Abstract
Microneedle patches coated with solid-state influenza vaccine have been developed to improve vaccine efficacy and patient coverage. However, dip coating microneedles with influenza vaccine can reduce antigen activity. In this study, we sought to determine the experimental factors and mechanistic pathways by which inactivated influenza vaccine can lose activity, as well as develop and assess improved microneedle coating formulations that protect the antigen from activity loss. After coating microneedles using a standard vaccine formulation, antigenicity was reduced to just 2%, as measured by hemagglutination activity. The presence of carboxymethylcellulose, which was added to increase viscosity of the coating formulation, was shown to contribute to vaccine activity loss. After screening a panel of candidate stabilizers, the addition of trehalose to the coating formulation was shown to protect the antigen and retain 48–82% antigen activity for all three major strains of seasonal influenza: H1N1, H3N2 and B. Influenza vaccine coated in this way also exhibited thermal stability, such that activity loss was independent of temperature over the range of 4 – 37°C for 24 h. Dynamic light scattering measurements showed that antigen activity loss was associated with virus particle aggregation, and that stabilization using trehalose largely blocked this aggregation. Finally, microneedles using an optimized vaccine coating formulation were applied to the skin to vaccinate mice. Microneedle vaccination induced robust systemic and functional antibodies and provided complete protection against lethal challenge infection similar to conventional intramuscular injection. Overall, these results show that antigen activity loss during microneedle coating can be largely prevented through optimized formulation and that stabilized microneedle patches can be used for effective vaccination.
Keywords: influenza virus, microneedle, inactivated virus vaccine, trehalose
1. Introduction
Influenza virus is one of the most common causes of serious respiratory illness leading to 20,000 to 50,000 deaths and 95,000 to 110,000 hospitalizations in the United States during a typical influenza season [1, 2]. A global pandemic could take millions of lives, as occurred during the 1918 influenza pandemic that resulted in 20 to 50 million deaths worldwide [3].
Vaccination is the most cost-effective public health measure to protect against influenza-related mortality. Although influenza vaccines have been available for over 60 years, high levels of protection and widespread patient coverage have been limited in part by the conventional subcutaneous or intramuscular routes of delivery [4]. Intradermal vaccination has been investigated to address these limitations and shown to offer immunologic advantages, such as a reduction of dose needed for protective immunity and an elevated immune response in the elderly, which is the population most susceptible to influenza-related morbidity and mortality [5–7]. However, intradermal immunization with vaccines in solution requires needle injection by medical personnel, which is an often painful, time consuming and unreliable, as well as associated with needle injuries and pathogen transmission [8, 9].
Vaccine delivery via the skin is an attractive approach, because the skin is replete with antigen-presenting cells such as Langerhans and dermal dendritic cells [10]. However, the efficacy of vaccine antigen delivery into the skin is severely limited by the barrier properties of the stratum corneum, which is skin’s outermost layer [11, 12]. To overcome the limitations of hypodermic needles, millimeter- and micron-scale hollow needles are being developed and shown in human trials to be effective for influenza vaccination [13, 14]. Other methods have also been investigated, such as skin abrasion [9, 15], gene gun and jet injection [16, 17], electroporation [18], thermal ablation [19, 20], dissolving implants [21] and use of a tattoo gun [22].
Microneedle patches have also been proposed for vaccine delivery to the skin. Although various microneedle designs have been developed [23], considerable study has emphasized solid metal needles measuring hundreds of microns in length that are coated with a vaccine formulation that rapidly dissolves off upon insertion into the skin. The advantage of such a microneedle patch is that it not only crosses the stratum corneum barrier to target dendritic cells in the skin, but does so using an inexpensive, disposable patch that is simple enough to be suitable for self administration by patients. Initial research addressed vaccination using ovalbumin as a model antigen in guinea pigs and showed that microneedle delivery to the skin required lower antigen dose compared to intramuscular injection [24, 25]. More recent studies in mice have examined immunization against influenza and demonstrated that microneedle patches yielded protective efficacies at least as good as intramuscular injection [26, 27].
These studies motivated us to more closely investigate the process by which microneedles are coated with vaccine and what factors might influence antigen stability. Initial studies showed that microneedles could be coated by dipping into a coating formulation containing a surfactant to improve coating uniformity and a viscosity enhancer to increase coating thickness [28]. However, previous studies have not considered the effect of vaccine formulation on antigen activity. As shown in this study, coating microneedles with influenza vaccine can severely damage vaccine activity, mainly due to the drying process. We hypothesized that addition of appropriate excipients could stabilize vaccine during microneedle coating by preventing antigen aggregation and found that trehalose was especially effective. Trehalose has been shown previously to protect other biologicals, such as microorganisms [29], yeast [30] and active retrovirus [31] from drying-induced damage by lowering membrane phase transition temperature, inhibiting aggregation between adjacent vesicles, and maintaining the lipid in fluid phase [32, 33]. We further studied the conditions to optimize coating formulations for delivery of microneedle influenza vaccines. Overall, this study sought to determine the effects of microneedle coating formulation on the stability of inactivated influenza virus vaccine using in vitro assays as well as on the induction of protective immunity by in vivo challenge infection.
2. Materials and methods
2.1. Preparation of inactivated influenza virus
Formalin-inactivated influenza A/PR/8/34 virus (A/PR8) was prepared as described previously [34]. For imaging experiments, inactivated virus was labeled with fluorescent dye. To carry out labeling, 200 µL of inactivated virus at a concentration of 3 mg/ml was mixed well with 10 µL of octadecyl rhodamine B chloride (R18, Invitrogen, Carlsbad, CA) and incubated at 25°C for 1 h. In order to remove unbound R18 molecules, the labeled inactivated virus was suspended in 10 mL of PBS and precipitated by ultracentrifugation (28,000 × g for 1h, Optima L-80 XP, Beckman Coulter, Fullerton, CA) with a 20% sucrose layer [35, 36]. The precipitated virus was again washed in PBS by ultracentrifugation to further wash out potential remaining R18 dye.
2.2. Fabrication, coating and imaging of microneedles
Rows of solid metal microneedles were fabricated by cutting needle structures from stainless sheets (SS304, 75µm thickness, McMaster-Carr, Atlanta, GA) using an infrared laser (Resonetics Maestro, Nashua, NH). The shape and orientation of the arrays were drafted in a CAD file, which was used by the laser control software. The laser beam traced the desired shape of the needle, which ablated the metal sheet and created the needles in the plane of sheet. The laser was operated at 1000 Hz at an energy density of 20 J/cm2. The metal sheet with needles on it was cleaned in hot, soapy water and rinsed with DI water. The needles were electropholished in a bath containing a 6:3:1 mixture by volume of glycerin, phosphoric acid, and water to remove debris, thereby reducing the needle thickness to 50 µm [28]. In the end, the microneedles measured 700 µm in length and 160 µm in width.
To apply a vaccine coating, microneedles were dipped six times at 25°C into coating solution using a dip-coating device described previously [28] and air dried. The coating solution was composed of 1% (w/v) carboxymethylcellulose (CMC) sodium salt (Carbo-Mer, San Diego, CA), 0.5% (w/v) Lutrol F-68 NF (BASF, Mt.Olive, NJ) with or without 15% (w/v) D-(+)-trehalose dihydrate (Sigma Aldrich, St.Louis, MO) and 1 mg/ml inactivated virus in phosphate buffered saline (PBS). Additional studies used stabilizers other than trehalose, including 15% (w/v) sucrose, glucose, inulin from dahlia tubers and chicory, and dextrans with molecular weights of 9 kDa and 36 kDa (Sigma–Aldrich).
Microneedles were imaged by scanning electron microscopy (LEO 1530, Carl Zeiss, Oberkochen, Germany), bright-field microscopy (Olympus SZX12 stereo microscope, Tokyo, Japan) with a CCD camera (Leica DC 300, Leica Microsystems, Wetzlar, Germany) and fluorescence microscopy (Olympus IX70) with a CCD camera (RT Slider, Diagnostic Instruments, Sterling Heights, MI). To image delivery of viral antigen into skin, microneedles coated with R18-labeled virus were inserted into human cadaver skin for 10 min and fixed by freezing in histology mounting compound (Tissue-Tek®, Sakura Finetek, Torrance, CA) for 10 min, after which microneedles were removed and skin was sectioned using a cryostat (Cryo-Star HM 560 MV, Microm, Hésingue, France) for imaging. This use of human skin was approved by the Georgia Tech Institutional Review Board.
