SUMMARY
Having the ability to coordinate the behavior of stem cells to induce regeneration of specific large-scale structures would have far reaching consequences in the treatment of degenerative diseases, acute injury, and aging. Thus, identifying and learning to manipulate the sequential steps that determine the fate of new tissue within the overall morphogenetic program of the organism is fundamental. We identified novel early signals, mediated by the central nervous system and 3 innexin proteins, which determine the fate and axial polarity of regenerated tissue in planarians. Modulation of gap junction-dependent and neural signals specifically induces ectopic anterior regeneration blastemas in posterior and lateral wounds. These ectopic anterior blastemas differentiate new brains that establish permanent primary axes re-established during subsequent rounds of unperturbed regeneration. These data reveal powerful novel controls of pattern formation and suggest a constructive model linking nervous inputs and polarity determination in early stages of regeneration.
Keywords: gap junctions, neural signals, regeneration, polarity, planaria
INTRODUCTION
Regeneration, or the ability to functionally restore structures lost to injury or disease, is widely distributed throughout metazoan phyla (Brockes and Kumar, 2008). A fundamental aspect of regeneration is the ability to sense the loss of specific components and activate cellular mechanisms that integrate new tissues within the organism. Understanding the signals that precisely orchestrate this morphogenetic process is a key prerequisite for unlocking the full potential of regenerative medicine.
Planarians possess an accessible adult somatic stem cell population (neoblasts) that maintains differentiated tissues during physiological cell turnover. Upon amputation, neoblasts proliferate and migrate to restore missing parts by forming a regeneration blastema that differentiates into missing structures (Reddien and Sánchez Alvarado, 2004). Individual cell behavior must be continuously coordinated with the large-scale morphogenetic state of the organism. This requires the stem cells to integrate information about the location/position of the damage (short-range) and recognition of remaining pre-existing tissue within the whole fragment (long-range). This type of modulation (by signals from the microenvironment and nervous/immune system activity) occurs in vivo to regulate stem cell behavior in a variety of species and tissues (Jones and Wagers, 2008; Mendez-Ferrer et al., 2008). The planarian is thus a highly tractable system within which to dissect signaling processes of very broad relevance to regenerative patterning.
How does the tissue remaining after an injury know what is missing and thus decide what must be rebuilt (e.g., head or tail)? Early molecular events during planarian regeneration are mostly unknown but the initial processes involve wound closure, dorsal-ventral interactions, and neoblast proliferation preceding blastema formation (Reddien and Sánchez Alvarado, 2004). Establishment of anterior-posterior (A/P) polarity during regeneration is affected by modulation of the Wnt signaling pathway (Adell et al., 2009; Gurley et al., 2008; Iglesias et al., 2008; Petersen and Reddien, 2008) or treatment with gap junction inhibitors, chick embryo extracts, colchicine, chloramphenicol, or etionine (Brøndsted, 1969; Nogi and Levin, 2005; Rodriguez and Flickinger, 1971). However, no existing molecular model integrates these data as a constructive algorithm for rebuilding large-scale morphology after amputation (Meinhardt, 2009). This is of particular relevance as the influence of systemic signals (e.g.: immune, nervous, and metabolic) is now beginning to be appreciated as an important regulator of stem cell behavior (Hsu and Drummond-Barbosa, 2009; Spiegel et al., 2008). Critical fate and axial polarity decisions around the injury site take place during the first day of regeneration and involve signals from both local and very distant tissue. Thus, molecules associated with rapid, long-range transmission of information are likely to be involved.
Gap junction (GJ) proteins are plasma membrane channels involved in direct cell-cell communication via physiological signals, regulating embryonic development, neoplasm, and tissue function in diverse contexts (Levin, 2007a; Phelan, 2005). GJ protein-mediated signals are known to regulate behavior of embryonic and adult stem cells (Wong et al., 2008) probably by modulating the exchange of information between undifferentiated cells and their surrounding microenvironment (Elias and Kriegstein, 2008; Oviedo and Levin, 2007b). Such short-range stem cell regulation by GJ proteins is essential for regeneration of complex structures in both vertebrates and invertebrates (Hoptak-Solga et al., 2008; Oviedo and Levin, 2007b).
The classical literature also suggests the nervous system as an essential component of vertebrate and invertebrate regeneration (Brøndsted, 1969; Singer, 1952). Despite recent advances (Kumar et al., 2007), the molecular connection and reciprocal influence between nerves and regeneration remains poorly understood. The planarian central nervous system (CNS) consists of a brain and a pair of longitudinal ventral nerve cords (VNC) running along the A/P axis (Supplemental Figure 1A) (Agata and Umesono, 2008; Cebrià, 2008). The planarian CNS has been suggested to control morphogenesis of posterior areas (Best et al., 1969; Flickinger and Coward, 1962; Lender, 1960). However, the mechanisms of such signaling are unknown.
Understanding the flow of long-range patterning information during regenerative morphogenesis is crucial for developing rational strategies to control stem cell behavior in vivo. First, while it was recently suggested that gap junctional communication (GJC) may be required for A/P patterning in planaria (Nogi and Levin, 2005), it is completely unknown which specific innexins (invertebrate GJ proteins) or mechanisms are involved in this process. Moreover, it is not known what tissue or structure transmits morphogenetic information from the organism to the neoblasts during regeneration. Our data revealed the existence and properties of instructive, long-range signals from the central nervous system that control pattern formation, and molecularly identified the specific GJ proteins that mediate this process. Strikingly, large-scale alterations in the animals’ body-plan, induced by short-term physiological modulation, persist across multiple cycles of regeneration, uncovering a mechanism by which physiological signals re-specify anatomy. We synthesize these data into a constructive, binary model integrating neural inputs and fate determination in early stages of regeneration.
MATERIAL AND METHODS
Planarian culture
A clonal strain of Dugesia japonica (GI) were kept and maintained as in (Oviedo et al., 2008a).
Antibody labeling and image collection
Planarians were processed for immunostaining as in (Oviedo et al 2008b). Primary antibody dilution: α-phosphorylated histone H3 (Upstate) 1:250, α-arrestin (gift from K. Watanabe) 1:10000, and synapsin 1:50 (Developmental Studies Hybridoma Bank). Incubation with 2ry antibody was performed overnight in goat anti-mouse Alexa488, 1:400. Representative images for each condition were processed as previously described (Oviedo and Levin, 2007b).
Whole-mount in situ hybridization
Animals were processed for whole-mount in situ hybridization as in (Nogi and Levin, 2005).
Drug treatment and amputations
Octanol solution (8-OH) was prepared by directly diluting 10μl of 1-Octanol (Sigma-Aldrich) into 500ml of commercial natural spring water (PS, Poland Spring Water), resulting in a final concentration of 127μM. Worm fragments were transferred into drug solutions immediately after amputation in PS.
dsRNA synthesis and microinjections
dsRNA synthesis and microinjections were performed as in (Oviedo et al., 2008a). Microinjection schedules extensively optimized until reproducible results were obtained and injections were performed as in (Oviedo et al., 2008b). Triple RNAi worms were injected with a mix (1:1) of Dj-Inx-5 + −13 dsRNA for three consecutive days followed by rest of 14 days and then two consecutive days of injections with Dj-Inx-12 dsRNA. For Dj-ßcatenin-B(RNAi), worms were injected for three consecutive days and amputations were performed one week after first injection.
Octanol incorporation in planarian tissue
Levels of octanol in planarian tissues were measured by gas chromatography mass spectrometry as previously described (Zada et al., 2002).
Mutagenesis analysis
Mutagenesis analysis was performed by luminometry as previously described (Min et al., 1999).
RESULTS
Long-range information is provided to wounded areas through the ventral nerve cord and gap junctions
Though not previously revealed by any pharmacological treatment (Brøndsted, 1969; Nogi and Levin, 2005) or genetic approaches (Adell et al., 2009; Gurley et al., 2008; Iglesias et al., 2008; Petersen and Reddien, 2008), non-local patterning cues have been long sought for building theoretical models of morphogenesis (Meinhardt, 2009) because neighboring cells adopt radically different fates (head or tail) after surgical bisection. We began by pharmacological loss-of-function studies targeting gap junctional communication as a primary candidate for long-range patterning signals during establishment of regenerative polarity.
