Abstract
A rapid event-related fMRI arrow flanker task was used to study aging-associated decline in executive functions related to interference resolution. Older adults had more difficulty responding to Incongruent cues during the flanker task compared to the young adults; the response time difference between the Incongruent and Congruent conditions in the older group was over 50% longer compared to the young adults. In the frontal regions, differential activation (“Incongruent – Congruent” conditions) was observed in the inferior and middle frontal gyri in within-group analyses for both groups. However, the cluster was smaller in the older group and the centroid location was shifted by 19.7 mm. The left superior and medial frontal gyri also appeared to be specifically recruited by older adults during interference resolution, partially driven by errors. The frontal right lateralization found in the young adults was maintained in the older adults during successful trials. Interestingly, bilateral activation was observed when error trials were combined with successful trials highlighting the influence of brain activation associated with errors during cognitive processing. In conclusion, aging appears to result in modified functional regions that may contribute to reduced interference resolution. In addition, error processing should be considered and accounted for when studying age-related cognitive changes as errors may confound the interpretation of task specific age-related activation differences.
Introduction
Human brain aging is a complex process, involving changes in anatomy, physiology, and cognition. Normal aging is generally associated with deterioration in frontal and parietal brain regions including both gray and white matter (Resnick et al., 2003) which may contribute to the declines in executive processing. As reflected by slower responses and/or reduced accuracy, older adults often have more difficulty with cognitive tasks that require selective attention and executive function compared to young adults (Treitz et al., 2007). Selective attention refers to the ability to direct processing at specific objects and/or spatial locations, and executive function can be generally defined as a process that directs thoughts and actions according to internal goals and readjusts goals when necessary (Cabeza and Kingstone, 2006). The decline in selective attention and executive function might be caused by the change of the functionality of the frontal-striatal neural circuit (Buckner, et al., 2006; Cummings, 1993). In this study, we focused on investigating age-related functional modifications of the frontal regions with an fMRI paradigm that targets selective attention and executive function.
One category of executive function that older adults have difficulty with is stimulus-response interference. In stimulus-response interference tasks, a distracter stimulus must be inhibited when attending to a target stimulus. These tasks include the flanker and Stroop color naming tasks (Botvinick et al., 1999; Carter et al., 2000; Casey et al., 2000; Colcombe et al., 2005; Colcombe et al., 2004; Erickson et al., 2004; Erickson et al., 2005; Milham et al., 2002; van Veen et al., 2001). Other types of inhibitory tasks include the Go/No-Go and Stop tasks (Rubia et al., 2001; Nielson et al., 2002; Langenecker and Nielson, 2003). The difficulty that older adults have with such tasks is consistent with the inhibitory deficit view of Hasher, Zacks, and colleagues (Hasher, Lustig, and Zacks, 2007; Hasher and Zacks, 1988; Hasher, Zacks, and May, 1999). This view posits that older adults have particular deficiencies in the inhibitory attentional control mechanisms that serve to prevent or stop the processing of task-irrelevant stimuli or stimulus dimensions (i.e., distraction). Consequences of older adults' less efficient suppression or inhibition mechanisms include increased processing of distraction and consequently greater interference from distraction. For example, in the modified Ericksen and Ericksen (1974) flanker task used in the current study, participants must respond to a central target (here reporting the pointing direction of a central arrowhead) in the face of “flankers” (other surrounding arrowheads pointing in the same or opposite directions). As expected from inhibitory deficit theory, relative to young adults, older adults show greater “flanker effects” such as greater reaction time increases and higher error rates when the flankers are inconsistent with the central target as compared to consistent or neutral stimuli. The current study replicates this behavioral finding and attempts to relate the increased distraction interference effect to age differences in brain areas serving executive functioning, particularly in stimulus-response interference tasks.
Studies using functional MRI (fMRI) have reported activation in several brain regions during inhibitory tasks including in the inferior, middle, and superior frontal gyri (IFG, MFG and SFG, respectively), inferior and superior parietal lobules (IPL and SPL, respectively), and the anterior cingulate cortex (ACC) (Casey et al., 2000; Colcombe et al., 2005; Colcombe et al., 2004; Erickson et al., 2004; Erickson et al., 2005; van Veen et al., 2001). Recently, Nee et al. (2007) performed a meta-analysis on 47 fMRI studies involving tasks related to interference resolution in young adults and found significant clusters bilaterally in the dorsolateral prefrontal cortex (DLPFC), IFG, ACC, and posterior parietal cortex. In particular for the six flanker fMRI studies, they found a significant cluster in the right DLPFC, and a smaller cluster in the right insula.
In our fMRI study, we used the arrow flanker task to examine age differences in brain activity, particularly in the frontal regions including IFG, MFG and SFG. Colcombe et al. (2005) carried out an fMRI study on age differences that used a similar Eriksen arrow flanker task but with a slow event-related design with fixed 14-sec inter-stimulus intervals. In response to Incongruent flanker trials, they found that both young and older adults demonstrated significant activity in the right MFG, bilateral ACC, and supplementary motor area (SMA); they also found that whereas the young adults showed right lateralized prefrontal gyrus activity, activity was more bilateral in the older adults. However, these findings were based on the analyses of Incongruent trials versus baseline. Differential brain activation (Incongruent versus Congruent stimulus conditions) was not reported, but would more clearly illustrate the brain activity due to interference resolution alone.