2.3. Vaccine stability test and size distribution
Hemagglutination (HA) activity was measured as an indicator of the functional integrity of hemagglutinin on the inactivated viral vaccine. We carried out screening experiments by aliquoting 2 µl of virus coating solution onto 3 mm × 3 mm chips of the same type of stainless steel used to make microneedles and letting it air dry at 25°C overnight to mimic the process of coating virus onto microneedles. Coating stainless steel chips produced coatings with antigen stability similar to coating stainless steel microneedles (data not shown), which suggests that using stainless steel chips is a suitable model system.
After drying, the metal chip was incubated in PBS lacking Mg2+ and Ca2+ for 12 h. To determine HA titers, 50 µl of dissolved coating in PBS was serially diluted in 50 µl of PBS mixed with an equal volume of a fresh 0.5% suspension of chicken red blood cells (Lampire Biological Laboratories, Pipersville, PA) and incubated for 1 h at 25°C. The titers were determined as the endpoint dilutions inhibiting the precipitation of red blood cells. Inactivated virus mass was determined using a protein assay kit (DC protein assay, Bio-Rad Laboratories, Hercules, CA).
Particle size distribution was determined by similarly dissolving virus coatings from metal chips and analyzing by dynamic light scattering (DynaPro, Wyatt, Santa Barbara, CA)
2.4. Immunization and viral challenge infection
BALB/c mice (n=6 animals per group, 8–10 week old, female) were anesthetized intramuscularly with 110 mg/kg ketamine (Abbott Laboratories, Abbott park, IL) mixed with 11 mg/kg xylaxine (Phoenix Scientific, St. Joseph, MO). The skin on the back of the mouse was exposed by removing hair with depilatory cream (Nair, Princeton, NJ), washed with 70% ethanol, and dried with a hair dryer. In our previous study, depilatory cream and 70% ethanol did not significantly effect skin permeability to inactivated virus [37]. An in-plane five-needle array of microneedles coated with 0.4 µg of inactivated influenza virus was manually inserted into the skin and left for 10 min to dissolve the vaccine coating in the skin. For an IM immunization control, mice were similarly treated, but no depilatory cream was applied, and 0.4 µg of unprocessed inactivated influenza virus in 100 µl PBS solution was injected intramuscularly into the upper quadriceps muscles of mice (50 µl per leg). The mock control mice received a similar treatment using microneedles coated with the coating solution excipiencts without influenza vaccine. To determine the amount of inactivated virus vaccine coated on microneedles, vaccine-coated microneedles were incubated in PBS solution for 12 h at 4°C, and the amount of released protein was measured by a BCA protein assay kit (Pierce Biotechnology, Rockford, IL). The vaccine delivery efficacy was determined by measuring the intensity of fluorescent-labeled vaccine released from the microneedle before and after insertion into the skin. Approximately 70% of vaccine coated on microneedles was delivered into skin.
For virus challenge, lightly anesthetized mice were intranasally infected with the mouse-adapted A/PR8 virus (50 µl of 20 LD50) five weeks after vaccination [34]. Mice were observed daily to monitor body weight changes and mortality rates. Mice were humanely euthanized if their body weight loss exceeded 25%. All animal studies were approved by the Emory University Institutional Animal Care and Use Committee (IACUC).
2.5. Antibody responses and hemagglutination inhibition titer (HAI)
Influenza virus-specific antibodies of different isotypes (IgG, IgG1, IgG2a and IgG2b) were determined by following the standard protocol for enzyme-linked immunosorbent assay (ELISA) as described previously [34]. Data are presented as optical densities read at 450 nm.
Hemagglutination-inhibition (HAI) titers were determined as previously described [34]. Briefly, serum samples were first treated with receptor-destroying enzyme in 1:3 ratio (Denka Seiken, Tokyo, Japan) overnight at 37°C and then incubated for 30 min at 56°C. Sera were then serially diluted in separate wells, mixed with 4 HA units (HAU) of influenza A/PR8 virus, and incubated for 30 min at room temperature prior to adding 0.5% chicken red blood cells. The highest serum dilution preventing hemagglutination was scored as the HAI titer.
2.6. Statistical Analysis
Every assay was measured using at least three replicate samples, from which the arithmetic mean and standard error of the mean were calculated (unless otherwise noted). A two-tailed Student’s t-test was performed when comparing two different conditions. When comparing three or more conditions, a one-way analysis of variance (ANOVA; α=0.05) was performed. In all cases, a value p<0.05 was considered statistically significant.
3. Results
3.1. Fabrication and coating of microneedles for influenza vaccine delivery into skin
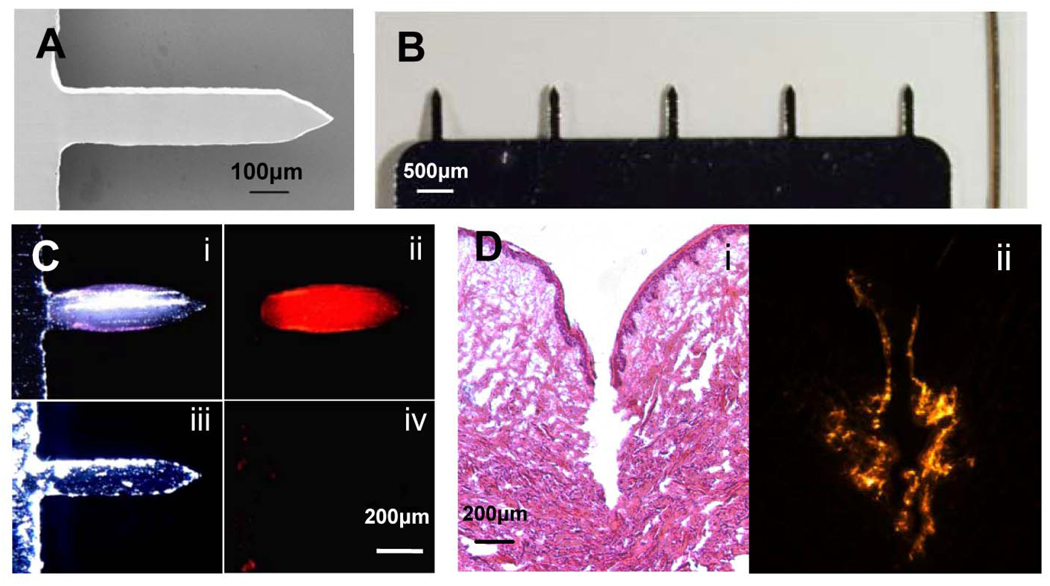
Microneedles fabricated by laser-cutting of stainless steel sheets (Fig. 1A and 1B) were designed to be long enough to penetrate through the stratum corneum and viable epidermis and into the superficial dermis by gentle manual insertion, but short enough to avoid pain [38]. Our delivery strategy involved coating solid microneedles with formulations of influenza vaccine that dissolve in skin. We developed aqueous coating formulations including a surfactant to facilitate uniform coatings by reducing surface tension, a viscosity enhancer to enable thicker coatings by increasing coating solution residence time during the drying process, and inactivated whole influenza virus (A/PR/8/34) as a vaccine antigen. Dip coating produced thick, uniform coatings localized to microneedle shafts (Fig. 1C). Insertion of microneedles into skin led to dissolution (Fig. 1C) and deposition (Fig. 1D) in skin within minutes.
Figure 1.
Microneedle vaccine delivery system. (A) Scanning electron micrograph of a microneedle (700 µm length, 160 µm width, 50 µm thickness; scale bar = 100 µm) (B) Comparison between an array of five microneedles and a human hair (scale bar = 500 µm) (C) Bright-field (i,iii) and fluorescence (ii,iv) micrographs of a microneedle coated with red-fluorescent inactivated influenza virus before (i,ii) and 10 min after (iii,iv) insertion into human cadaver skin (scale bar = 200µm). (D) Histologic section of human cadaver skin fixed after insertion of a vaccine-coated microneedle imaged by (i) bright-field microscopy with H&E staining showing skin deformation and needle track across the epidermis and into superficial dermis and (ii) fluorescence microscopy showing deposition of red-fluorescent vaccine coating in skin (scale bar = 200µm).