GJ blockers have been extensively used to characterize functional roles of gap junctional signaling in the absence of general disruption of membrane integrity of cell housekeeping functions (Abdelmohsen et al., 2005; Burnside and Collas, 2002; Jin et al., 2008; Levin and Mercola, 1998; Levin and Mercola, 1999; Levin and Mercola, 2000; Schilling et al., 2008). Among n-alcohols, heptanol and octanol are best known for their potent and specific inhibition of GJC in vertebrate and invertebrate models (Adler and Woodruff, 2000; Anderson and Woodruff, 2001; Brooks and Woodruff, 2004; Chanson et al., 1989; Christ et al., 1999; Ehrlich and Diez, 2003; Garcia-Dorado et al., 1997; Mire et al., 2000; Momose-Sato et al., 2005; Waksmonski and Woodruff, 2002; Weingart and Bukauskas, 1998). In planarians, treatment with heptanol or octanol, but not hexanol (which does not block GJC), leads to a consistent alteration of A/P polarity during regeneration, suggesting that the effects of these compounds on planarian regeneration correlate with their ability to block GJs ((Nogi and Levin, 2005), Supplemental Figure 1C). By optimizing an octanol dose that induced consistent alterations of A/P polarity during regeneration but did not shut down all gap junctions simultaneously, we avoided generalized toxicity and alteration of neoblast maintenance (Oviedo and Levin, 2007b), allowing us to specifically investigate patterning phenotypes in the context of normal neoblasts (Supplemental Figure 1B). Because of the unique suitability of pharmacological compounds for testing spatio-temporal properties, we used this loss-of-function reagent for the initial characterization of GJC’s role in regeneration; molecular validation using RNAi (which cannot be targeted or turned off in timing experiments) is presented further below.
A series of transverse amputations, resulting in 2 blastemas, were performed at different levels along the A/P axis followed by octanol exposure (Figure 1A and 1B). Both untreated and octanol-exposed amputated fragments developed normal anterior blastemas that differentiated into heads. However, fragments treated with octanol often formed ectopic anterior blastemas at posterior-facing wounds that developed into heads, resulting in animals with heads at both ends of the A/P axis (i.e.: bipolar head regeneration). This abnormality in polarity was never observed in untreated animals, and the fragments were much bigger than the thin slices that have been reported to sometimes exhibit A/P abnormalities. Furthermore, susceptibility to developing the bipolar-headed phenotype gradually increased as the plane of amputation (posterior wound) was moved towards the posterior end, reaching maximum effect (~100%) at the post-pharyngeal area (Figure 1A). Thus, dependence of the blastema upon GJ-mediated signals exists as a gradient along the A/P axis, being highest at the post-pharyngeal region.
FIGURE 1. Neural cues mediate polarity during regeneration.
(A) Schematic of transverse amputations in the presence of octanol. The incidence of bipolar head phenotype gradually increases toward posterior areas. Inset is representative of bipolar-head regenerate (white arrows indicate heads). Red dotted lines indicate level of amputation. Data are presented as mean +/− confidence intervals (95%). (B) Schematic representation of transverse amputations along the A/P axis to obtain fragments with a pre-existing head (left). Fragments generated after amputations exhibited very few bipolar-head regenerate. (C) Planarians were dissected in different ways (illustrative representation is shown for each case within columns) and the regeneration pattern was recorded after 2 weeks of regeneration. Animals reestablishing the original pattern were considered normal, while worms developing abnormalities were classified into seven categories by macroscopic observations, A/P polarity, and CNS morphological abnormalities. Seven abnormalities were: middle head; side head; bipolar + middle head; side protrusion; bipolar head and double side head (color-coded at the top of the graph). Percentage of animals regenerating each pattern is displayed along with the number of animals (within parentheses) assayed in each condition (Roman numbers). Untreated animals always regenerated normally (not shown for simplicity). Most cases (3/4) where dissections did not disrupt VNC integrity at pre-pharyngeal level regenerated 100% normal animals (conditions I, II, VII and X). Conversely, almost all cases (7/8) where VNC integrity was disrupted in pre or post-pharyngeal area led to regeneration of abnormalities characterized by alterations in CNS patterning and polarity (conditions III-IX). These results suggest that VNC integrity and GJC play important role in regenerative patterning and polarity. Since all animals regenerated tissues and developed blastemas, treatment with GJ inhibitor does not alter normal response to wound damage but rather instructively influences the identity of the newly formed tissue. Importantly only animals with disrupted VNC integrity and octanol treated led to novel phenotypes (D) characterized by multiple heads (white arrows) and pharynxes (gray arrows). Representative images of immunostaining with anti-synapsin antibody (green signal) over a pseudocolored red background are shown. In all cases original anterior end is to the top. Bars represent 200μm.
The planarian brain is capable of preventing regeneration of secondary head within the same animal (Flickinger and Coward, 1962; Lender, 1960). Our data, showing that the head fragment produced the fewest bipolar-headed animals, support this model. However, the mechanism of this inhibition is not known. We sought to determine whether pre-existing head tissues in regenerating fragments could prevent regeneration of an ectopic head in the presence of GJC inhibitor. Cuts along the A/P axis of octanol-treated animals, always including the original head region (Figure 1B), resulted in >95% single-head (normal) animals. Although posterior regenerating fragments require GJs for correct assignment of blastema identity, GJ inhibition in fragments containing brain does not result in bipolar head regeneration. Thus, we sought an additional mechanism by which the blastema can sense the presence of the head at long distances.
Neural projections of the planarian CNS reach all parts of the worm (Cebrià, 2008). To test the hypothesis that the CNS may mediate long-range signaling, we made cuts that did or did not disrupt ventral nerve cord (VNC) continuity along the A/P axis (Figure 1C, specificity of cuts confirmed in Supplemental Figure S2). All (octanol treated and untreated) animals formed blastemas, suggesting that damage recognition and basic wound healing were not altered by this procedure. Strikingly, VNC-interrupted worms treated with octanol developed multiple A/P axes (Figure 1C and 1D). In all (3/3 conditions) where tissue removal did not affect VNC contiguity, regeneration proceeded normally (classes I, II and X in Fig. 1C). However, when amputation produced disruption of VNC integrity (7/8 conditions), regeneration of ectopic heads, pharynxes, and abnormal protrusions occurred (Figure 1C and 1D), revealing a new set of 6 phenotypes including some with multiple A/P axes (Figure 1D). For a direct comparison of the effects upon regeneration pattern, please compare classes II, VI and VIII (Figure 1C). Thus, disruption of VNC continuity, together with GJ blockade, result in the loss of correct blastemal A/P identity (formation of ectopic heads) in fragments containing an intact brain.
These results show that sensing the presence of brain tissue at long distances by the blastema can occur if the VNCs is un-interrupted or if GJC is functional. Taken together, these data reveal two parallel pathways by which a blastema surveys the remaining anatomy within the pre-existing tissue for instructive cues during regenerative morphogenesis.