In this study, we tested the main hypothesis that the differential activation in Incongruent versus Congruent conditions at IFG, MFG and SFG would be modified with aging, corresponding with the increased differential response time for these two conditions. We employed a rapid event-related design in young and older adults to determine age-related differences in interference resolution. We presented the subjects with three conditions: Congruent (“>>>>>>>” or “<<<<<<<”), Incongruent (“>>><>>>” or “<<<><<<”) and Neutral (“□□□>□□□” or “□□□<□□□”). Each trial was presented for 2.5 sec. The subject's task was to identify the direction of the central arrowhead by pressing the buttons under the index fingers of two keypads for each trial. Response accuracy and response time were recorded. We compared differential brain activation between the Incongruent and Congruent conditions within each group. Besides the improved time efficiency, a rapid event-related design was chosen so that the subject's general attentiveness level was kept relatively constant, and thus the differential activation could more clearly unveil the neural network that contributes specifically to conflict resolution during the stimulus-response inhibition task. The results of the current study may help to unravel age-related changes in activation that lead to reduced performance, particularly in interference resolution.
With this study, we also investigated whether older adults had increased left frontal hemispheric activation compared to young adults. This phenomenon (hemispheric asymmetry reduction in older adults (HAROLD)) was initially described by Cabeza (2002). The HAROLD pattern has been reported for working memory tasks as well as inhibitory tasks (Go/No-Go) (Cabeza et al., 2002; Dolcos et al., 2002; Reuter-Lorenz et al., 2000; Nielson et al., 2002). While this pattern has been observed in older adults, the cognitive impact of the additional recruitment is currently being debated. Increased recruitment may result in improved performance supporting a compensation theory (Reuter-Lorenz et al., 2000). In opposition, the increased recruitment may serve as a marker of cognitive decline when it does not result in improved performance supporting a dedifferentiation theory (Logan et al., 2002; Li and Lindenberger, 1999). We hypothesized that older adults would have greater frontal left hemispheric activation compared to younger adults during the resolution of stimulus-response interference.
Error treatment in aging studies has recently gained attention based on findings by Murphy and Garavan (2004). They found that, at least on certain tasks, activation patterns may differ considerably on correct (successful) versus incorrect (error) response trials in older adults. As such, even though performance accuracy was quite high in the current flanker task (mean ≥ 90% in all conditions), the main analyses of the fMRI data are based on successful trials modeled separately from error trials. To understand the effect and importance of error treatment, we also present post-hoc analyses without separating the errors from the successful trials and on error trial activation within a sub-group of older adults.
Materials and Methods
Subjects
Twenty-three young adults and 26 older adults participated in this study. All subjects self-reported that they were free of neurological disorders. Data from one young subject and three older adults were discarded due to vision problems and/or highly irregular anatomical structure or a diagnosis of past stroke. Data from an additional older adult were discarded due to very low correct response rates during the flanker task (48.7% accuracy). Twenty-two young adults (11 males, age 20 ± 3 yrs) and 22 older adults (9 males, age 74 ± 6 yrs) were included in the data analyses. All subjects signed consent forms approved by the Michigan State University Institutional Review Board. All 22 younger subjects were students from Michigan State University. All 22 older subjects were recruited from Michigan State University and surrounding communities. These older subjects were well-educated with a mean of 16.4 (± 3.6) years of education for the 18 subjects who provided this information.
Imaging Acquisition
The experiment was conducted on a GE 3T Signa® HDx MR scanner (GE Healthcare, Waukesha, WI) with an 8-channel head coil. During each session, images were first acquired for the purpose of localization, and then the first and higher-order shimming procedures were carried out to improve magnetic field homogeneity (Kim et al., 2002). To study brain function, echo planar images (EPI), starting from the most inferior regions of the brain, were then acquired with the following parameters: 34 contiguous 3-mm axial slices in an interleaved order, TE = 27.7 ms, TR = 2500 ms, flip angle = 80°, FOV = 22 cm, matrix size = 64 × 64, ramp sampling, and with the first four data points discarded. Each volume of slices was acquired 164 times during each of the four functional runs while a subject viewed the stimuli and pressed a button to indicate the pointing direction of the central arrow, resulting in a total of 656 volumes of images over the course of the entire experiment. After the functional data acquisition, high-resolution volumetric T1-weighted spoiled gradient recalled (SPGR) images with cerebrospinal fluid suppressed were obtained to cover the whole brain with 120 1.5-mm sagittal slices, 500 ms time of inversion, 8° flip angle and 24 cm FOV. These images were used to identify anatomical locations.
Paradigm for fMRI
This study employed a flanker task with a rapid event-related design, including a total of 128 trials for each of the three conditions described earlier: Incongruent, Congruent and Neutral. Each of the four 7-minute runs started with a 10-second baseline condition (a fixation cross) followed by the stimulus trials (32 for each condition) presented in random order and with randomized inter-stimulus intervals (ISI) at multiples of 2.5 sec (mean of ISI = 4.27 sec). A fixation cross was presented as the baseline condition between the stimuli. The stimuli for all trials and the fixation cross were in white and were presented on a black background. Each stimulus array was presented for 2.5 sec, during which time the participant pressed a button to identify the direction of the central arrow head. Subjects were naïve to the flanker task but performed a 2-minute practice flanker task immediately before the imaging. Although the task included the Neutral condition described above, we used Incongruent-Congruent (IC-C) condition differences, rather than Incongruent-Neutral (IC-N) condition differences, as our main measure of interference resolution. The IC-C comparison is arguably the more typical and stronger comparison and, in the present case, it has the advantage of holding constant the stimulus features in the two conditions.
The stimulus trials were randomized with the “RSFgen” program in AFNI software (Cox, 1996) for optimizing the calculation of the hemodynamic response function for each stimulus condition and for the contrasts between them. For each stimulus condition, targets and flankers were presented in the two possible directions an equal number of times. Stimuli were displayed on a 640×480 LCD monitor mounted on top of the RF head coil. The LCD subtended 12°×16° of visual angle. The paradigm was controlled by an IFIS-SA system (Invivo Corp., Gainesville, FL). A pair of 5-button MR-compatible keypads with this system was used to record participant responses.