3.2. Stabilization of solid microneedle influenza vaccines by trehalose
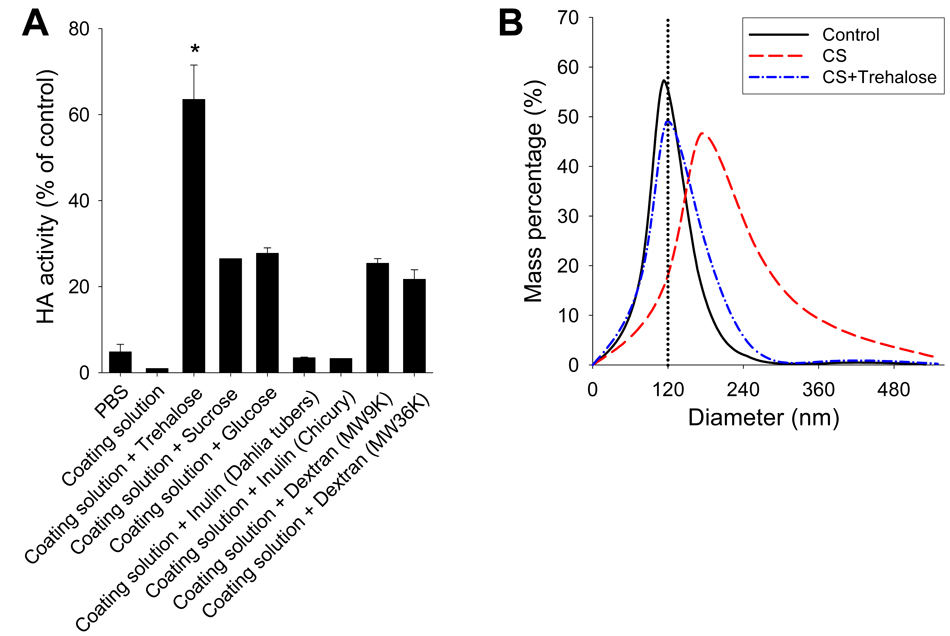
Coating solid microneedles with vaccine involves a phase change of the antigen from liquid solution to a solid coating containing dried antigen and excipients. This phase change could cause stresses on the vaccine leading to denaturation, aggregation or other mechanisms of damage to anitigen activity. We therefore used red blood cell hemagglutination (HA) activity, which measures the receptor binding function of hemagglutinin [39], as a measure of the activity of our inactivated influenza virus vaccine. Suspension of virus in coating solution had no effect on HA activity. However, after drying to form a solid coating, the reconstituted vaccine showed dramatically reduced HA activity to below 2% (Fig. 2A).
Figure 2.
Stabilization of influenza vaccine coatings. (A) Effects of various carbohydrates on retaining the HA activity of inactivated influenza virus during drying. Influenza vaccines in coating solution containing various carbohydrates were dried on pieces of stainless steel mimicking microneedles and then reconstituted to determine the HA activity. All carbohydrates were added at a concentration of 15% (w/v). Data are presented as the percent HA activity compared to the same amount of unprocessed inactivated whole virus in PBS solution without drying (n=4, *p <0.05 for pair wise comparisons between the “coating solution + trehalose” formulation and all other formulations). (B) Particle-size distribution determined by dynamic light scattering in coating solution (control) or reconstituted microneedle coatings prepared using coating solution (CS) with or without 15% trehalose.
To prevent this loss in vaccine stability, we screened various carbohydrates including trehalose, sucrose, glucose, inulin, and dextran known to protect biomolecules from drying or freezing damage [40]. While a number of these candidate stabilizers protected the antigen, trehalose was the most effective (Fig. 2A). Further optimization enabled coating up to 1 µg virus onto a row of five microneedles, while maintaining 65% HA activity, which suggests that coating tens of micrograms is reasonable for patches containing hundreds of microneedles. This study’s target dose of 0.4 µg whole inactivated virus vaccine was easily coated onto five-microneedle arrays.
We hypothesized that HA activity loss during coating was due to aggregation of virus antigens. Indeed, dynamic light scattering showed that drying in coating solution without trehalose substantially increased the size of virus particles, consistent with virus aggregation, whereas coating with trehalose retained particle size similar to non-dried vaccine controls, which suggests that trehalose prevented aggregation (Fig. 2B). These results indicate that coating microneedles with influenza vaccine can lead to particle aggregation affecting the conformation and biological activity of the antigen resulting in loss of influenza vaccine integrity. Importantly, trehalose molecules can substantially contribute to maintaining the integrity of influenza vaccine by preventing particle aggregation which is believed to preserve the hemagglutinin functional activity during the drying process.
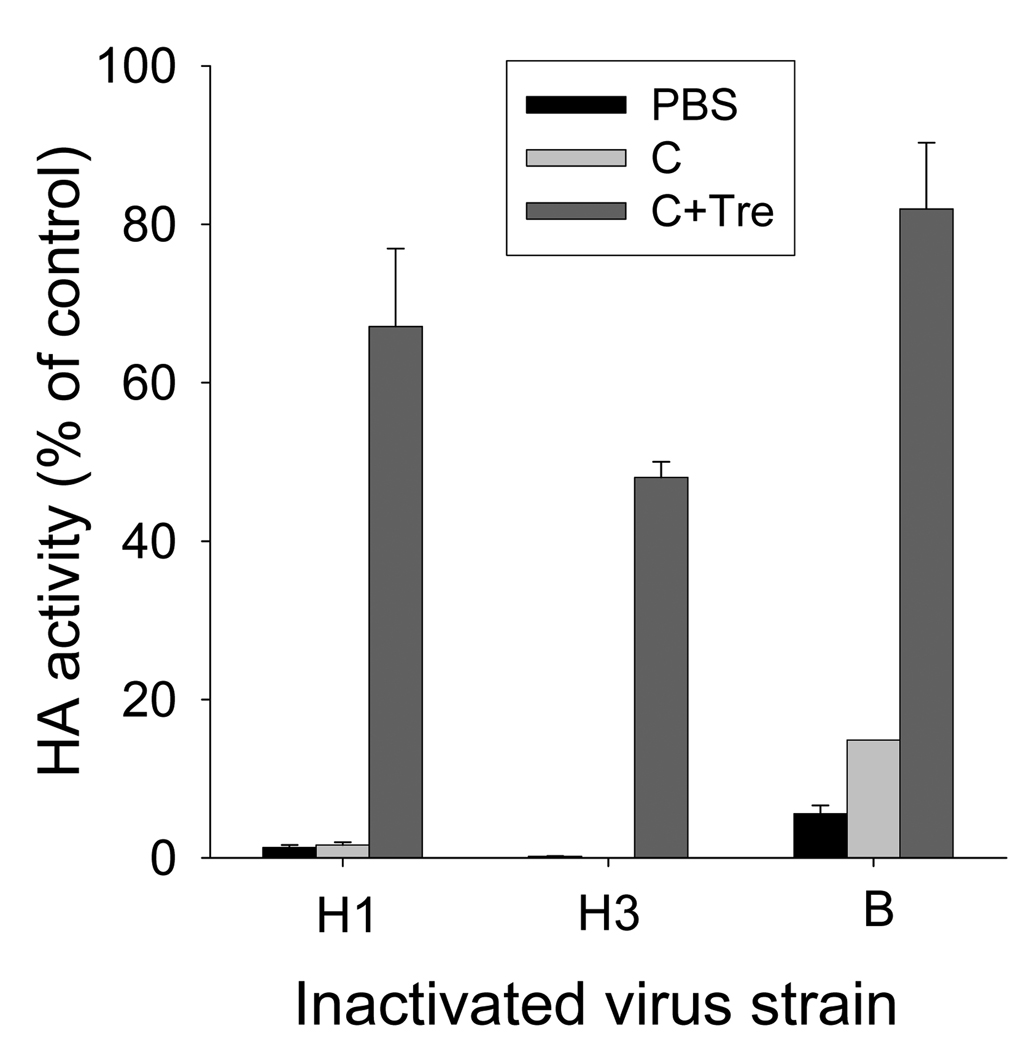
3.3. Trehalose effects on stability of different subtypes of influenza virus
Licensed influenza vaccines are trivalent, containing H1 and H3 subtype strains of influenza type A and a strain of influenza type B virus. We therefore measured HA activity after coating with H3 and B influenza virus strains too (Fig. 3). Similar to the H1 subtype influenza strain, the H3 strain was also vulnerable to the drying process in either PBS buffer or coating solution. In contrast, the influenza B strain was less vulnerable, showing 6 or 15% HA activity after drying in PBS or coating solution, respectively. Most importantly, however, HA activities were retained at approximately 67 to 82% for H1 and B nfluenza viruses and at 48% for the H3 influenza virus with the addition of the trehalose stabilizer. These results demonstrate that inclusion of trehalose in the coating solution significantly contributed to maintaining HA activity of diverse influenza strains, although the extent of stabilization depended on the influenza strain to a certain degree.