CNS- and GJ-mediated inputs function during very early regeneration
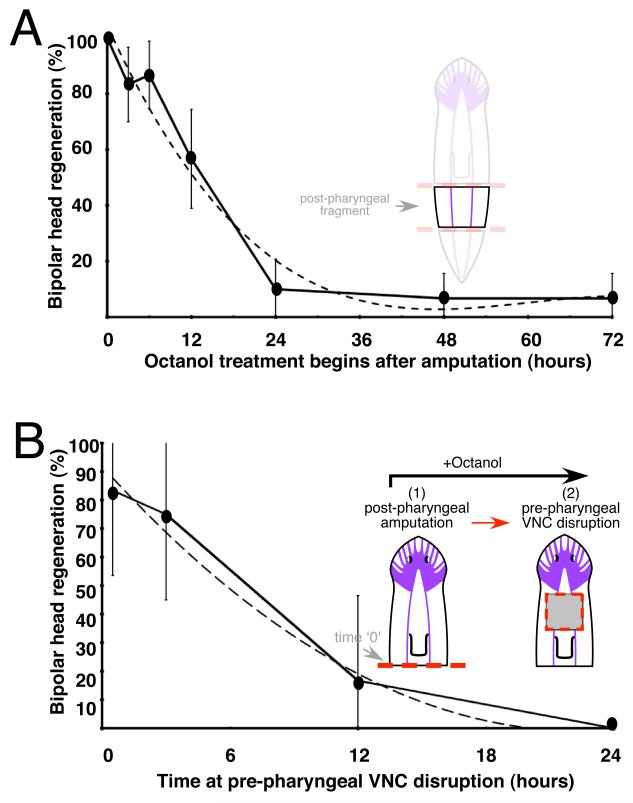
To characterize the temporal properties of this novel signaling system, we performed experiments combining disruptions of GJ signaling (octanol) and VNC continuity (surgical cuts) at specific time points during regeneration (Figure 2). Uncoupling GJC with octanol is known to happen very fast (< 1 min after exposure) and the effects are reversible after the reagent is removed from the media (Chanson et al., 1989). Analyses of the incidence of A/P polarity duplications were performed in two different fragments (with or without pre-existing head) (Figure 2). Regenerating tissue without head (post-pharyngeal fragment) was treated with GJ inhibitor at different time-points after amputation (Figure 2A). Starting the exposure at progressively later intervals is a technique that does not rely on drug wash-out, and revealed that the most significant effect results from starting GJ blockade within the first 3-6 hours post-amputation. The effect on A/P patterning dropped significantly (<60%) for treatments beginning >12 hours of regeneration, and reached insignificant levels thereafter. We conclude that GJC-mediated signals are fundamental for establishment of polarity within post-pharyngeal area, and that the primary role of GJ-mediated information occurs within 12 hours of amputation.
FIGURE 2. CNS-mediated inputs and GJC are required early during phases of regeneration.
(A) The percentage of bipolar-head regeneration was recorded from post-pharyngeal fragments in which octanol treatment began at different time points post-amputation. The incidence of bipolar-head phenotype was sharply reduced when GJ blocker (octanol) treatment began a few hours post-amputation, suggesting that critical decisions to identify missing parts and set A/P polarity take place early (within first 3-6 hours) during regeneration. (B) Planarians were amputated at post-pharyngeal level, treated with octanol, and subjected to disruption of VNC at anterior areas (red dotted line in pre-pharyngeal area) at different time points after initial post-pharyngeal cut (time 0). Significant A/P duplications were observed when VNC contiguity was disrupted prior to 12 hours after amputation. Numbers in parenthesis indicate the sequence of amputations, being first post-pharyngeal and then VNC disruption at pre-pharyngeal level; all animals were treated with octanol. For each time point n≥ 10 animals. In all cases data are represented as mean +/− confidence intervals (95%) and curve fitting is shown with dotted line.
We next investigated the temporal properties of GJ/VNC-mediated signals during regeneration of fragments with a pre-existing head (Figure 2B). Planarians were treated with octanol and amputated at post-pharyngeal level (time 0), followed by VNC disruption at different time points. These experiments revealed a dramatic decrease (4-fold) in the incidence of A/P defects if the VNC was disrupted after the first 3 hours post-amputation. These data suggest that information provided to the blastema via the CNS (existence of the head at long range, indicating a tail fate for new tissue) is largely transferred very early after injury (~6 hours) and is consistent with timeframe of CNS mediated inputs in regenerating post-pharyngeal fragments shown in Figure 2A.
Innexin genes (Dj-Inx-5, −12 and −13) mediate information crucial for morphogenesis and polarity determination during regeneration and tissue maintenance
Although previous work demonstrated planarian innexins to be important for stem cell function and blastema formation (Oviedo and Levin, 2007b), it is not known which (or how many) of the dozen innexins actually mediate instructive A/P patterning information during regeneration. Compensation among GJ protein family members has made it difficult to identify phenotypes associated with individual connexins or innexins in mammalian models (Elias and Kriegstein, 2008; Levin, 2007a) and few studies have targeted more than one GJ gene at the same time. We used gene-specific knockdown to abolish the expression of multiple innexin genes simultaneously. Our microinjection strategy involved more than 600 animals and targeted, via RNA interference (RNAi), up to four innexins simultaneously (Supplemental Table S1). No phenotype was found with single RNAi of CNS-associated innexins. Permutations with simultaneous down-regulation by RNAi of two innexins revealed that 4 of 12 (4/12) combinations led to abnormal behavior characterized by abnormal response to light, locomotion problems, and ruffled edges and regeneration-associated phenotypes. All experiments (n=5) in which three innexins were down-regulated simultaneously by RNAi consistently led to such phenotypes in intact and regenerating animals (Supplemental Table S1). We therefore focused on characterization of simultaneous RNAi of three innexins, as macroscopic phenotypic abnormalities were consistent with those induced by octanol treatment.
Triple RNAi of Dj-Inx-5, −13, and −12 led to the most consistent and strong phenotypes (Supplemental Table S1). Endogenous expression of these 3 transcripts is mostly found overlapping within the CNS as well as in sub-epithelial cell populations throughout the animal, and is up-regulated within regeneration blastemas ((Nogi and Levin 2005), Supplemental Figure S3). Sequential down-regulation of these genes was obtained from an optimized double-stranded (dsRNA) microinjection schedule (five dsRNA microinjections over two weeks, Figure 3A). dsRNA microinjections of DJ-Inx-5 and 13 were administered together for 3 consecutive days followed by a resting period of two weeks and two more microinjections of dsRNA of DJ-Inx-12 [i.e.: Dj-Inx-5+13, −12(RNAi)]. About 30 days after the first dsRNA microinjection, intact planarians showed behavioral changes (see Supplemental Movie 1) characterized by slow movements and random contractions (~95%, n=137) similar to those observed in intact animals after octanol treatment.
FIGURE 3. Loss-of-function by RNAi of three innexin genes led to A/P polarity phenotype during homeostasis and regeneration.
(A) Microinjection schedule of double-stranded RNA (dsRNA) in intact planarians. A total of five injections (arrows between green squares) were performed over 19 days. Injecting a mix (1:1) of Dj-Inx-5 + 13 dsRNA for 3 consecutive days and 2 injections of Dj-Inx-12 ds-RNA 2 weeks after first injections led to strong and reproducible phenotypes ~14 days after last injection. Control animals were always run in parallel and injected with water under same schedule. (B) Representative phenotypes following 30-35 days since first injection. Conditions: control (water injected) and Dj-Inx-5 + 13, −12(RNAi). Control animals (left column) did not display any behavioral or anatomical abnormalities as assayed by markers of CNS (anti-synapsin antibody), mitotic cells (anti-H3P antibody), proliferative neoblasts (Dj-Inx-11 antisense probe), and digestive system (Dj-Inx-7 antisense probe). In contrast, animals subjected to Dj-Inx-5 + 13, −12(RNAi) displayed obvious behavioral and morphological changes (right column). Random contractions, ectopic photoreceptor pigmentation (yellow arrow), and extra pharynxes (blue arrows) were evident, especially in post-pharyngeal areas. Ectopic pharynxes, brain tissue (white arrows) and striking alterations in the distribution of neoblasts and the digestive system were observed. Remarkably, the orientation of ectopic brain and pharynx were sometimes reversed with respect to the original A/P axis, and the distribution of neoblasts and digestive system adopted anatomical changes consistent with the new structures developed (e.g.: no mitotic activity surrounding brain and pharynx and formation of primary intestinal branch in post pharyngeal area followed by posterior bifurcation around ectopic pharynx). Bar is 500 μm. (C) Microinjection dsRNA schedule for worms amputated (red arrow) after ~25-28 days of first injection. (D) To analyze the roles of Dj-Inx-5 + 13, −12(RNAi) during regeneration, injected animals were amputated at different levels along the A/P axis producing 5 fragments. Pharyngeal and post-pharyngeal fragments regenerated animals with polarity problems (inset representative picture). Data are represented as mean +/− confidence intervals (95%) and red dotted line indicates plane of amputation. (E) Summary illustration of phenotypes obtained in intact and regenerating of Dj-Inx-5 + 13, −12(RNAi) animals. In both cases, polarity problems and ectopic organs appeared (as after pharmacological inhibition of GJs). The original anterior ends are shown to the left.