Behavioral Analysis
Flanker task performance was compared between groups and across conditions using mixed-model ANOVA in which flanker condition (Incongruent versus Congruent) was the repeated-measures factor and age group was the between-group factor. Accuracy (number of correct responses) and response speed (for the correct responses) were analyzed. A Greenhouse-Geisser factor was used to correct for sphericity. Statistical significance was set at p < 0.05.
fMRI Data Pre-processing and Analysis
All fMRI data pre-processing and analyses were conducted with AFNI software (Cox, 1996). For each subject, the acquisition timing difference was first corrected for different slice locations. With the first functional image as the reference, rigid-body motion correction was done in three translational and three rotational directions. The amount of motion in these directions was estimated and then the estimations were used in data analysis. For each subject, spatial blurring with a full width half maximum of 4 mm was used to reduce random noise (Parrish et al., 2000), and also to reduce effects of inter-subject anatomical variation and Talairach transformation variation during group analysis. For the group analysis, all images were converted to Talairach coordinate space (Talairach and Tournoux, 1988) with an interpolation to 1 mm3 voxels. Throughout the paper, the coordinates of brain activity are presented in Talairach space in the format of (RL, AP, IS) in mm, where R = Right, L = Left, A = Anterior, P = Posterior, I = Inferior, and S = Superior.
Individual Subject fMRI Analysis: Flanker Activation from Successful Trials
For the data analysis of each individual subject, six trial types, including both successful (correct) and error (incorrect) trials, were modeled (correct trials in Incongruent, Congruent and Neutral conditions, and incorrect trials in Incongruent, Congruent and Neutral conditions). Incorrect trials were modeled separately from correct trials to improve the accuracy of the estimation of the activation pattern associated with correct trials (Murphy and Garavan, 2004). Therefore, up to six conditions were modeled, and the impulse response function (IRF) at each voxel with respect to each stimulus condition was resolved with multiple linear regressions using the “3dDeconvolve” software in AFNI (Ward, 2000a). However, because of collinearity problems, the “3dDeconvolve” software has difficulty with cases in which a participant produces only a single error in a presentation type. This occurred in one condition for five young and one older subject and in two conditions for a second older subject. Although these errors could not be modeled separately, their small number (8) relative to the large number of trials overall (128 per condition for each of 44 subjects) suggests a negligible effect on the analyses. In the “3dDeconvolve” procedure, MRI signal modeling also included the subject motion estimations in the three translational and the three rotational directions, and the constant, linear and quadratic trends for each of the four functional runs. The IRFs were resolved to seven points from zero to 15 sec at the resolution of 2.5 sec (TR). The BOLD signal change was calculated based on the area under the IRF curve. The equivalent BOLD percent signal change relative to the baseline state was then calculated.
Whole-Brain Analysis for fMRI: Flanker Activation from Successful Trials
After the percent signal change was estimated with respect to each stimulus condition for each subject, an ANOVA was performed over the 22-subject dataset for each group for group analysis with a mixed-effect two-factor model. The stimulus condition at correct trials (three levels) was the first factor and was modeled as a fixed effect. Subject was the second factor and was modeled as a random effect. Then the difference of the BOLD percent signal changes (IC – C) obtained based on correct trials at each voxel was estimated for each subject. The between-group ANOVA was performed on these differences with a mixed-effect two-factor model. The age group category was modeled to provide fixed effects and subject was modeled as a random effect.
Correction for Multiple Comparisons
Monte Carlo simulation of the effect of matrix and voxel sizes of the imaging volume, spatial blurring, voxel intensity thresholding, masking, and cluster identification was used to estimate the overall statistical significance with respect to the whole brain (Ward, 2000b). Based on these estimations, the ANOVA results above were further corrected for multiple comparisons based on the following criteria: The active voxel selection criteria required that the voxels had voxel-based p ≤ 5 × 10−3 and were nearest-neighbor and within a cluster size of 248 mm3. Based on application of these criteria to the whole brain, the voxel-based p ≤ 5 × 10−3 was corrected to be an equivalent whole-brain corrected p ≤ 0.021.
Post-hoc Analyses
Whole-Brain Analysis for fMRI: Flanker Activation from Successful and Error Trials Together
In order to explore the impact of error trials we also analyzed the data without separating out the error trials. In this analysis, three flanker stimulus conditions (Incongruent, Congruent, Neutral) were modeled for both the young and older groups. The activation in each condition would then include the effect of both successful (correct) and error (incorrect) trials modeled together. Activation was analyzed with whole-brain analysis (described above, and with voxel based p ≤ 5 × 10−3 and whole-brain corrected p ≤ 0.021).
Whole-Brain Analysis for fMRI: Flanker Activation from Error Trials in Older Adults
The best approach to examine error processing is to analyze the activation from the error trials directly. Ideally this analysis would be done on a full data set and each subject would have some (minimum) number of errors. However, the flanker task used in the current study was not designed to induce a high number of errors and in fact more than half of the subjects in the older group had near perfect accuracy (mean accuracy in all conditions = 99%, n = 13). Nonetheless, we were able to extract data from a subset of older adults (n = 9) who made at least five errors in both Incongruent and Congruent conditions (mean accuracy in all conditions = 84%) to further investigate the effect and impact of error treatment. The BOLD percent signal changes were from individual subject analysis already discussed with error trials modeled separately (Analysis of Successful Trials). We used the whole brain analysis procedure with four stimulus conditions (correct Incongruent and Congruent conditions, and incorrect Incongruent and Congruent conditions). We analyzed the contrast (voxel-based p ≤ 5 × 10−3, uncorrected) between incorrect Incongruent versus correct Congruent conditions. The correct Congruent condition was selected in this contrast in accordance with the main analysis; main analysis contrast = correct Incongruent versus correct Congruent.