Figure 3.
Vaccine stability as a function of vaccine strain and storage temperature. HA activities of three different inactivated influenza virus strains dried in PBS or coating solution with or without 15% trehalose. H1 = inactivated influenza A/PR/8/34 (H1N1) virus; H3 = inactivated influenza A/Aichi/68 (H3N2). B = influenza B virus. CS= coating solution. Tre=trehalose.
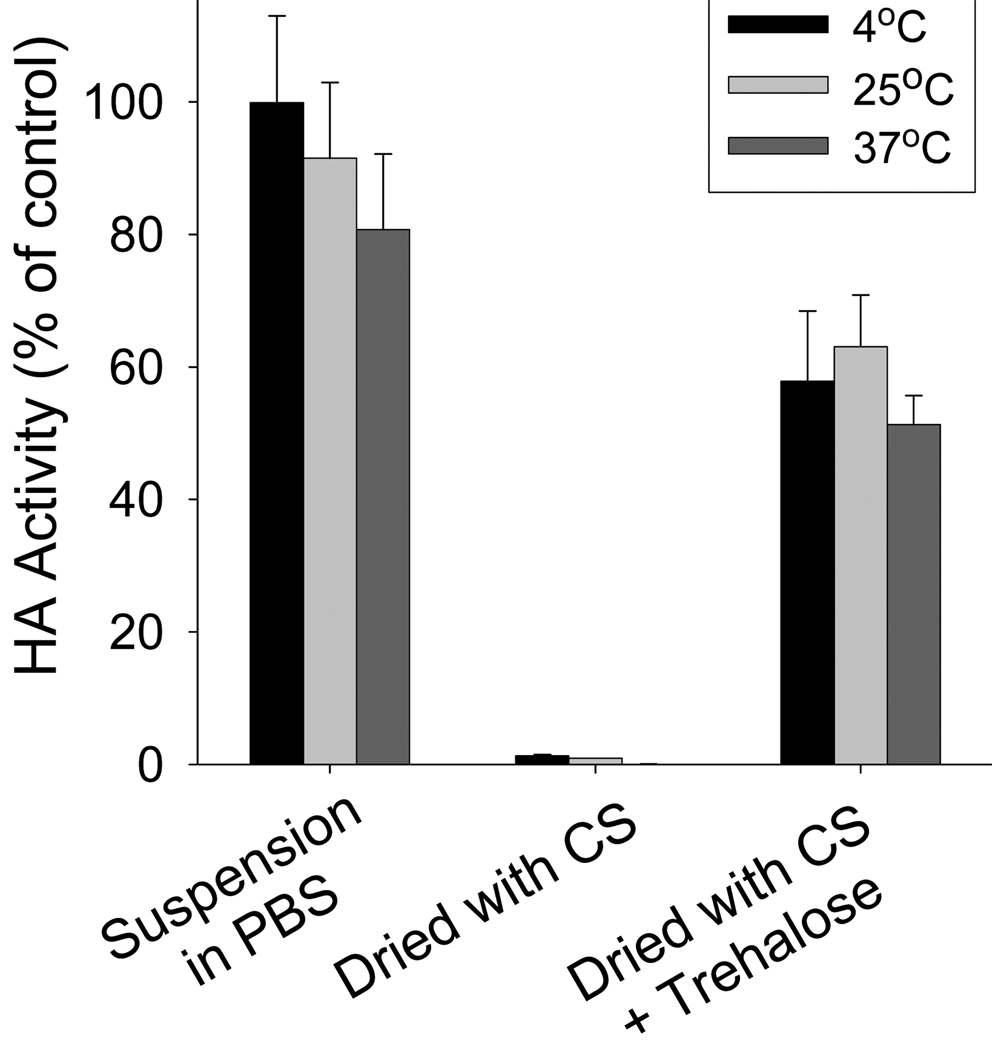
3.4. Temperature effect on vaccine stability
To facilitate vaccine distribution, vaccines should have thermal stability. We tested the stability of our inactivated influenza virus after storage for 24 h at three different temperatures: 4°C, 25°C, and 37°C. As shown in Fig. 4, inactivated virus in suspension retained 100% HA activity at 4°C and slightly lower activity at 25 °C and 37 °C (p>0.05). When inactivated virus vaccine was dried in coating solution without trehalose, it lost almost all HA activity upon drying; after storage for 24 h, activity remained similarly low. When inactivated virus vaccine was dried with coating solution including 15% trehalose, HA activity immediately dropped to 64%, but then showed no significant further drop after storage at all three temperatures studied. These results indicate that our inactivated virus vaccine after coating showed good stability over 24 h, even at elevated temperature of 37 °C.
Figure 4.
Vaccine stability as a function of storage temperature. HA titers of coated inactivated virus after drying and storage at different temperatures for 24 h. CS = coating solution.
3.5. The effect of trehalose concentration on HA activity and coating mass
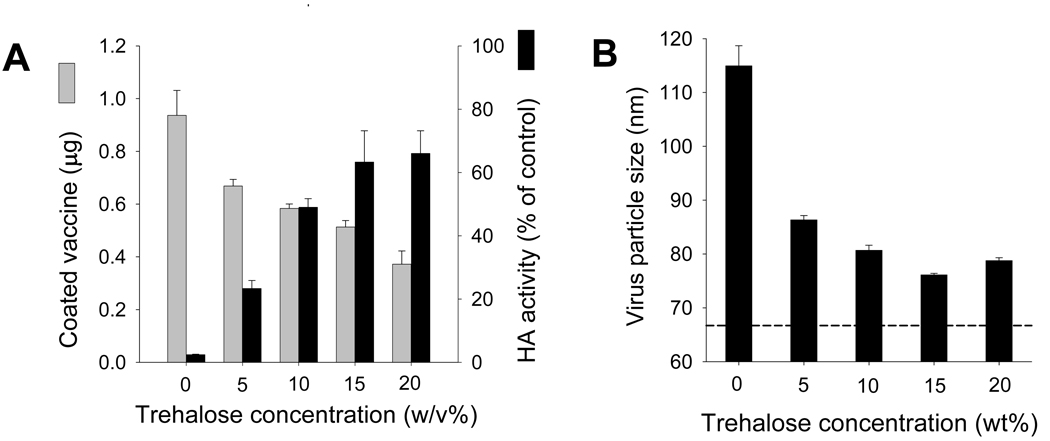
We next investigated the effect of trehalose concentration on HA activity and found that increasing trehalose concentration in the coating solution from 0% to 15% increased retention of HA activity from 3% to 64% (ANOVA, p=0.0002); increasing trehalose concentration further had no additional effect (p=0.74) (Fig. 5A, black bar). Similarly, increasing trehalose concentration also decreased the extent of virus aggregation, as measured by the average virus particle size (p<0.05) (Fig. 5B).
Figure 5.
Effect of trehalose concentration on vaccine coating. (A) Effect of trehalose concentration in coating solution on ( ) mass (per 5 microneedles) and (
) mass (per 5 microneedles) and ( ) HA activity of coated inactivated influenza virus after reconstitution (n=4). (B) Effect of trehalose concentration in coating solution on the size of coated inactivated influenza virus particles after reconstitution (n=4).
) HA activity of coated inactivated influenza virus after reconstitution (n=4). (B) Effect of trehalose concentration in coating solution on the size of coated inactivated influenza virus particles after reconstitution (n=4).