Molecular analyses with markers of CNS, neoblasts, and intestine clearly demonstrated the presence of ectopic brain tissue, multiple pharynxes, and altered distribution of neoblasts and digestive system (Figure 3B). Ectopic brain and photoreceptors generally developed in the post-pharyngeal area. Remarkably, in all cases (n=14), two new pharynxes appeared, and were aligned correctly with respect to the new brain. One of the two ectopic pharynxes showed a characteristic inversion of A/P polarity with respect to the original pre-existing axis, indicating that not only ectopic pharynxes, but also changes in polarity, were induced. All of these morphogenetic changes occurred in intact animals (i.e., no amputation-induced regeneration was involved), suggesting that this signaling system is required for complex tissue maintenance. We conclude that the down-regulation of 3 specific innexins leads to the development of ectopic primary axes and organs with consistent internal A/P polarity but exhibiting a failure to conform to the normal (1-headed) pattern of the organism. This is consistent with the phenotype observed in regenerating fragments after pharmacological blockade of GJ, confirming the importance of GJs in the establishment of large-scale A/P patterning and identifying a specific subset of the innexin family involved in this process.
In control animals, proliferative neoblasts are scattered throughout the body but absent in tissue in front of the photoreceptors and around the pharynx (Newmark and Sanchez Alvarado, 2000). We found that this arrangement was consistent in Dj-Inx-5+13,+12(RNAi) worms; however, the presence of two ectopic brains and pharynxes revealed additional areas without mitotic activity surrounding them (Figure 3B). The normal pattern of the digestive system was also dramatically altered, exhibiting fusion of posterior branches (post-pharyngeal area) followed by bifurcation around the new pharynxes, suggesting that modifications in this tissue also resulted from signals from the new morphogenetic arrangement during tissue maintenance. Further, amputation of Dj-Inx-5+13,+12(RNAi) animals (Figure 3C,D) led to worms with inverted A/P axis and bipolar head after regeneration from pharyngeal (20%, n=21) and post-pharyngeal (80%, n=18) fragments, which is anatomically consistent with pharmacological inhibition of GJ or RNAi of these innexins in intact animals. These results demonstrate that the GJ-dependent signaling system is required for proper A/P establishment during regeneration and firmly implicate three specific CNS-associated GJ genes (DJ-Inx-5, −12 and −13) as mediating important morphogenetic signals and polarity determination during tissue maintenance and regeneration (Figure 3E).
Polarity in regenerating lateral wounds is also controlled by CNS/GJ signaling
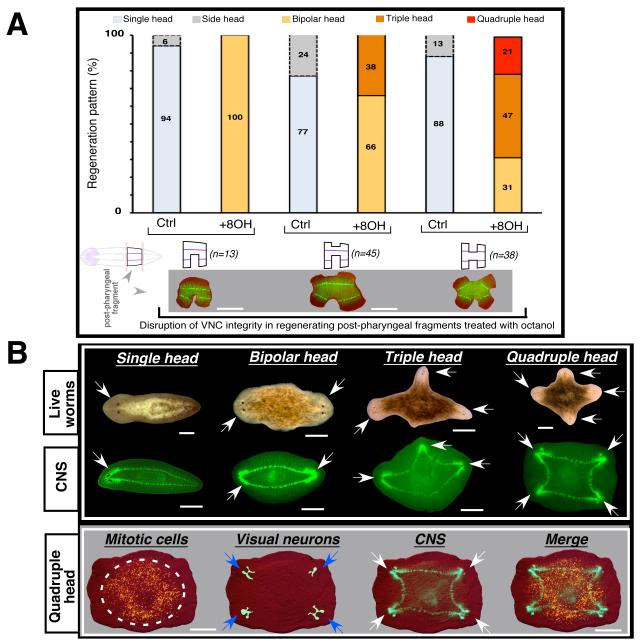
We asked whether information provided by the CNS was also used by posterior-facing wounds. Post-pharyngeal fragments were treated with octanol and single or multiple cuts were produced laterally so as to disrupt VNC integrity or leave it intact (Supplemental Figure S4A). Each amputation resulted in blastema formation; unexpectedly, all of these organisms (n>100) exhibited behavioral changes (i.e.: multiple sides pulling in different directions) and the appearance of photoreceptor pigmentation in two, three, or four different blastemas within the same fragment, suggesting that the alterations of normal behavior result from ectopic anteriorization of multiple areas. Indeed, these animals ultimately formed triple or quadruple heads (Figure 4A). These striking morphogenetic changes were more frequent in those fragments with simultaneous lateral cuts (Figure 4A). Although the majority of untreated post-pharyngeal regenerating fragments formed single headed worms (77-94%), in some cases (6-24%) duplication of head structures (side head) were observed (Figure 4A). Interestingly, none of these resulting animals displayed reversal of polarity (see side head in Figure 1D). These results in both treated and untreated post-pharyngeal fragments are consistent with our observations of two different mechanisms involving GJ proteins and the ventral cord respectively.
FIGURE 4. GJ-mediated and neural information prevents ectopic and permanent axes formation in lateral and posterior-facing wounds.
(A) Post-pharyngeal fragments were cut laterally (once or twice simultaneously) in the presence of octanol or not (control, untreated). In all cases (n>90) treated animals developed A/P polarity problems; when both lateral sides were simultaneously cut (schematics and synaptotagmin immunostaining are shown under graph), the fragment produced bipolar or triple heads. Additionally, lateral amputations disrupting the VNC led some animals (~20%, n=38) to produce anterior structures in all 4 blastemas. Note that untreated (control) animals mostly regenerated single headed worms; in some cases where head duplication was observed, this never affected the polarity of the animal. (B) Representative images of animals with single, bipolar, triple, and quadruple heads (white arrows). Triple immunostaining (CNS, mitotic cells, and visual neurons) in quadruple-headed planarians revealed that additional brains were linked by common VNCs (n>10 each). Further, immunostaining in with anti-H3P antibody (recognizes mitotic cells) indicated that mitotic cells were restricted to the new pre-pharyngeal area of each new axis. Visual neurons connecting photoreceptors with brain (assayed by anti-VC-1 antibody) also differentiated within each new brain. Bars are 500 μm.
Analysis with molecular markers revealed differentiation of ectopic brains and pharynxes, and changes in neoblast distribution and digestive system morphology (Figure 4B and Supplemental Figure S4B, C). The number of ectopic organs and their orientation was always consistent with the number of heads that establishes new A/P axes, suggesting that similar key morphogenetic regulation observed during regeneration of single animals occurred when multiple axes formed in the same body. Taken together, these results indicate that CNS/GJ-mediated signaling supplies crucial cues to both posterior and lateral wounds, informing blastemas about the presence of anterior structures (brain) within the pre-existing tissue and during early stages of regeneration.
Morphogenetic changes persist for several rounds of regeneration after GJ inhibition
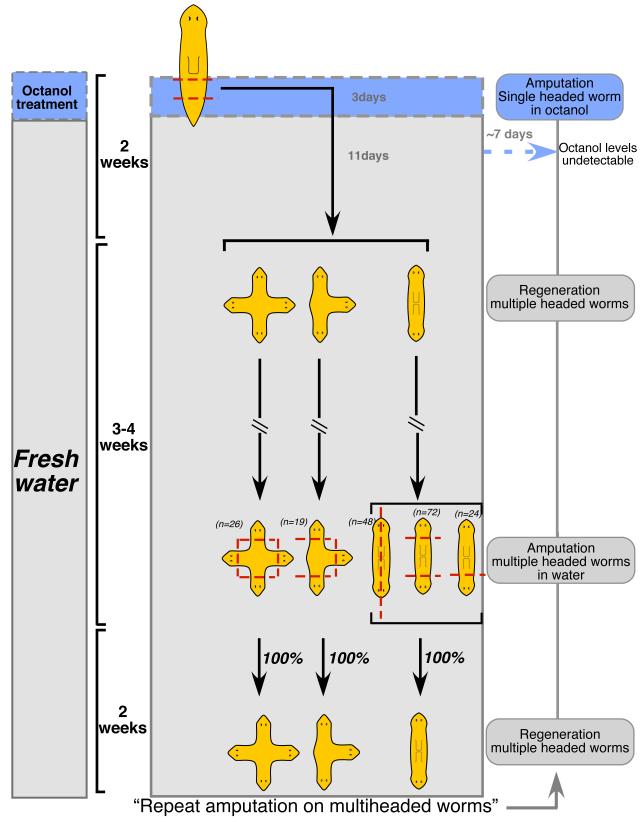
Animals with bipolar, triple, and quadruple heads are viable, providing a unique opportunity to ask whether it is possible to permanently re-set the target morphology (i.e., the complex 3-dimensional pattern that is recreated after injury and determines the termination point of regenerative processes). While it has been suggested that induction of the head could serve as a permanent organizing center, no systematic analysis of this type of signaling has been published. We performed 2 types of amputations on multi-headed animals without further octanol treatment: (i) removing one, two, three, or four heads simultaneously (regeneration of multiple anterior wounds), and (ii) longitudinal cut along the A/P axis providing a long regenerative surface (Figure 5 and Supplemental Figure S5). In all cases (>200 amputations) involving anterior and longitudinal wounds, the new (ectopic head) state of the worm was regenerated.