Results
Behavioral Analysis
As can be seen in Table 1, the response times of the older participants were slower than those of the young adults across all conditions, but especially in the Incongruent condition. (The results for the Neutral condition are included only for informational purposes.) A 2 (Young versus Old) × 2 (Incongruent versus Congruent conditions) ANOVA confirmed these observations by showing significant Age and Condition main effects, F(1, 42) = 11.73, p = 0.001, and F(1, 42) = 83.105, p < 0.001, respectively, and, importantly, a significant Age × Condition interaction, F(1, 42) = 4.267, p = 0.045, due to the older adults' differentially slow response times in the Incongruent compared to the Congruent condition. A similar analysis of the accuracy data showed a significant Condition main effect F(1, 42) = 9.105, p = 0.004, and a trend for an age main effect, F(1, 42) = 3.67, p = 0.061, but a non-significant Age × Condition interaction, F(1, 42) = 2.984, p = 0.091. These findings, notably in the response times, indicate increased difficulty in the older group with the Incongruent condition, presumably reflecting difficulty with interference resolution.
Table 1.
Flanker Task Response Time and Accuracy Results
| Young Group | Old Group | |
|---|---|---|
| Response Time (ms)+ | ||
| Neutral (N)* | 671 ± 120 | 775 ± 98 |
| Congruent (C) | 675 ± 119 | 789 ± 99 |
| Incongruent (IC) | 805 ± 188 | 995 ± 206 |
| Flanker effect (IC-C) | 130 ± 92 | 206 ± 146 |
| Accuracy (% correct)+ | ||
| Neutral (N)* | 97.5 ± 5.2 | 94.3 ± 9.3 |
| Congruent (C) | 97.8 ± 6.1 | 93.7 ± 10.0 |
| Incongruent (IC) | 96.8 ± 6.7 | 90.0 ± 13.9 |
| Flanker effect (IC-C) | −1.0 ± 1.8 | −3.7 ± 7.0 |
Notes:
Neutral condition results are included only for informational purposes
Data are presented as means ± standard deviation; response time results include only correct responses.
Whole Brain Analysis for fMRI: Flanker Activation from Successful Trials
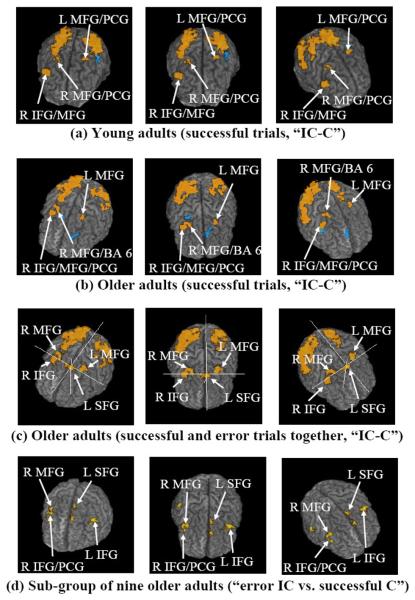
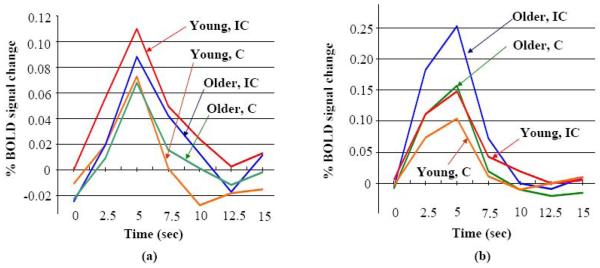
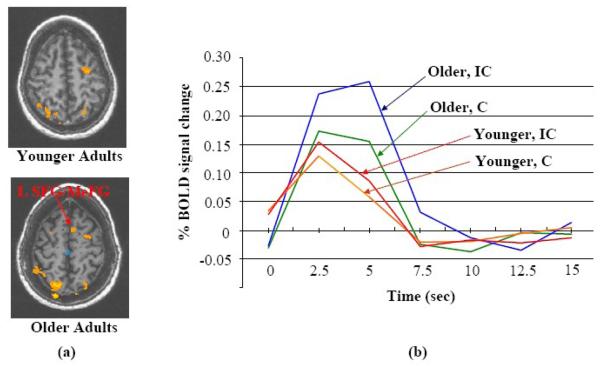
The whole-brain within-group ANOVA (Table 2 and Fig. 1(a, b)) shows that the young and older groups share some common differentially (IC-C) active frontal regions (based on differences of less than 10 mm in Talairach space), including the right and left MFG. Both groups activated the right IFG and precentral gyrus (PCG), but the older group had smaller clusters and the specific location of the frontal activation was different for the older group. The largest differentially active cluster at the frontal region for the young adults was at the right IFG/MFG (1271 mm3, centroid coordinate (R49, A18, S24), including 556 mm3 at IFG). The largest differentially active cluster at the frontal region for the older adults was at the right IFG/MFG/PCG (849 mm3, centroid coordinate (R39, A3, S32), including 321 mm3 at IFG and 362 mm3 at PCG). The distance between these two centroids was 19.7 mm. The difference between these two IFG/MFG/PCG clusters is further demonstrated by the mean IRFs in Incongruent and Congruent conditions (Fig. 2). For each age group, the amplitude difference (IC-C) was comparatively larger at the location of that age group's largest cluster in the right IFG/MFG/PCG. Also, a 153 mm3 cluster at the left SFG/MeFG (medial frontal gyrus) (centroid coordinate (L6, A9, S51)) was found before whole-brain correction for the older group, but was not found for the younger group, even at a p ≤ 0.05 before whole-brain correction (Fig. 3). At this cluster location, the IRF amplitude difference (IC-C) was larger for the older adults (Fig.3(b)).