In contrast, the mass of antigen coated onto microneedles showed the opposite dependence on trehalose concentration. The amount of virus coated onto microneedles decreased with increasing trehalose concentration from 0.94 µg per five-needle array without trehalose to 0.37 µg with 20% trehalose (ANOVA, p=0.0002). (Fig. 5A, gray bar). This is probably because the presence of additional trehalose occupies space in the microneedle coating film that displaces coated virus. HA activity was inversely proportional to the mass of virus coating when varying trehalose concentration (Fig. 5A). These results indicate that trehalose concentration needs to be optimized, where 15% trehalose may be optimal, because it provides the maximum retention of HA activity (i.e., increased from 3% to 64%), while reducing virus mass by 46%. It should also be noted that in this study we coated less than 1 µg of vaccine per 5 microneedles array, because that was sufficient for the low dose needed for this vaccine. However, we have coated up to 7–8 µg of inactivated influenza virus per 5 microneedles array in separated study (data not shown) and have coated more than 7.86 µg per 5 microneedles array using other model drug (vitamin B) [28].
3.6. The effect of CMC concentration on HA activity
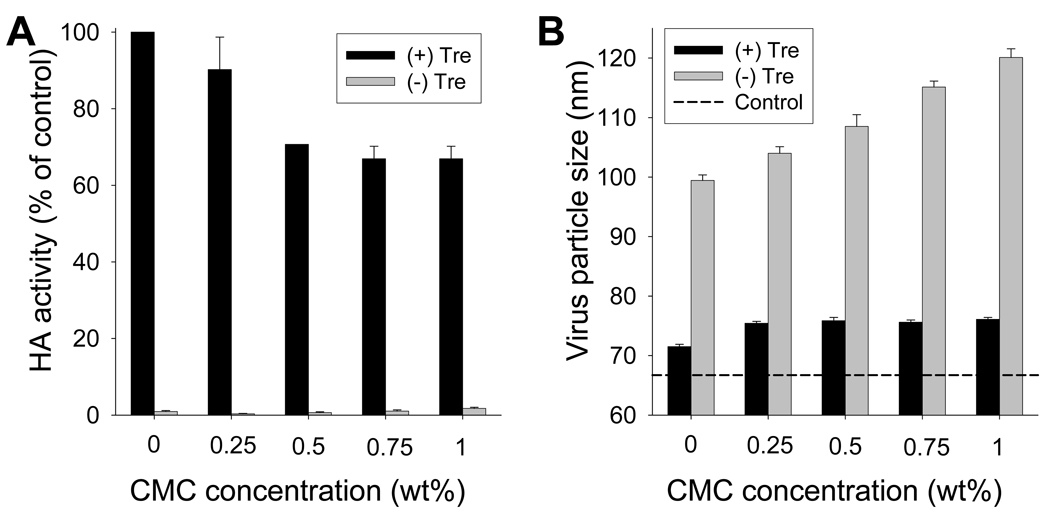
Drying in coating solution caused more damage to HA activity than drying in PBS. Therefore, we assessed the effect of concentration of one of the coating excipients, CMC, on vaccine stability with and without trehalose (Fig. 6A). In the absence of trehalose, HA activity of all samples was extremely low independent of CMC concentration (p<0.001). When 15% trehalose was included, CMC concentration had a significant effect, such that without CMC, 100% HA activity was retained, but increasing CMC concentration to 0.5% lowered HA activity to 71% (ANOVA, p<0.05); increasing CMC concentration further had no additional effect (p>0.05) (Fig. 6A). Correspondingly, virus aggregation increased with CMC concentration in samples coated without trehalose (ANOVA, p<0.05), but showed no dependence on CMC concentration in samples coated with trehalose (ANOVA, p>0.05) (Fig. 6B).
Figure 6.
Effect of CMC concentration in coating solution ( ) with or (
) with or ( ) without 15% trehalose on (A) HA activity and (B) size of coated inactivated influenza virus particles after reconstitution
) without 15% trehalose on (A) HA activity and (B) size of coated inactivated influenza virus particles after reconstitution
Although removing CMC from the coating formulation allowed us to retain 100% HA activity in the presence of trehalose, CMC was nonetheless required to produce thick coatings. Without CMC, the mass of virus coated on microneedles was reduced by more than an order of magnitude (data not shown). Further studies are needed to find new excipients that do not cause damage to influenza vaccine stability but still maintain appropriate viscosity of the coating solution to facilitate coating efficiency. As a final note, the other excipient in the coating solution was the surfactant Lutrol F-68 NF. Varying its concentration over the range of 0% to 0.5% had no significant effect on HA activity (data not shown).
3.7. Induction of systemic and functional antibody responses after influenza vaccination using coated microneedles
Our in vitro analysis suggested that antigen activity can largely be retained after coating onto microneedles in the presence of trehalose. Although HA activity is expected to correlate with protective antibody responses in vivo, we validated this expectation by testing influenza vaccine efficacy via microneedle delivery to mice in vivo. Groups of mice (n=6, BALB/c mice) received a single dose immunization via skin delivery of a microneedle array coated with 0.4 µg of inactivated whole virus with or without trehalose as a stabilizer. As a positive control, we intramuscularly (IM) immunized mice (n=6) with 0.4 µg of inactivated whole virus vaccine. As a negative control, an additional group (n=6) was mock-treated via microneedle delivery of coating solution without influenza vaccine.
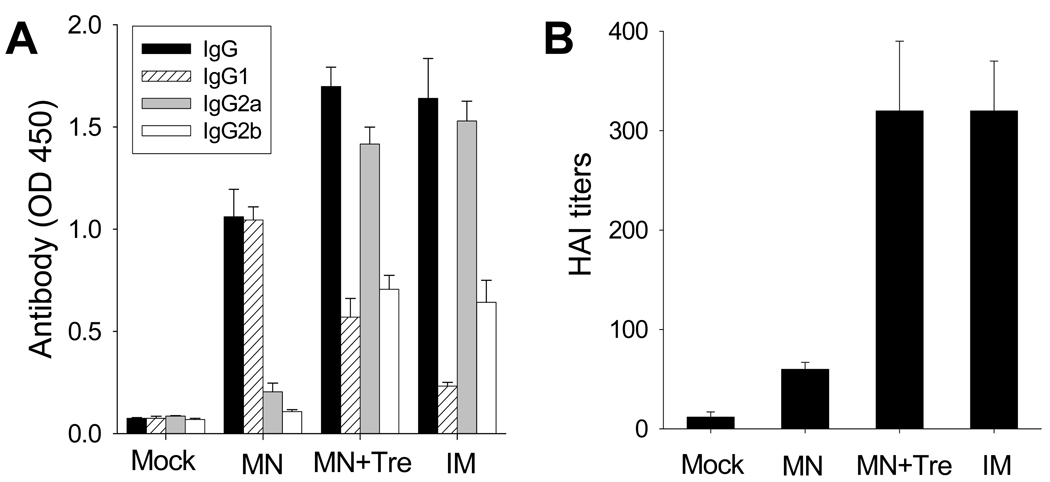
Four weeks after vaccination, levels of total serum (IgG) and subtype (IgG1, IgG2a, IgG2b) antibodies were measured. As expected, IM immunized mice showed robust antibody responses (Fig. 7A). In comparison, mice immunized with microneedles coated with the trehalose formulation (MN+Tre) showed IgG, IgG2a and IgG2b levels equal to those found in the IM group (Student’s t-test, p<0.05). Interestingly, IgG1 levels were higher after immunization using microneedles with trehalose (MN+Tre) compared to IM (Student’s t-test, p<0.05), suggesting a shift to a stronger Th2 response. Among mice immunized using microneedles without trehalose (MN), IgG, IgG2a and IgG2b levels were all significantly lower than the other immunized groups (IM, MN+Tre) (Student’s t-test, p<0.05), showing the important role of trehalose to stabilize the vaccine. However, IgG1 levels were notably higher (Student’s t-test, p<0.05) in the MN group, suggesting that the drying-induced changes in the antigen (e.g., aggregation) may be associated with a stronger IgG1 response. Mock immunized mice showed very weak antibody levels, as expected. As a final note, additional data show that IM injection of our influenza vaccine mixed in solution with 15% trehalose yielded the same antibody response as IM injection without trehalose, which suggests that trehalose itself does not have significant adjuvant effects and probably acts primarily to stabilize the viral antigen (data not shown).