FIGURE 5. Morphogenetic changes persist for several rounds of regeneration in absence of GJ inhibitor.
Schematic illustrating that short treatment with octanol (3 days, blue area) is able to induce morphogenetic changes that persist for weeks, and across multiple rounds of regeneration. Multi-headed worms were obtained from post-pharyngeal regenerating fragments amputated in presence of octanol. The period of time in which animals were in fresh water is depicted with gray background. About one month after first amputation, multiheaded worms were amputated in different planes (transversally or longitudinally, dotted lines) along the A/P axis in plain water (in absence of octanol). Transverse amputations produced single or multiple simultaneous decapitations and longitudinal cuts divided bipolar worms in two mirror images fragments. In all cases, the ectopic heads were regenerated reconstituting multiple headed worms; from longitudinal cuts, two double-headed worms were formed. Total number of decapitations are represented within parentheses. This procedure was repeated two more times and morphogenetic changes persisted (not shown).
The use of a pharmacological GJ blocker enabled time-limited exposure experiments, which are not feasible with RNAi. Two more rounds of re-amputation of octanol-induced multiple headed animals were performed in plain water (no further octanol exposure). We used gas chromatography mass spectrometry (GC-MS) to directly assay the presence of octanol in worm tissues after the initial exposure (Supplemental Figure S6). Octanol levels in worm tissues decreased rapidly, and reached undetectable levels (orders of magnitude below those needed to induce the phenotypes), within mere hours after removing the animal from octanol-containing media. Strikingly, these multiple-headed planarians re-created the altered axial configuration of the animal (Figure S5A) at each round of amputation, consistent with permanent establishment of new axes by a one-time inhibition of GJ-dependent signals (Figure 5). The amputations were carried out 3-6 weeks after octanol removal, demonstrating that altered A/P organization was re-created by the blastemas at a time long after the last detectable traces of blocker compound left the animal. Thus, once altered by an early, temporary blockade of GJ-dependent signals, the new large-scale axial morphology persists through further rounds of regeneration.
We next asked whether this permanent change of large-scale morphology in the worms was likely due to alteration of DNA sequence by octanol. A standard mutagenicity assay (Min et al., 1999) indicated that the octanol concentration used in all experiments was not mutagenic (Supplemental Figure S7), suggesting strongly that the observed long-term effects could not have been due to changes in DNA. The above data reveal a remarkable phenomenon: a brief inhibition of a signaling system (GJC) results in the alteration of A/P polarity that is re-asserted upon subsequent episodes of regeneration weeks after the GJ inhibitor is removed. These data identify a novel physiological system that permanently alters the target morphology that guides (must be restored by) regeneration.
DISCUSSION
In order to re-establish pre-existing morphology, regenerating fragments require that the wound site have access to 2 pieces of information: what shape is the animal supposed to be “target morphology” (the morphology that must be achieved before regeneration can terminate), and what shape it is now (what has been removed by injury and what’s left). Our data address both components, revealing novel molecular pathways of remote sensing of the presence of head tissues, and illustrating how target morphology can be re-set by transient modulation of physiological signals.
Central nervous system and gap junctions direct the establishment of anterior-posterior polarity
Correct morphogenesis (avoiding ectopic head formation) requires either the presence of an un-interrupted ventral nerve cord or normal gap-junctional communication. Only when both are inhibited do ectopic heads appear (despite the presence of brain tissue within the regenerating fragment), suggesting that blastemas receive information via at least two complementary pathways. The presence of overlapping, complementary pathways are consistent with the remarkably robust regenerative abilities in this model.
What instruction is being passed to morphogenetic organizers such as the blastema during regeneration? The data suggest that these long-range pathways are signaling the presence or nascent formation (as a very early event during regeneration) of head elsewhere in the animal (Lender, 1960). Both posterior and side wounds make use of this information very early during the regenerative process; this is reasonable as decisions of fundamental identity needs to be made in the blastema before significant morphogenesis occurs. Previous work in planarians (Cebria et al., 2002) suggested that FGF signaling may participate in a brain-inducing circuit. However, this phenotype has not led to insight into several of the issues addressed here: polarity establishment, time-course of brain signaling to adjacent tissues, and long-range signaling between brain and other regions. While FGF-related signals seem to restrict brain to anterior areas, they do not affect polarity of any organ as does GJ blockade.
Our data also illustrate a link between position along the A/P axis and polarity (direction). Posterior areas have higher tendency to develop A/P polarity problems when GJ-dependent signals are blocked, revealing that the degree of dependence of a blastema’s head/tail identity upon GJ signals is a function of position along the A/P axis. A gradient has not yet been observed in either pharmacological treatments or genetic manipulations. This provides a tractable entrypoint into the mechanisms that link position to direction along a major body axis – a key puzzle in morphogenesis of many structures (Aw and Levin, 2009; French et al., 1976).
Our results suggest a model (Fig. 6) for determination of blastema identity. The default identity for wounded surfaces is “head formation”. In the absence of specific signals, informing instructing the neoblasts that a head already exists elsewhere, information reaches the blastema via 2 routes: through the VNC, and innexin proteins. The latter is most likely a long-range gap-junctional transfer of small molecule signals as has been shown in other patterning systems (Levin, 2007a; Levin and Mercola, 1999).
FIGURE 6. Schematic model of GJ-mediated and neural signals during regeneration.
(A) Post-pharyngeal amputation generates a regenerating worm with pre-existing head and a posterior wound. Our data suggest that long-range signals –informing about the presence of a head within the fragment-travel from anterior (brain in yellow) to the posterior wound (white arrowheads) through two pathways: ventral nerve (VNC, red line) and GJ-mediated signals associated with VNC (blue line) and parenchymatic cells (light blue background). In all, untreated animals or animals exposed to GJ blocker (octanol) and animals with VNC disruption regeneration of the missing posterior area is recreated without polarity problems. However, in animals where both octanol treatment and VNC disruption take place, signals coming from anterior areas are disrupted, leading to abnormalities in polarity (bipolar animals). (B) Blastema fate determination in regenerating post-pharyngeal fragments with anterior and posterior-facing wounds. Instructive signals from anterior wound travel to posterior wound through the same pathways as in “A” but in this case the information carried is to inform about the anterior blastema formation that instruct neoblasts to form a posterior blastema. In animals exposed to octanol this information is altered and animals regenerate bipolar heads. In the case of VNC disruption alone most animals regenerate without problems. If both VNC are disrupted and GJ are inhibited, animals regenerate four heads. Specific cuts for VNC disruption are represented for each case.
In addition to molecular-biological induction/repression pathways, which themselves do not determine a specific morphology (could be applied to many different arrangements of cells and tissues), it is important to develop algorithmic models that illustrate how complex morphogenesis occurs. While we are still far from a detailed picture of how all types of fragments recreate an entire worm, we propose an algorithmic model that shows what decisions are made by the various blastemas in determining the identity to adopt (Supplemental Figure. S8).