Table 2.
Differential Activation from the Whole-brain Within-group Analyses (Incongruent > Congruent)
| Younger Group | Older Group | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R/L | Cluster | Size (mm3) |
Centroid Coordinate (RL, AP, IS) |
Max t value |
IC % Signal Change |
C % Signal Change |
R/L | Cluster | Size (mm3) |
Centroid Coordinate (RL, AP, IS) |
Max t value |
IC % Signal Change |
C % Signal Change |
|
| Frontal/Anterior Regions | ||||||||||||||
| R | IFG, MFG | 1271 | (R49, A18, S24) | 5.094 | 0.255 | 0.021 | R | |||||||
| IFG, MFG, PCG |
849 | (R39, A3, S32) | 4.525 | 0.501 | 0.234 | |||||||||
| MFG, PCG | 318 | (R30, P2, S45) | 4.128 | 0.249 | 0.132 | MFG, BA 6 | 437 | (R29, P1, S54) | 4.611 | 0.380 | 0.193 | |||
| L | MFG, PCG | 398 | (L25, P7, S50) | 4.544 | 0.268 | 0.154 | L | MFG | 263 | (L25, P2, S51) | 3.991 | 0.324 | 0.178 | |
| Posterior Regions | ||||||||||||||
| R | IOG, MOG | 4795 | (R36, P74, I0) | 5.918 | 0.793 | 0.494 | R | IOG, MOG, BA 18, ITG, MTG, SOG, Precuneus, SPL |
10229 | (R32, P71, S19) | 6.276 | 0.750 | 0.371 | |
| Cuneus, SOG, Precuneus |
496 | (R32, P83, S33) | 4.464 | 0.503 | 0.196 | |||||||||
| Precuneus, IPL, SPL |
4281 | (R28, P56, S45) | 5.378 | 0.454 | 0.213 | IPL, SPL | 669 | (R40, P49, S52) | 4.530 | 0.495 | 0.153 | |||
| L | Lingual Gyrus, IOG, MOG, FG, BA 18, ITG, BA 19 |
9710 | (L32, P80, I0) | 5.727 | 0.836 | 0.562 | L | MOG, IOG, Cuneus |
3215 | (L32, P81, S10) | 7.280 | 0.680 | 0.412 | |
| MOG, FG | 361 | (L45, P71, I10) | 4.650 | 1.187 | 0.746 | |||||||||
| MOG | 292 | (L26, P72, S25) | 4.085 | 0.293 | 0.149 | IOG, MOG | 258 | (L38, P63, I6) | 4.012 | 0.691 | 0.424 | |||
| SPL, Precuneus |
791 | (L21, P64, S46) | 4.107 | 0.554 | 0.271 | |||||||||
Differential activation was for “IC – C”. IC = Incongruent and C = Congruent. Coordinates are in Talairach space in the format of (RL, AP, IS). R = Right, L = Left, A = Anterior, P = Posterior, I = Inferior and S = Superior. Voxel based p ≤ 0.005 and whole brain corrected p ≤ 0.021.
IFG = inferior frontal gyrus, MFG = middle frontal gyrus, PCG = precentral gyrus, BA = Brodmann area, IOG = inferior occipital gyrus, MOG = middle occipital gyrus, SOG = superior occipital gyrus, ITG = inferior temporal gyrus, MTG = middle temporal gyrus, IPL = inferior parietal lobule, SPL = superior parietal lobule, FG = fusiform gyrus.
Fig. 1.
The spatial extents of differential activation (“Incongruent – Congruent”) in the whole-brain within-group analyses shown through color maps (voxel base p value ≤ 0.005 and whole-brain corrected p value ≤ 0.021, red or orange = IC is more active than C, and blue = C is more active than IC.) for (a) young adults' successful trials, (b) older adults' successful trials, and (c) older adults' successful and error trials together. The cross hair indicates the existence of the L SFG cluster in (c) but not in (b). The contrast of error IC versus successful C trials at voxel base p value ≤ 0.005 (not whole-brain corrected, but with cluster size ≥ 150 mm3) is shown for the sub-group of nine older adults who made reasonable number of IC and C errors (d). Some key active regions are indicated. IC = Incongruent, C = Congruent, R = right, L = left, IFG = inferior frontal gyrus, MFG = middle frontal gyrus, PCG = precentral gyrus, and BA = Brodmann area.
Fig. 2.
The within-group whole-brain differential activation during successful flanker trials (“Incongruent – Congruent”) (voxel-based p value ≤ 0.005 and whole-brain corrected p value ≤ 0.021) revealed a location shift of the IFG/MFG cluster with aging. The mean impulse response functions for older and younger group in conditions IC (Incongruent) and C (Congruent) are plotted at (a) the right IFG/MFG cluster found from the younger group, and (b) the right IFG/MFG/PCG cluster found from the older group.
Fig. 3.
The within-group whole-brain differential activation during successful flanker trials (“Incongruent – Congruent”) (voxel-based p value ≤ 0.005 but whole-brain uncorrected) revealed a cluster that is more active for the Incongruent condition at the left SFG/MeFG region with older adults but this cluster was not found at the younger adults (a). The mean impulse response functions for older and younger group in conditions IC (Incongruent) and C (Congruent) are plotted at this cluster (b).