Figure 7.
Antibody responses in mice after vaccination. (A) Virus-specific total IgG and isotype antibodies after a single vaccination. Influenza virus-specific serum antibody responses were determined at week 4 post-immunization. Antibody levels in 100× diluted serum samples were determined by ELISA using inactivated virus as a coating antigen and presented as optical densities (OD at 450 nm). (B) At week 4 after a single vaccination, hemagglutination inhibition (HAI) titers in sera were determined. Mock = microneedle immunization with coating solution only; MN = microneedle immunization with influenza vaccine formulated in the absence of trehalose; MN+Tre = microneedle immunization with influenza vaccine formulated in the presence of trehalose (15%); IM = intramuscular immunization with liquid influenza vaccine. (n=6 per group)
Sera were also tested for hemagglutination inhibition (HAI) activity, which is the serological correlate for protection that is typically accepted by regulatory agencies to establish vaccine efficacy; an HAI titer greater than 40 is generally associated with protection [41]. HAI titers in mice vaccinated using microneedles with trehalose (MN+Tre) and IM immunization were extremely strong and statistically indistinguishable from each other (Student’s t-test, p<0.05) (Fig. 7B). In contrast, mice vaccinated using microneedles without trehalose (MN) had significantly lower HAI titers (p<0.05), which were nonetheless higher than the mock-immunized negative controls (p<0.05).
Altogether, these results show that microneedles coated with a trehalose-stabilized vaccine formulation yielded antibody responses at least as good as IM injection. Moreover, microneedles without trehalose gave weaker antibody responses, consistent with expectations from in vitro HA activity measurements. This confirms the expectation that in vitro HA activity correlates with levels of in vivo functional antibody responses and that inclusion of the trehalose stabilizer is critical to maintaining vaccine immunogenicity.
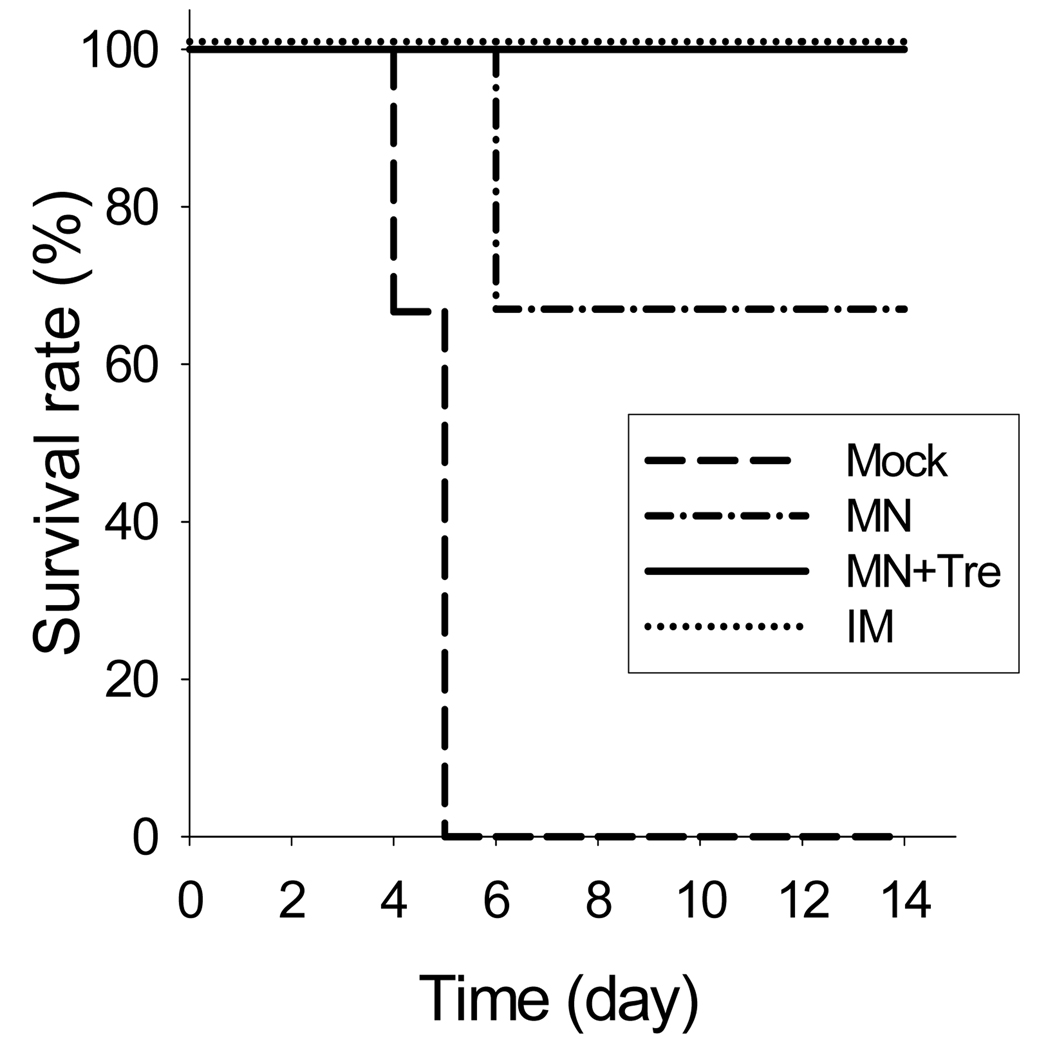
3.9. Protection against lethal challenge infection
As a final test to assess the efficacy of microneedle vaccination, we measured protection of mice after challenge with a lethal dose of influenza A/PR8 virus (20 × LD50) five weeks after one immunization (Fig. 8). Mice immunized using either microneedles with trehalose (MN+Tre) or IM injection were 100% protected. In contrast, mice immunized using microneedles without trehalose (MN) showed only 67% survival (Fig. 8) and significant body weight loss (data not shown). The mock immunized group died or had to be euthanized by day 5. These results further show that microneedles coated with a trehalose-stabilized formulation can deliver an immunogenic vaccine that provides protective efficacy.
Figure 8.
Protection of mice after a single vaccination. Immunized mice were challenged with a lethal dose (20×LD50) of a highly pathogenic A/PR8 influenza virus 5 weeks after a single vaccination (n=6). Animal survival was monitored daily for 14 days (n=6). Groups of mice are as described in the legend of Figure 7.
4. Discussion
The efficacy and coverage of influenza vaccination are below recommended levels [1] and need to be improved. Therefore, development of easy and effective methods to increase vaccine coverage is a priority. Transdermal microneedle vaccination offers an attractive approach to develop a simple influenza vaccination method that could increase patient coverage [26, 27]. To keep costs down during seasonal vaccination and to avoid rationing and vaccination delays during a pandemic, influenza vaccine dose should be minimized, which means that loss of vaccine immunogenicity during micorneedle coating must likewise be minimized. In this study, we describe the development of a solid microneedle coating formulation that largely retained vaccine antigen stability as measured by hemagglutinin functional analysis. In addition, we demonstrated the importance of maintaining in vitro HA activity of the influenza vaccine in order to achieve strong antibody responses and protection against viral challenge in vivo. These results indicate that vaccination using microneedles is not inferior to IM injection in inducing protective host immune responses and that microneedles can offer a promising alternative method to simplify influenza vaccination.
Hemagglutinin is the most abundant influenza virus surface glycoprotein, which is composed of trimeric molecular spikes and is responsible for binding of virus to the receptor sialic acid on target cells [42, 43]. In this study, we observed that microneedle coating with inactivated influenza virus vaccine resulted in the functional loss of HA activity of the vaccine. Use of stabilizers for pharmaceutical compounds or vaccines during the formulation of a dry powder has been previously described [40]. For example, vesicular lipid bilayer systems can be stabilized by incorporating sugars such as trehalose or inulin on both sides of the lipid bilayer [32, 33]. Carbohydrates such as trehalose, inulin, and dextran have also been demonstrated to exhibit similar stabilizing effects on an influenza hemagglutinin subunit protein vaccine [40, 44]. In this study, a number of different carbohydrate excipients helped stabilize our inactivated influenza virus vaccine, but trehalose was found to be most effective.