Novel pathways for long-range signaling during regenerative morphogenesis
There have been no molecular data associating specific organs or tissues with polarity or fate determination in planarians. Likewise, the alteration in axial patterning resulting from grafting pieces of head tissue into the tail of a host is not understood at the molecular level (Kobayashi et al., 1999). These data extend the understanding of regenerative morphogenesis in several ways. For the first time, we demonstrate novel roles in patterning for a distinct anatomical structure (VNC) and 3 specific innexin proteins. Moreover, the data reveal that long-range signaling from differentiated tissues instructs the blastema through multiple overlapping pathways.
The GJ-dependent signals are mediated by Dj-Inx-5, −13, and −12, as their RNAi knockdown phenocopied the effects of pharmacological inhibition by octanol. Combinatorial analysis of individual innexin genes by RNAi revealed a number of phenotypes in intact and regenerating animals. The expression of most of these innexins was associated with CNS in intact animals and during regeneration (Nogi and Levin, 2005), and indeed most of the RNAi phenotypes included behavioral abnormalities. A/P patterning problems resulted only by targeting Dj-Inx-5, −13, and −12 simultaneously, and did not result from targeting single innexins or from other combinations of up to four innexins. Thus, it suggests that this phenotype is specifically related to Dj-Inx-5, −13, and −12 function and that these GJ protein family members normally compensate for one another.
When do these signaling pathways operate? Nine hours post-amputation, up-regulation of noggin-like gene expression near the wound site is observed (Ogawa et al., 2002). Gurley et al. concluded that important fate decisions take place as early as 12 hours post amputation (Gurley et al., 2008). However, the nature of signals instructing such early gene expression was largely unknown. Time-course inhibition of GJC and disruption of VNC contiguity revealed the window (<3-6 hours post amputation) of critical fate decisions during blastema formation. These effects cannot be explained by differential permeability of the regenerating fragments to octanol because similarly-sized anterior and posterior regenerating fragments exhibit different phenotypes upon exposure to GJ inhibitors. Blocking CNS-mediated early inputs did not affect wound closure, neoblast proliferation, or blastema formation; uniquely, isolating the blastema from long-range CNS inputs altered the identity and polarity of the new tissue, removing the normal morphogenetic coordination with pre-existing structures.
Performing Dj-Inx-5,−13,−12(RNAi) on intact animals led to striking morphogenetic changes characterized by the presence of additional sets of brains and pharynxes along with a new distribution of neoblasts and digestive system which are similar to changes induced with pharmacological inhibition of GJ proteins. Both RNAi and pharmacological inhibition of GJ genes indeed led to alterations in normal pattern morphology. However, the phenotypes are not identical, due to the fact that heptanol targets GJ post-translationally, in a reversible way that rapidly saturates, while RNAi results in a more permanent RNA-based down regulation of their function lasting for more than a month. Although further analysis is required to characterize the relevant GJ-permeable signaling molecules, we propose a scenario in which information mediated by this set of GJ proteins functions during tissue maintenance: a continuous “cellular inventory” is performed, allowing the animal to sense and integrate different anatomical components during morphostasis and remodeling. Therefore, it is possible that in the wounded area, absence of GJ-mediated signals associated with “presence of head” would trigger differentiation of a new brain and anterior identity. While the presence of ectopic brains is associated with the region where worms predominantly fission during asexual reproduction (post-pharyngeal area), it is unlikely that morphogenetic changes observed in this phenotype were associated with fission. During fissioning, no reversal of polarity is observed and only one brain is regenerated per animal; in contrast, in this phenotype we observed three brains within an intact animal. It is not clear what factors determine the polarity of the new brain, but it is evident that it can be established in an orientation independent of that of the host.
Recently, components of the Wnt signaling pathway were associated with determination of A/P polarity during regeneration and morphostasis – the first molecular information linking polarity and regeneration in planarians (Adell et al., 2009; Gurley et al., 2008; Iglesias et al., 2008; Petersen and Reddien, 2008). Nevertheless, how A/P polarity is determined and maintained in adult tissues is poorly understood (Meinhardt, 2009). To determine the relationship between the GJ/VNC system and the Wnt-dependent signals, we identified and characterized a novel clone (Dj-βCatenin-B) of the Wnt signaling pathway in D. japonica. Its RNAi-mediated knockdown revealed the same phenotypes previously reported for its homolog in S. mediterranea. Importantly, these phenotypes differed significantly from those we report here for inhibition of GJC or VNC connectivity (Supplemental Note 1 and Figure 9). While both the GJ/VNC and Wnt pathways are required for A/P polarity, down-regulation of the physiological pathways revealed several important differences from the Wnt pathway phenotypes, suggesting that these are distinct regulatory mechanisms. First, the defects arising from the innexin phenotype reveal a gradient of sensitivity (maximal at post-pharyngeal areas). In Wnt signaling loss-of-function, defects were observed mostly uniform along the A/P axis. Second, the post-pharyngeal area is the most susceptible to A/P mispatterning after 3 innexins are downregulated, while in Wnt signaling disruption, ectopic anteriorization is commonly observed at the tip of the tail and randomly in lateral edges leading to more extreme cases of radial-like hypercephalized animals that were not observed in any innexin-RNAi phenotype. Third, RNAi targeting innexins led to a general correspondence between the number of ectopic brains and pharynxes that were always aligned with each other and with similar polarity. However, in RNAi of different components of Wnt signaling there is no correspondence between the number of ectopic heads and pharynxes suggesting that GJ-related signals activate large-scale patterning modules (e.g., organizers) leading to well-synchronized and orchestrated downstream morphogenetic events. The ability to induce morphogenetic changes that are coherent, without having to micromanage the patterning of substructures, is a very desirable property for the purposes of regenerative intervention.
Long-term alteration of target morphology by transient, physiological modulation
Once disrupted by a transient suppression of these physiological signals, the multi-headed phenotype persists across many weeks and additional rounds of regeneration occurring in the absence of any additional reagents. Direct measurements reveal that the GJ blocker is gone from tissues within days. The striking failure of subsequent regeneration to correct the altered pattern weeks after the blockade of GJC reveals that the GJ-dependent signals have reset the “target morphology” of the animal (the pattern that serves as a comparison template for making decisions about when regeneration is to be initiated and terminated). These results cannot easily be explained by a simple induction of a head with organizer properties (Broun and Bode, 2002) because it occurs in tissue distal to the ectopic head, even after the head is removed. How biological structures encode the morphology to be recreated through regeneration is a fascinating question. These data provide the first molecular entrypoint into the physiological pathway that allows the long-term (or permanent) morphology of a complex adult bodyplan to be experimentally re-specified.
While it is unlikely that random mutagenic effects could consistently cause a formation of a perfect head, we show through a standard mutagenicity assay that the GJ blocker does not change DNA sequence. Normal GJC is known to be restored very quickly after removal of blockers (Chanson et al., 1989), and the half-life of mature gap junctions have been estimated to be less than five hours (Laird, 1996) making it extremely unlikely that any effect on GJ is present in the cuts taking place more than a month after the initial 3-day exposure to octanol. It is not yet known whether the effect is truly permanent or simply long-lived, nor how well multi-headed animals would fare in the wild. However, it should be noted that this physiological modulation results in a drastic alteration of the normal planarian body-plan and behavior that persists across the dominant reproductive mode of this animal (bisection and regeneration). These data may have significant implications for the role of epigenetic change in evolution.
The results highlight the importance of systemic signals for the regulation of stem cell behavior in adult tissues. Moreover, our findings also indicate that GJ proteins regulate stem cell behavior not only through short-range signaling (Oviedo and Levin, 2007a), but also through long-range signals involving neural tissue, allowing the dissection of the long-sought neural regulation of regeneration. Because the complexity of tissue regeneration cannot be fully understood from the perspective of transcriptional networks alone, the novel physiological modulation we reveal here opens novel avenues in which to explore these signals in the complexity of the whole animal.
Our findings provide mechanistic insights into the initial process of regeneration. The data reveal long-range communication between stem cells (neoblasts) and differentiated tissues mediated by the central nervous system and gap junction coupled cells throughout the worm. Similar long-range regulatory mechanisms have been recently demonstrated to actively modulate the natural microenvironment of bone marrow stem cells (Mendez-Ferrer et al., 2008; Spiegel et al., 2008). The characterization of physiological signals, and their influence upon regenerative morphogenesis, offers unprecedented opportunities for rational modulation of somatic and stem cell behavior in guiding pattern formation in biomedical settings (Levin, 2007b; Sundelacruz et al., 2008; Zhao et al., 2006).