In the posterior regions of the brain (Fig. 1(a, b), Table 2), differential activation was found in the right IPL, right SPL, right precuneus, right and left inferior and middle occipital gyri, and right superior occipital gyrus in both groups. The clusters at the posterior part of the brain were large and tended to cover multiple anatomical regions.
Overall, the active volume (IC > C) for the whole brain was 21,561 mm3 (11,161 mm3 in the right and 10,400 mm3 in the left hemispheres) for the young adults, and was 17,072 mm3 (12,184 mm3 in the right and 4,888 mm3 in the left hemispheres) for the older adults. In the frontal regions, both groups appeared to have activation lateralized to the right. The total active volume in the frontal region of the brain was 1,987 mm3 (1,589 mm3 at the right and 398 mm3 at the left hemispheres) for the young adults, and was 1,549 mm3 (1,286 mm3 at the right and 263 mm3 at the left hemispheres) for the older adults. This asymmetry with older adults was also observed before the correction for multiple comparisons at voxel based p ≤ 0.005 and at even more liberal p ≤ 0.01. The between-group analysis did not yield significant differences between young and older adults.
Whole-Brain Analysis for fMRI: Flanker Activation from Successful and Error Trials Together
We turn now to analyses based on all the trials (errors as well as successful trials). The whole-brain within-group ANOVA showed that the right IFG and the right and left MFG in the frontal region were differentially active (IC-C) in both age groups, as in the analysis of only the successful trials. In the posterior regions of the brain, differential activation was found in the left SPL, right and left precuneus, right and left middle occipital gyri in both groups, also resembling the activation from successful trials. However, there were some notable differences in activation. There was an overall increase in activated volume estimated for the whole brain; 29,283 mm3 (15,719 mm3 in the right and 13,564 mm3 in the left hemispheres) for the young adults and 23,321 mm3 (15,557 mm3 in the right and 7,764 mm3 in the left hemispheres) for the older adults. In the frontal regions, the activation in younger adults was lateralized to the right as was the case with successful trials, but activation in the older adults was more bilateral. The total active volume in the frontal region of the brain was 4,080 mm3 (3,580 mm3 at the right and 500 mm3 at the left) for the young adults, and was 3,621 mm3 (1,946 mm3 at the right and 1,675 mm3 at the left) for the older adults. Besides the overall pattern difference, there were also some specific regional differences. A cluster at the right IFG/insula (742 mm3, centroid coordinate (R40, A18, S1)) was found differentially active only in young adults. Notably, a significant cluster at the left SFG/MeFG (575 mm3, with 486 mm3 in SFG, centroid coordinate (L5, A9, S52)) was found to be differentially active only in the older group. A smaller cluster at the right SFG/MeFG (211 mm3, centroid coordinate (R6, A9, S53)) was also found to be differentially active only in the older group.
Whole-Brain Analysis for fMRI: Flanker Activation from Error Trials in Older Adults
The contrast between the incorrect (errors) Incongruent versus the correct (successful) Congruent trials from the older adult subset (those who made a high number of errors) showed two large differentially active clusters (Fig. 1(d)): a 620 mm3 cluster at the left IFG with centroid (L43, A10, S23), and a 264 mm3 cluster at the right IFG/MFG with centroid (R44, A11, S29). These two clusters survived the whole-brain correction for multiple comparisons. A differentially active cluster (161 mm3) was found at the left SFG with centroid (L6, A9, S53) (Fig. 1(d)), at the location where a left SFG/MeFG cluster was found in older adults for the IC – C contrast in the analysis of successful trials (not corrected for whole brain) and in the analysis of successful and error trials together (corrected for whole brain). In the frontal region, the differentially active volumes (uncorrected for whole brain) for the contrast of incorrect Incongruent versus correct Congruent trials were 801 mm3 at the right and 1320 mm3 at the left.
Discussion
Common Areas of Brain Activation during Interference Resolution in Young and Older Adults
The results of our flanker fMRI study show an overall agreement on the brain regions involved in selective attention and executive function with the findings from the meta-analysis on flanker studies by Nee et al. (2007) and other fMRI studies on interference resolution on a similar population (Botvinick et al., 1999; Colcombe et al., 2005; Colcombe et al., 2004; Erickson et al., 2005; Hazeltine et al., 2000; Nielson et al., 2002). The common differentially active (IC-C) regions (Table 2 and Fig. 1(a, b)) of the two age groups include the right IFG, bilateral MFG, right IPL and right SPL. The IPL and SPL were likely activated for their role in visual attention and processing as they are anatomically connected to areas associated with visual processing (precuneus and occipital gyri). Differential activation in IPL and SPL is consistent with the result of Erickson et al.'s (2005) structural equation modeling analysis of attentional control in a flanker task. The network relationship between various regions, such as IPL, SPL, IFG and MFG, supports attention control. Our results showed smaller regions of activation in the IFG and MFG in the older adults suggesting an age-related decline in this supporting network.
