear memory was tested 24 h after fear conditioning in a novel context (modular test chambers; Med Associates Inc.). The mice were exposed to 3 (1-h test), 15 (24- and 72-h tests), and 50 (extinction and retention tests) CS tones with a 1.5-min ITI. Freezing during the tone presentations was measured with FreezeView software (Coulbourn Instruments). Extinction retention tests occurred 24 h after extinction training.
In Situ Hybridization and Histology.
Mice were killed, and brains were collected, flash frozen on dry ice, sectioned on a cryostat (16 μm/section), and stored at −80 °C. Cre expression and BDNF deletion were confirmed with in situ hybridization (28). In brief, slides were pretreated and hybridized with 35S-UTP labeled with antisense riboprobes for BDNF (exon 5) or Cre. After a stringent wash protocol, slides were exposed to Biomax MR film (Eastman Kodak Co.). For LacZ/XGal staining, slide-mounted tissue was fixed in 4% paraformaldehyde and incubated at 37 °C overnight in 1 mg/mL X-Gal. For Nissl staining, slide-mounted tissue was counter stained with Cresyl violet.
Western Immunoblotting.
The BDNF knockout mice were injected with 5 mg/kg 7,8-DHF i.p., and 2 h later, they were killed; cortex tissue was homogenated, lysed in lysis buffer, and centrifuged, and the supernatant was collected. The normalized proteins were subjected to SDS/PAGE analysis and transferred to a nitrocellular membrane. Western blotting analysis was performed with anti-TrkB Y816 (gift from Moses Chao, NYU School of Medicine, New York, NY) and TrkB antibodies (Cell Signaling Technology Inc.), both diluted at 1:1,000.
Statistical Analyses.
Fear acquisition, expression, and extinction data were analyzed by two-way or three-way ANOVA with repeated measures, where appropriate. Other behavioral tests were analyzed by ANOVAs or Student’s t test, where appropriate. Statistically significant main effects or interactions by ANOVA were followed by post hoc least-squares difference tests for multiple comparisons. Data are presented as mean ± SEM; significance was set at P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Mary Nguyen-Choi for her expertise with the preparation of manuscript figures and Dr. Lisa Stanek for her experimental and technical expertise. Support was provided by National Institutes of Health (MH085443, MH088467, NS055077, and DA019624), National Science Foundation (GRFP DGE-0234618), the Center for Behavioral Neuroscience (National Science Foundation agreement IBN-987675), National Alliance for Research on Schizophrenia and Depression, and the Burroughs Wellcome Fund. This work was supported by a National Institutes of Health/National Center for Research Resources base grant (P51RR000165) to Yerkes National Primate Research Center.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909359107/DCSupplemental.
References
- 1.Milad MR, et al. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Milad MR, et al. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci USA. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- 4.Davidson RJ. Anxiety and affective style: Role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- 5.Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- 6.Quirk GJ, Gehlert DR. Inhibition of the amygdala: Key to pathological states? Ann N Y Acad Sci. 2003;985:263–272. doi: 10.1111/j.1749-6632.2003.tb07087.x. [DOI] [PubMed] [Google Scholar]
- 7.Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: Contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- 8.Garcia R, Vouimba RM, Baudry M, Thompson RF. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402:294–296. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- 9.Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herry C, Garcia R. Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J Neurosci. 2002;22:577–583. doi: 10.1523/JNEUROSCI.22-02-00577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 12.Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- 13.Paré D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- 14.Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- 15.Rosen JB, et al. Lesions of the perirhinal cortex but not of the frontal, medial prefrontal, visual, or insular cortex block fear-potentiated startle using a visual conditioned stimulus. J Neurosci. 1992;12:4624–4633. doi: 10.1523/JNEUROSCI.12-12-04624.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Runyan JD, Moore AN, Dash PK. A role for prefrontal cortex in memory storage for trace fear conditioning. J Neurosci. 2004;24:1288–1295. doi: 10.1523/JNEUROSCI.4880-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J Neurosci. 2005;25:6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baeg EH, et al. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cereb Cortex. 2001;11:441–451. doi: 10.1093/cercor/11.5.441. [DOI] [PubMed] [Google Scholar]
- 21.Gilmartin MR, McEchron MD. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behav Neurosci. 2005;119:1496–1510. doi: 10.1037/0735-7044.119.6.1496. [DOI] [PubMed] [Google Scholar]
- 22.Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 24.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 25.Black IB. Trophic regulation of synaptic plasticity. J Neurobiol. 1999;41:108–118. [PubMed] [Google Scholar]
- 26.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 27.Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear condition-ing. J Neurosci. 2004;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rattiner LM, Davis M, Ressler KJ. Differential regulation of brain-derived neurotrophic factor transcripts during the consolidation of fear learning. Learn Mem. 2004;11:727–731. doi: 10.1101/lm.83304. [DOI] [PubMed] [Google Scholar]
- 29.Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9:870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ou LC, Gean PW. Regulation of amygdala-dependent learning by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol-3-kinase. Neuropsychopharmacology. 2006;31:287–296. doi: 10.1038/sj.npp.1300830. [DOI] [PubMed] [Google Scholar]
- 31.Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 33.Chhatwal JP, Hammack SE, Jasnow AM, Rainnie DG, Ressler KJ. Identification of cell-type-specific promoters within the brain using lentiviral vectors. Gene Ther. 2007;14:575–583. doi: 10.1038/sj.gt.3302898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rios M, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 35.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 36.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 37.Jang SW, et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci USA. 2009;107:2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maguschak KA, Ressler KJ. Beta-catenin is required for memory consolidation. Nat Neurosci. 2008;11:1319–1326. doi: 10.1038/nn.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laurent V, Westbrook RF. Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learn Mem. 2009;16:520–529. doi: 10.1101/lm.1474609. [DOI] [PubMed] [Google Scholar]
- 40.Pezawas L, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montag C, Reuter M, Newport B, Elger C, Weber B. The BDNF Val66Met polymorphism affects amygdala activity in response to emotional stimuli: Evidence from a genetic imaging study. Neuroimage. 2008;42:1554–1559. doi: 10.1016/j.neuroimage.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- 43.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd Ed. San Diego: Academic Press; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.