Supplementary Material
TABLE S1. Summary of screen of RNAi innexin genes and phenotypes during tissue maintenance and regeneration.
dsRNA microinjections of different DJ-Inx genes were administered individually or in combination of up to four with an scheme similar to the one shown in Figures 3A and 3C. In all cases but one [Dj-Inx-5+12, 2+4(RNAi)], multiple RNAi led to behavioral changes in intact animals; however, in most cases this was not followed by abnormalities during regeneration. These behavioral signs were mostly observed about 30 days after first dsRNA microinjection, when intact planarians began to show behavioral changes characterized by slow movements and random contractions. The strongest behavioral changes were observed in worms subjected to Dj-Inx-5+13, −12(RNAi) (~95%, n=137). Behavioral changes in this group were mostly followed by fissioning in pre or post-pharyngeal areas (~43%, n=137), mortality (~ 37% mostly from small fissioning fragments. i.e.: generalized lyses), or development of ectopic photoreceptor-like pigmentation and pharynxes (~ 50%, n=30) (Figure 3B and supplemental Movie M1). Note: RNAi was performed in all cases by microinjection in the pre-pharyngeal area of dsRNA that consisted of single dsRNA or combination of dsRNA of different genes up to four different genes.
FIGURE S1. Planarian CNS, schedule of gap junction inhibitors treatment and the effect of n-alcohols on polarity during regeneration.
(A) Planarian CNS and pharynx are illustrated (left); representative image of immunostaining with anti-synapsin antibody (right). (B) Schedule of gap junction inhibitor (octanol) treatment (green line). (C) Different n-alcohols used to treat regenerative fragments and their effects on polarity. Note that abnormalities in anteroposterior (AP) polarity during regeneration were observed only after exposure to heptanol and octanol but not hexanol, which, is consistent with effective closing of GJ observed in diverse in vitro and in vivo models for heptanol and octanol but not hexanol. Further, among n-alcohols octanol is recognized as the most potent and effective gap junction blocker in different invertebrate and vertebrate animals; this is consistent with its effects during regeneration. Together this strongly supports the notion that treatment with these drugs specifically target gap junction proteins properties. In intact animals these drugs have transitory behavioral effects, which the animals survive without other noticeable changes.
FIGURE S2. Different planes of dissection, with or without disruption of VNC integrity, and confirmation of cut by marker analysis.
To confirm the predicted cut targeted the region of interest, a group of planarians were processed shortly after amputation with an antibody that recognizes planarian CNS structures (Cebrià, 2008). Top row, representation of the planarian CNS and pharynx showing eleven different dissection planes (Roman numerals) intended to disrupt the VNC or leave intact. Bottom row, representative images from immunostaining performed with anti-synapsin antibody (CNS marker, green signal) in planarians that were dissected as shown above. Worms (n>10 per condition) were fixed approximately 3 hours post dissection for later processing with immunostaining. Note that analysis of actual animals confirms the targeting of each class of surgery. Scale bar = 200 μm.
FIGURE S3. Schematic representation of gene expression of innexin 5, 12 and 13.
Illustrative design of the spatial expression of innexin genes is based upon data previously published (Nogi and Levin 2005). In intact animals expression of Dj-Inx-5, −12 and −13 is mostly found associated with CNS structures (color coded for each gene). Different expression domains for each gene along the CNS can be recognized being Dj-Inx-5, and −13 present in both brain and VNC while Dj-Inx-12 is mostly restricted to VNC in post-pharyngeal areas. For simplicity Dj-Inx-5 expression associated with sub-epithelial cells is not represented here. Interestingly, simultaneous RNAi of these three genes led to strong phenotypes associated with A/P polarity determination and ectopic organ formation.
FIGURE S4. Different versions of multiple headed worms obtained by blocking GJ proteins and disrupting VNC integrity in post-pharyngeal fragments.
(A) Dissection of post-pharyngeal fragments was performed to additionally disrupt VNC structural integrity in presence or absence of octanol. Top row shows schematics of different amputations performed. Bottom row shows representative pictures of fragments fixed three hours post-amputation that were processed for immunostaining with anti-synapsin antibody (green signal over a pseudocolored red background) for each respective dissection (n>10 per condition). Notice that immunostaining experiments with CNS marker revealed a close resemblance between the two rows, suggesting that the intended dissection specifically targeted the tissue of interest. Scale bar = 500μm. (B) Representative images of phenotypes obtained after regeneration of post-pharyngeal fragments with lateral dissections in presence of octanol. Three phenotypes were characterized by ectopic heads and pharynx formation and A/P polarity problems. These animals were classified into 3 categories (i.e.: bipolar head plus, triple head, and quadruple head). CNS abnormalities are indicated with arrows. Animals considered within the category of “bipolar head plus” effectively developed polarity problems (bipolar head) but formation of additional head was aborted leading to VNC with abnormal morphology. In all conditions, CNS abnormalities developed; in only one case this led to formation of ectopic heads in dorsal ventral axis (triple head animal at the bottom row); this was associated with the type of dissection that originated such changes (see window cut in post-pharyngeal fragment −right most in A). Scale bar = 500μm. (C) Spatial gene expression profile of single and quadruple headed worms (n≥5 each case) with markers of digestive system (Dj-Inx-7), CNS (Dj-PC2), posterior marker (Dj-Abd-BA), and neoblasts (Dj-Inx-11). Notice that in all cases, dramatic morphological changes were induced in quadruple headed worms. This suggests that each head is followed by new A/P axis that organizes new distribution of organ and system irrespective of the original polarity within the fragment. Scale bar = 200μm. Interestingly, all heads resulting from decapitations always regenerated as normal worms. Altogether these results suggest that recovery to original A/P axis information can be induced if amputation of multi-headed worms involves post-pharyngeal areas, which is consistent with not fully differentiation of post-pharyngeal areas within bipolar-headed planarians. As for the head fragments that regenerated normally, this is consistent with reversion of octanol effects and that these changes are unlikely due to alterations in DNA sequence.
FIGURE S5. Different planes of amputation in multiheaded worms
(A) Representative images of decapitation in multiheaded worms. Removal of one, two, three and even four head simultaneously always lead to regeneration of the missing head. (B) Different amputation along the A/P axis in bipolar-headed planarians led to variable results. All amputations of multiheaded worms were performed in absence of octanol (i.e.: no gap junction inhibitor in the media and after 3-4 weeks of first amputation). In all cases (3/3) that amputation plane traverse post-pharyngeal area a number of animals regenerated either as normal worm or bipolar-headed animal and the percentage for each case were variable (numbers within parenthesis are amputations).
FIGURE S6. Octanol incorporation within tissue of regenerating planarian.
Worms were treated with octanol (as indicated at the bottom of the graph) and every 24 hours for seven consecutive days a pool of animals (n>10) were subjected to rapid-freeze and processed for GC-MS. Concentration of incorporated octanol was calculated per gram of planarian tissue (nmol/g) and was observed to steadily decrease within the first three days of treatment, reaching low levels that persisted subsequently.
FIGRUE S7. Octanol treatment used to block gap junction proteins does not alter DNA sequence.
Chemical-induced genotoxicity was detected by monitoring bioluminescent emission under different octanol dilutions. Experiment was run for about 48 minutes. Increases in biolumiscence are associated with DNA damage. (A) Dose-dependent response curves for different octanol dilutions; note that even high octanol concentrations (1000 ppm) did not induce notable increases in bioluminescent emission. Experiments described in this manuscript used octanol dilutions of 1/30000, shown to not be mutagenic by this standard test. (B) For comparison, dose-dependent response curves for different dilutions of Mitomycin C (which is known as a typical mutagen) from (Min et al., 1999). Note that even low dilutions of this compound induce dramatic changes of signal in the same assay by 60-100-fold (compare to the signal for octanol which is < 2). Measurements were run in triplicates and error bars represent standard deviation.
FIGURE S8. Summary schematic of early events during planarian regeneration involving GJ-mediated neural signals.