Age-related Differences in Brain Activation during Interference Resolution
The main goal of our study was to identify specific age-related modification at the frontal region for interference resolution. Older adults showed relatively smaller total volume of differential activation for the whole brain. This might be explained by the change of the neurovasculature with aging (Takahashi et al., 2005). An important finding with aging is the change of the location of the differential activation in the right IFG and MFG, the regions that have been shown to be involved in this type of executive processing (Colcombe et al., 2005; Colcombe et al., 2004; Erickson et al., 2005; Nee et al., 2007). For both groups, the right IFG/MFG cluster was the largest in the frontal area. This suggests recruitment of functionally similar regions in older adults to accomplish inhibition processing. The shift in location may occur to offset the reduced activation in the specific locations observed within the younger group. However, as the older adults showed slower inhibitory processing, this recruitment at a shifted region did not result in equivalent performance when compared to the younger adults. Others have shown that older adults recruit additional areas during successful cognitive performance to compensate for otherwise ineffective processing in traditional brain regions. These studies show an advantage of recruiting additional areas by showing improved performance when compared to older adults who do not recruit additional brain regions (Cabeza et al., 2002; Grady, 2000). Our results also do not provide direct support for other theories of cognitive aging such as dedifferentiation as the shifted activation in the current study is located within brain regions that are generally associated with inhibitory processing. Nonetheless, these results do show that older adults use a different pattern of brain activation compared to young adults during inhibitory processing and an activation pattern that is confined to smaller regions. Although the additional analyses including error trials were post-hoc, we were quite surprised to find such a large effect of modeling error trials given the small number of errors committed by both young and older adults. Error-related processing was associated with added activation in the left frontal hemisphere, notably at IFG, SFG and MeFG. This also explains why the left SFG/MeFG cluster survived the correction for multiple comparisons if the error trials were included in the data, but not if only successful trials were analyzed. In fact, the additional activation observed with errors has been shown in another inhibitory processing task (Murphy and Garavan, 2004). If the successful trials were not separated from the error trials, higher overall active volumes were obtained, also consistent with findings by Murphy and Garavan (2004).
A secondary aim of the study was to investigate age-related changes in frontal hemispheric asymmetry (e.g. reduction in asymmetry) that has been reported by others (Colcombe et al., 2005; Nielson et al., 2002; Langenecker and Nielson, 2003; Cabeza et al., 2002). During successful inhibition, there was no loss of asymmetry with either voxel based p ≤ 5 × 10−3 (and whole-brain corrected p ≤ 0.021) or at the much more liberal voxel-based p ≤ 0.01 (whole-brain uncorrected). Our data do provide support for reduced asymmetry when error trials were included, and suggest that this asymmetry reduction was specifically associated with error processing. However, it should be emphasized that this finding is from a limited number of subjects and contains bias in selecting the sub-group; the current study was not designed or powered to adequately address error processing during aging. This will be an interesting topic to explore in the further investigations of cognitive aging. Future studies should consider additional task difficulty to explore error related activation changes with aging.
Rationale for the Flanker Paradigm and Rapid Event-Related Design in This Study
The flanker paradigm used in the current study was intentionally chosen to emphasize stimulus-response inhibitory processing, while it is recognized that the task may also include selective attention. The use of a rapid event-related presentation of stimuli is expected to elicit constant general attention as the subject is consistently, but randomly, presented with task displays and control fixation cues. This study showed greater differential behavior responses for older adults providing support that this type of stimulus-response inhibitory task is affected by age, beyond that of general age-related slowing.
An event-related design was chosen over a block design for several reasons. Despite a high detectability for differential activation due to an overall high BOLD signal to noise ratio, a block design needs to present multiple trials with the same stimulus type in each block of time. After the first trial in each block, a subject can easily predict the stimulus type for the rest of the trials in the block which can reduce the effect of interference resolution. In a more traditional (slow) event-related design (Colcombe et al., 2005; Garavan et al., 1999), the hemodynamic response to a stimulus is allowed to return to the baseline before another stimulus is presented, commonly with an ISI longer than 12 sec. This design suffers from time inefficiency and needs more data points (or a longer scan time) to achieve a good level of statistical power. In addition, one must assume that the subject is able to maintain a similar attention level over an extended period of time. Any variation in attention level over an extended period of time can be a confounding factor in the results. One advantage of a slow-event related design is that the hemodynamic response to an error trial can be easily identified and removed from the analysis.
A rapid event-related design allows the subject's general attentiveness level to be kept relatively constant, and thus differential activation patterns can more clearly unveil the neural network that contributes specifically to conflict resolution during the stimulus-response inhibition task. In a rapid event-related design, stimuli are presented at a much higher frequency than in a more traditional event-related design. However, the hemodynamic responses convolve to each other and this makes it difficult to identify the raw image signal data points corresponding to error trials and to remove them from analysis. The estimation of the average hemodynamic response for each trial type relies on the deconvolution analysis (Glover, 1999; Ward, 2000a). The error trials need to be modeled in the analysis and can introduce some variation in IRF estimation.
We must also address the limited data that we have on our participants' demographic, general cognitive, and health characteristics. Subject information was limited to self reports on health, task performance and anatomical images. It is possible that physical health issues, such as cardiovascular conditions, and poor mental status may have contributed to the group difference seen in the BOLD signal, but it should be noted that the older sample comes from a population that we know from other studies (e.g., in RTZ's lab) has generally good physical and psychological health.
In summary, our study found an age-associated shift in location in executive-function related regions, notably at the right IFG and MFG. The left SFG/MeFG also appeared to be specifically recruited by older adults for interference resolution, albeit this activation was partially driven by incorrect trials presumably arising from brain activation during error processing. These modifications with aging might play a direct role in impaired interference resolution, corresponding to the longer response time. Lastly, while our study did not find concrete evidence to support frontal hemispheric asymmetry associated with aging in successful flanker interference resolution, we show bilateral activation during error processing in older adults.
Acknowledgements
The authors want to thank student assistants Stephen Kemsley, Devon Witherell, Alena Patsenka and Lisa Kelly during various stages of this project. The authors also want to thank the valuable suggestion from the reviewers on the necessary treatment of the error trials.