Although the exact mechanisms are unknown, prevention of virus particle aggregation in the presence of trehalose appears to be involved, as shown by the dynamic light scattering assay. We hypothesize that the formation of a trehalose-based sugar matrix may act as a physical barrier isolating particles and preventing particle aggregation during the dehydration process [45]. In a recent study, the addition of trehalose prevented physical changes in secondary and tertiary structure of hemagglutinin during a freezing process [44, 46]. Alternatively, during the drying process, a sugar molecule such as trehalose that contains multiple hydroxyl groups can replace water molecules in the hydrogen-bonding interaction with the active material, which can preserve the structural integrity of the drug or vaccine [47, 48]. Therefore, the effects of trehalose on preserving HA activity of microneedles coated with influenza vaccine might involve several factors including stabilization of lipid bilayers and hemagglutinin structure, as well as prevention of virus particle aggregation. Further studies are needed to better understand the role of trehalose stabilization in maintaining microneedle vaccine stability.
Currently used influenza vaccines are trivalent, which consist of three different strains of influenza [4]. It is significant to find that trehalose was able to at least partially retain HA activity of all three influenza strains studied, although the degree of stabilization depended on the strain. Therefore, trehalose-mediated stabilization of microneedle vaccines may be applicable to clinically relevant influenza vaccines, and possibly other vaccines too. However, it is not clear why the degree of stabilization depended on the subtypes of influenza viruses. The HA proteins from influenza A subtypes H1 and H3, and influenza B virus are significantly different in terms of the amino acid sequence and structure so that there are no cross-reactivities among these viruses. These differences in HA proteins among different subtypes of influenza viruses are more likely to contribute to the degree of stability [49] since the vaccine stability was based on the functional activity of HA glycoproteins. Further studies are needed to further delineate the underlying mechanisms of influenza vaccine stability during the drying process.
Despite the promising results demonstrating microneedle-based influenza vaccination, there are several challenging problems that need to be overcome before developing effective microneedle vaccines. For example, while addition of trehalose protected vaccine activity, it also decreased coating efficiency and thereby reduced the vaccine dose coated per needle. This requires the use of more microneedles to achieve a given target dose, which may not be a problem for vaccines that are typically of low microgram doses, but may become limiting for other therapeutics that require larger doses. An approach to improve coating efficiency would be to optimize formulation and coating conditions further, possibly reducing the amount of stabilizer by replacing trehalose with another more potent stabilizer, by replacing CMC with a less damaging viscosity enhancing agent, by developing a more controlled drying process and other possible changes. For example, carbohydrates including trehalose were sufficient to stabilize influenza vaccines from freeze-drying at concentrations as low as 1.7% [46].
Most vaccinations are given by the IM or subcutaneous (SC) route using hypodermic needles or, in some cases, jet injection devices, which requires trained personnel, is time consuming during mass vaccinations and is painful for patients. Intradermal immunization has received recent attention due to expected dose sparing and increased immunogenicity [5, 6, 14]. However, intradermal injection is even more difficult and unreliable than IM or SC injection in clinical practice and, for research purposes, is especially difficult in the thin skin of rodents, whose skin is often thinner than the bevel on the tip of a hypodermic needle [50]. For these reasons, microneedle delivery may be a significant advance that not only enables simple and reliable intradermal delivery for animal research and eventual clinical use, but also provides potential advantages over conventional IM or SC injection due to the expected speed, simplicity, and efficacy of microneedle vaccination. Therefore, it will be important to further investigate in detail the locations of vaccine deposition and delivery efficiency in the skin using different types and length of microneedles.
Acknowledgments
This work was carried out at the Emory Vaccine Center and the Georgia Tech Center for Drug Design, Development and Delivery and Institute for Bioengineering and Biosciences. It was supported in part by NIH grants R01-EB006369 (M.R.P.), AI0680003 (R.W.C.), AI074579-01 (R.W.C), SERCEB (R.W.C) and the Korea Ginseng Society (S.M.K). We thank Dr. Vladimir Zarnitsyn for microneedle fabrication, Dr. Huan Nguyen for mouse-adapted influenza virus A/PR8/34 strain, and Dr. Mark Allen for use of his laser microfabrication facilities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention. ACIP. Prevention and control of influenza, recommendations of the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report (MMWR) 2008;57:1–60. [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, Brammer I, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. J.Ameri.Med.Assoc. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 3.Palese P. Influenza: old and new threats. Nat.Med. 2004;10:S82–S87. doi: 10.1038/nm1141. [DOI] [PubMed] [Google Scholar]
- 4.Hoelscher M, Gangappa S, Zhong WM, Jayashankar L, Sambhara S. Vaccines against epidemic and pandemic influenza. Expert Opin. Drug Deliv. 2008;5:1139–1157. doi: 10.1517/17425247.5.10.1139. [DOI] [PubMed] [Google Scholar]
- 5.Belshe RB, Newman FK, Cannon J, Duane C, Treanor J, Van Hoecke C, Howe BJ, Dubin G. Serum antibody responses after intradermal vaccination against influenza. New Engl J Med. 2004;351:2286–2294. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
- 6.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. New Engl J Med. 2004;351:2295–2301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 7.Holland D, Booy R, De Looze F, Eizenberg P, McDonald J, Karrasch J, McKeirnan M, Salem H, Mills G, Reid J, Weber F, Saville M. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: A randomized controlled trial. J. Infect. Dis. 2008;198:650–658. doi: 10.1086/590434. [DOI] [PubMed] [Google Scholar]
- 8.Howard A, Mercer P, Nataraj HC, Kang BC. Bevel-down superior to bevel-up in intradermal skin testing. Ann. Allergy Asthma Immunol. 1997;78:594–596. doi: 10.1016/s1081-1206(10)63222-x. [DOI] [PubMed] [Google Scholar]
- 9.Laurent PE, Bonnet S, Alchas P, Regolini P, Mikszta JA, Pettis R, Harvey NG. Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system. Vaccine. 2007;25:8833–8842. doi: 10.1016/j.vaccine.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Glenn GM, Flyer DC, Ellingsworth LR, Frech SA, Frerichs DM, Seid RC, Yu J. Transcutaneous immunization with heat-labile enterotoxin: development of a needle-free vaccine patch. Expert Rev. Vaccines. 2007;6:809–819. doi: 10.1586/14760584.6.5.809. [DOI] [PubMed] [Google Scholar]
- 11.Babiuk S, Baca-Estrada M, Babiuk LA, Ewen C, Foldvari M. Cutaneous vaccination: the skin as an immunologically active tissue and the challenge of antigen delivery. J. Control. Release. 2000;66:199–214. doi: 10.1016/s0168-3659(99)00274-6. [DOI] [PubMed] [Google Scholar]
- 12.Celluzzi CM, Falo LD. Epidermal dendritic cells induce potent antigen-specific CTL-mediated immunity. J. Invest. Dermatol. 1997;108:716–720. doi: 10.1111/1523-1747.ep12292095. [DOI] [PubMed] [Google Scholar]
- 13.Leroux-Roels I, Vets E, Freese R, Seiberling M, Weber F, Salamand C, Leroux-Roels G. Seasonal influenza vaccine delivered by intradermal microinjection: A randomised controlled safety and immunogenicity trial in adults. Vaccine. 2008;26:6614–6619. doi: 10.1016/j.vaccine.2008.09.078. [DOI] [PubMed] [Google Scholar]
- 14.Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27:454–459. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 15.Mikszta JA, Alarcon JB, Brittingham JM, Sutter DE, Pettis RJ, Harvey NG. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat. Med. 2002;8:415–419. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]