Financial Support:
Financial support for the current study was provided by the Departments of Radiology and Psychology at Michigan State University, the Core Analytical Facility Fund from Michigan State University, and the NIA R37 AGO 4306 award (RTZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Lustig C. Brain changes in aging: a lifespan perspective. In: Bialystok E, Craik FIM, editors. Lifespan Cognition: Mechanisms of Change. Oxford University Press; New York: 2006. pp. 27–42. [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Kingstone A. Handbook of Functional Neuroimaging of Cognition. 2nd ed. MIT Press; Cambridge, Mass: 2006. [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Thomas KM, Welsh TF, Badgaiyan RD, Eccard CH, Jennings JR, Crone EA. Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proc Natl Acad Sci U S A. 2000;97:8728–8733. doi: 10.1073/pnas.97.15.8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P. The implications of cortical recruitment and brain morphology for individual differences in inhibitory function in aging humans. Psychol Aging. 2005;20:363–375. doi: 10.1037/0882-7974.20.3.363. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Rice HJ, Cabeza R. Hemispheric asymmetry and aging: right hemisphere decline or asymmetry reduction. Neurosci Biobehav Rev. 2002;26:819–825. doi: 10.1016/s0149-7634(02)00068-4. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Milham MP, Colcombe SJ, Kramer AF, Banich MT, Webb A, Cohen NJ. Behavioral conflict, anterior cingulate cortex, and experiment duration: implications of diverging data. Hum Brain Mapp. 2004;21:98–107. doi: 10.1002/hbm.10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Ringo Ho MH, Colcombe SJ, Kramer AF. A structural equation modeling analysis of attentional control: an event-related fMRI study. Brain Res Cogn Brain Res. 2005;22:349–357. doi: 10.1016/j.cogbrainres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Eriksen B, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception and psychophysics. 1974;16:143–149. [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH. Deconvolution of impulse response in event-related BOLD fMRI. Neuroimage. 1999;9:416–429. doi: 10.1006/nimg.1998.0419. [DOI] [PubMed] [Google Scholar]
- Grady CL. Functional brain imaging and age-related changes in cognition. Biol Psychol. 2000;54:259–281. doi: 10.1016/s0301-0511(00)00059-4. [DOI] [PubMed] [Google Scholar]
- Hasher L, Lustig C, Zacks RT. Inhibitory mechanisms and the control of attention. In: Conway AR, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in Working Memory. Oxford University Press; New York: 2007. pp. 227–249. [Google Scholar]
- Hasher L, Zacks RT, May CP. Inhibitory control, circadian arousal, and age. In: Koriat A, Gopher D, editors. Attention and Performance XVII, Cognitive Regulation of Performance: Interaction of Theory and Application. MIT Press; Cambridge, MA: 1999. pp. 653–675. [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension and aging: a review and a new view. In: Bower GH, editor. The Psychology of Learning and Motivation. Academic Press; San Diego, CA: 1988. pp. 193–225. [Google Scholar]
- Hazeltine E, Poldrack R, Gabrieli JD. Neural activation during response competition. J Cogn Neurosci. 2000;2(12 Suppl):118–129. doi: 10.1162/089892900563984. [DOI] [PubMed] [Google Scholar]
- Kim DH, Adalsteinsson E, Glover GH, Spielman DM. Regularized higher-order in vivo shimming. Magn Reson Med. 2002;48:715–722. doi: 10.1002/mrm.10267. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Nielson KA. Frontal recruitment during response inhibition in older adults replicated with fMRI. Neuroimage. 2003;20:1384–1392. doi: 10.1016/S1053-8119(03)00372-0. [DOI] [PubMed] [Google Scholar]
- Li S-C, Lindenberger U. Cross-level unification: A computational exploration of the link between deterioration of neurotransmitter systems and dedifferentiation of cognitive abilities in old age. In: Nilsson LG, Markowitsch HJ, editors. Cognitive Neuroscience of Memory. Hogrefe & Huber; Seattle, WA: 1999. pp. 103–146. [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ. Attentional control in the aging brain: insights from an fMRI study of the stroop task. Brain Cogn. 2002;49:277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Murphy K, Garavan H. Artifactual fMRI group and condition differences driven by performance confounds. Neuroimage. 2004;21:219–228. doi: 10.1016/j.neuroimage.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Langenecker SA, Garavan H. Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychol Aging. 2002;17:56–71. doi: 10.1037//0882-7974.17.1.56. [DOI] [PubMed] [Google Scholar]
- Parrish TB, Gitelman DR, LaBar KS, Mesulam MM. Impact of signal-to-noise on functional MRI. Magn Reson Med. 2000;44:925–932. doi: 10.1002/1522-2594(200012)44:6<925::aid-mrm14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SC, Giampietro V, Andrew CM, Taylor E. Mapping motor inhibition: conjunctive brain activations across different versions of the go/no-go and stop tasks. NeuroImage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamaguchi S, Kobayashi S, Yamamoto Y. Effects of aging on regional cerebral blood flow assessed by using technetium Tc 99m hexamethylpropyleneamine oxime single-photon emission tomography with 3D stereotactic surface projection analysis. AJNR Am J Neuroradiol. 2005;26:2005–2009. [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: an Approach to Cerebral Imaging. Georg Thieme Verlag; New York: 1988. [Google Scholar]
- Treitz FH, Heyder K, Daum I. Differential course of executive control changes during normal aging. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2007;14:370–393. doi: 10.1080/13825580600678442. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage. 2001;14:1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- Ward B. Deconvolution analysis of fMRI time series data. Biophysics Research Institute, Medical College of Wisconsin; Milwaukee, WI: 2000a. [Google Scholar]
- Ward B. Simultaneous inference for fMRI data. Biophysics Research Institute. Medical College of Wisconsin; Milwaukee, WI: 2000b. [Google Scholar]