- 16.Fynan EF, Webster RG, Fuller DH, Haynes JR, Santoro JC, Robinson HL. DNA Vaccines - Protective Immunizations by Parenteral, Mucosal, and Gene-Gun Inoculations. Proc. Natl. Acad. Sci. U. S. A. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren S, Li M, Smith JM, DeTolla LJ, Furth PA. Low-volume jet injection for intradermal immunization in rabbits. BMC Biotech. 2002;2:10. doi: 10.1186/1472-6750-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Widera G, Austin M, Rabussay D, Goldbeck C, Barnett SW, Chen MC, Leung L, Otten GR, Thudium K, Selby MJ, Ulmer JB. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J. Immunol. 2000;164:4635–4640. doi: 10.4049/jimmunol.164.9.4635. [DOI] [PubMed] [Google Scholar]
- 19.Bramson J, Dayball K, Evelegh C, Wan YH, Page D, Smith A. Enabling topical immunization via microporation: a novel method for pain-free and needle-free delivery of adenovirus-based vaccines. Gene Ther. 2003;10:251–260. doi: 10.1038/sj.gt.3301886. [DOI] [PubMed] [Google Scholar]
- 20.Garg S, Hoetscher M, Belser JA, Wang C, Jayashankar L, Guo Z, Durland RH, Katz JM, Sambhara S. Needle-free skin patch delivery of a vaccine for a potentially pandemic influenza virus provides protection against lethal challenge in mice. Clin. Vaccine Immunol. 2007;14:926–928. doi: 10.1128/CVI.00450-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirschberg HJHB, de Wijdeven GGPV, Kelder AB, van den Dobbelsteen GPJM, Kerstena GFA. Bioneedles (TM) as vaccine carriers. Vaccine. 2008;26:2389–2397. doi: 10.1016/j.vaccine.2008.02.067. [DOI] [PubMed] [Google Scholar]
- 22.Eriksson E, Yao F, Svensjo T, Winkler T, Slama J, Macklin MD, Andree C, McGregor M, Hinshaw V, Swain WF. In vivo gene transfer to skin and wound by microseeding. J. Surg. Res. 1998;78:85–91. doi: 10.1006/jsre.1998.5325. [DOI] [PubMed] [Google Scholar]
- 23.Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. In: Compans RW, Orenstein WA, editors. Current Topics in Microbiology and Immunology: Vaccines for Pandemic Influenza. Berlin/Heidelberg: Springer-Verlag; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matriano JA, Cormier M, Johnson J, Young WA, Buttery M, Nyam K, Daddona PE. Macroflux® microprojection array patch technology: A new and efficient approach for intracutaneous immunization. Pharm. Res. 2002;19:63–70. doi: 10.1023/a:1013607400040. [DOI] [PubMed] [Google Scholar]
- 25.Widera G, Johnson J, Kim L, Libiran L, Nyam K, Daddona PE, Cormier M. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006;24:1653–1664. doi: 10.1016/j.vaccine.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 26.Koutsonanos DG, Martin MdP, Zarnitsyn VG, Sullivan SP, Compans RW, Prausnitz MR, Skountzou I. Transdermal influenza immunization with vaccine-coated microneedle arrays. PLoS ONE. 2009;4(3):e4773. doi: 10.1371/journal.pone.0004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu QY, Zarnitsyn VG, Ye L, Wen Z, Gao Y, Pan L, Skountzou I, Gill HS, Prausnitz MR, Yang Y, Compans RW. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc. Natl. Acad. Sci. U. S. A. 2009;106:7968–7973. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J. Control. Release. 2007;117:227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leslie SB, Israeli E, Lighthart B, Crowe JH, Crowe LM. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl. Environ. Microbiol. 1995;61:3592–3597. doi: 10.1128/aem.61.10.3592-3597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leslie SB, Teter SA, Crowe LM, Crowe JH. Trehalose lowers membrane phase-transitions in dry yeast-cells. Biochim. Biophys. Acta. 1994;1192:7–13. doi: 10.1016/0005-2736(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 31.Bieganski RM, Fowler A, Morgan JR, Toner M. Stabilization of active recombinant retroviruses in an amorphous dry state with trehalose. Biotech. Progress. 1998;14:615–620. doi: 10.1021/bp980057d. [DOI] [PubMed] [Google Scholar]
- 32.Crowe JH, Crowe LM, Carpenter JF, Wistrom CA. Stabilization of dry phospholipid-bilayers and proteins by sugars. Biochem. J. 1987;242:1–10. doi: 10.1042/bj2420001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun WQ, Leopold AC, Crowe LM, Crowe JH. Stability of dry liposomes in sugar glasses. Biophys. J. 1996;70:1769–1776. doi: 10.1016/S0006-3495(96)79740-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quan FS, Compans RW, Nguyen HH, Kang SM. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J.Virol. 2008;82:1350–1359. doi: 10.1128/JVI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Connolly SA, Lamb RA. Paramyxovirus fusion: Real-time measurement of parainfluenza virus 5 virus-cell fusion. Virology. 2006;355:203–212. doi: 10.1016/j.virol.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 36.Ludwig K, Korte T, Herrmann A. Analysis of delay times of hemagglutinin-mediated fusion between influenza-virus and cell-membranes. Eur. Biophy. J. 1995;24:55–64. doi: 10.1007/BF00211399. [DOI] [PubMed] [Google Scholar]
- 37.Skountzou I, Quan FS, Jacob J, Compans RW, Kang SM. Transcutaneous immunization with inactivated influenza virus induces protective immune responses. Vaccine. 2006;24:6110–6119. doi: 10.1016/j.vaccine.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin. J. Pain. 2008;24:585–594. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vyas GN, Shulman NR. Hemagglutination assay for antigen and antibody associated with viral hepatitis. Science. 1970;170:332–333. doi: 10.1126/science.170.3955.332. [DOI] [PubMed] [Google Scholar]
- 40.Amorij JP, Huckriede A, Wischut J, Frifflink HW, Hinrichs WLJ. Development of stable influenza vaccine powder formulations: Challenges and possibilities. Pharm. Res. 2008;25:1256–1273. doi: 10.1007/s11095-008-9559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hobson D, Curry RL, Beare AS, Wardgard A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J. Hygiene. 1972;70:767–777. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cox RJ, Brokstad KA, Ogra P. Influenza virus: Immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand. J. Immunol. 2004;59:1–15. doi: 10.1111/j.0300-9475.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- 43.Nicholson KG, Wood JM, Zambon M. Influenza. Lancet. 2003;362:1733–1745. doi: 10.1016/S0140-6736(03)14854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Jonge J, Amorij JP, Hinrichs WLJ, Wiischuta J, Huckriedea A, Frijlinkb HW. Inulin sugar glasses preserve the structural integrity and biological activity of influenza virosomes during freeze-drying and storage. Eur. J. Pharm. Sci. 2007;32:33–44. doi: 10.1016/j.ejps.2007.05.112. [DOI] [PubMed] [Google Scholar]
- 45.Allison SD, Molina MDC, Anchordoquy TJ. Stabilization of lipid/DNA complexes during the freezing step of the lyophilization process: the particle isolation hypothesis. Biochim. Biophys. Acta. 2000;1468:127–138. doi: 10.1016/s0005-2736(00)00251-0. [DOI] [PubMed] [Google Scholar]
- 46.Amorij JP, Meulenaar J, Hinrichs WLJ, Stegmann T, Huckriede A, Coenen F, Frijlink HW. Rational design of an influenza subunit vaccine powder with sugar glass technology: Preventing conformational changes of haemagglutinin during freezing and freeze-drying. Vaccine. 2007;25:6447–6457. doi: 10.1016/j.vaccine.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 47.Carpenter JF, Crowe JH. An infrared spectroscopic study of the interactions of carbohydrates with dried proteins. Biochemistry. 1989;28:3916–3922. doi: 10.1021/bi00435a044. [DOI] [PubMed] [Google Scholar]
- 48.Carpenter JF, Martin B, Crowe LM, Crowe JH. Stabilization of phosphofructokinase during air-drying with sugars and sugar transition-metal mixtures. Cryobiology. 1987;24:455–464. doi: 10.1016/0011-2240(87)90049-6. [DOI] [PubMed] [Google Scholar]
- 49.Steinhauer DA. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology. 1999;258:1–20. doi: 10.1006/viro.1999.9716. [DOI] [PubMed] [Google Scholar]
- 50.Azzi L, El-Alfy M, Martel C, Labrie F. Gender differences in mouse skin morphology and specific effects of sex steroids and dehydroepiandrosterone. J. Invest. Dermatol. 2005;124:22–27. doi: 10.1111/j.0022-202X.2004.23545.x. [DOI] [PubMed] [Google Scholar]