(A) An algorithm based on bimodal decisions was derived, representing in a simplified manner how information mediated by GJ proteins and VNC affect regenerative pattern. The key inputs provided to a wound at early stages of regeneration are schematized in a hypothetical case in which transverse amputations produce 3 fragments (*, “PW” = long fragment including pre-existing brain and a posterior wound, ** = post-pharyngeal fragment with anterior (AW) and posterior (PW) wounds, and *** = tail fragment with only AW. Time post-amputation is shown in the vertical left scale from 0 to >24 hours. As soon as the wound surface is minimized by mechanical contractions, instructive inputs from differentiated tissue are required at the wound to begin to establish polarity and identity of the regenerate. Key signals include GJ-mediated signals and VNC integrity functioning within the first 3-6 hours of regeneration. Information provided to the wounded area likely includes the presence of brain tissue in the animal’s body. Since anterior wounds regenerate heads despite GJ inhibition, additional mechanisms must also exist. In a long fragment (*), both GJ-mediated signals and VNC integrity inform the PW about the presence of brain and that no anterior blastema is needed; thus, neoblasts surrounding PW are specified by other means (e.g.: intercalary regeneration) to form a posterior blastema. If no brain is detected within the regenerating fragment, this narrows down the possibilities to fragments below the head region (e.g.: post-pharyngeal** or tail***). A critical step for the blastema is to identify whether there is only one wound within the pre-existing tissue. If so (it is a fragment only with an anterior wound *), then the neoblasts must be instructed to form an anterior blastema. Conversely, if there are two wounds in the fragment (e.g.: ** post-pharyngeal) the subsequent information instructing neoblasts at those wounds concerns the determination of wound location (i.e.: AW or PW). For AW, alternative cues independent of GJs are in place. Anterior blastemas form faster (Morgan, 1898); thus they can prevent (via neural/GJ-dependent signaling) the differentiation of additional anterior structures (black dotted line). Thus, inputs at this point allow sensing of anterior blastema formation and the PW is instructed to form a posterior blastema that integrates with pre-existing A/P axis. Fate decisions during this process can be altered by blocking neural GJC and disrupting VNC integrity (which affect the determination of head tissue within the fragment). It is also formally possible that other tissues (e.g.: mesenchyme, intestine) may influence polarity determination. Nevertheless, our model and the early timeline revealed by Fig. 2 are consistent with proposed anterior regenerative process based on gene expression (Agata and Umesono, 2008).
FIGURE S9. Molecular conservation of ß-catenin isoforms between planarians species, and down-regulation of Dj-ßcatenin-B leads to anteroposterior polarity defects.
(A) Alignment of deduced amino-acid sequences of novel clone Dj-ßcatenin-B (accession number BP190419) and known Smed-ßcatenin-1 (accession number EU082826). Similarity is 80% within this C-terminal region. Black: identical residues. Grey: conserved substitutions. (B) Dj-ßcatenin-B(RNAi) induces A/P polarity defects during regeneration. Notice that anterior regenerating fragments reach maximum polarity defects, suggesting that most anterior region in the worm are not structurally restricted to polarity defects. Animals subjected to Dj-ßcatenin-B(RNAi) and treated with octanol during regeneration also displayed AP polarity abnormalities but this treatment was toxic for (killed all) fragments. Interestingly, post-pharyngeal fragments exhibited increased incidence of AP polarity defects during regeneration, suggesting an additive effect between these two pathways.
ACKNOWLEDGEMENTS
We thank K. Watanabe for VC-1 antibody, D. Qiu and WHA de Jong for technical assistance, and Kelly Tseng and other members of the Levin Lab for discussion and suggestions. N.J.O. was supported by NIH under Ruth L. Kirschstein National Research Service Award (F32 GM078774). P.W. was supported by a Herzog-Carl-Stipend from the University of Hohenheim and thanks M. Blum for his support. This work was supported by NSF grant IBN#0347295, NHTSA grant DTNH22-06-G-00001, and NIH grant HD055850 to M.L.
Footnotes
COMPETING INTEREST STATEMENT The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdelmohsen K, Stuhlmann D, Daubrawa F, Klotz LO. Dicumarol is a potent reversible inhibitor of gap junctional intercellular communication. Arch Biochem Biophys. 2005;434:241–247. doi: 10.1016/j.abb.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Adell T, Salo E, Boutros M, Bartscherer K. Smed-Evi/Wntless is required for {beta}-catenin-dependent and -independent processes during planarian regeneration. Development. 2009;136:905–910. doi: 10.1242/dev.033761. [DOI] [PubMed] [Google Scholar]
- Adler EL, Woodruff RI. Varied effects of 1-octanol on gap junctional communication between ovarian epithelial cells and oocytes of Oncopeltus fasciatus, Hyalophora cecropia, and Drosophila melanogaster. Arch Insect Biochem Physiol. 2000;43:22–32. doi: 10.1002/(SICI)1520-6327(200001)43:1<22::AID-ARCH4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Agata K, Umesono Y. Brain regeneration from pluripotent stem cells in planarian. Philos Trans R Soc Lond B Biol Sci. 2008;363:2071–2078. doi: 10.1098/rstb.2008.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KL, Woodruff RI. A gap junctionally transmitted epithelial cell signal regulates endocytic yolk uptake in Oncopeltus fasciatus. Dev Biol. 2001;239:68–78. doi: 10.1006/dbio.2001.0433. [DOI] [PubMed] [Google Scholar]
- Aw S, Levin M. Is left-right asymmetry a form of planar cell polarity? Development. 2009;136:355–366. doi: 10.1242/dev.015974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JB, Goodman AB, Pigon A. Fissioning in planarians: control by the brain. Science. 1969;164:565–566. doi: 10.1126/science.164.3879.565. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Kumar A. Comparative aspects of animal regeneration. Annu Rev Cell Dev Biol. 2008;24:525–549. doi: 10.1146/annurev.cellbio.24.110707.175336. [DOI] [PubMed] [Google Scholar]
- Brooks RA, Woodruff RI. Calmodulin transmitted through gap junctions stimulates endocytic incorporation of yolk precursors in insect oocytes. Dev Biol. 2004;271:339–349. doi: 10.1016/j.ydbio.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Broun M, Bode HR. Characterization of the head organizer in hydra. Development. 2002;129:875–884. doi: 10.1242/dev.129.4.875. [DOI] [PubMed] [Google Scholar]
- Brøndsted HV. Planarian Regeneration. 1st ed Pergamon Press; London: 1969. [Google Scholar]
- Burnside AS, Collas P. Induction of Oct-3/4 expression in somatic cells by gap junction-mediated cAMP signaling from blastomeres. Eur J Cell Biol. 2002;81:585–591. doi: 10.1078/0171-9335-00286. [DOI] [PubMed] [Google Scholar]
- Cebrià F. Organization of the nervous system in the model planarian Schmidtea mediterranea: an immunocytochemical study. Neurosci Res. 2008;61:375–384. doi: 10.1016/j.neures.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Cebria F, Kobayashi C, Umesono Y, Nakazawa M, Mineta K, Ikeo K, Gojobori T, Itoh M, Taira M, Alvarado AS, Agata K. FGFR-related gene noudarake restricts brain tissues to the head region of planarians. Nature. 2002;419:620–624. doi: 10.1038/nature01042. [DOI] [PubMed] [Google Scholar]
- Chanson M, Bruzzone R, Bosco D, Meda P. Effects of n-alcohols on junctional coupling and amylase secretion of pancreatic acinar cells. J Cell Physiol. 1989;139:147–156. doi: 10.1002/jcp.1041390121. [DOI] [PubMed] [Google Scholar]
- Christ GJ, Spektor M, Brink PR, Barr L. Further evidence for the selective disruption of intercellular communication by heptanol. Am J Physiol. 1999;276:H1911–1917. doi: 10.1152/ajpheart.1999.276.6.H1911. [DOI] [PubMed] [Google Scholar]
- Ehrlich HP, Diez T. Role for gap junctional intercellular communications in wound repair. Wound Repair Regen. 2003;11:481–489. doi: 10.1046/j.1524-475x.2003.11616.x. [DOI] [PubMed] [Google Scholar]
- Elias LA, Kriegstein AR. Gap junctions: multifaceted regulators of embryonic cortical development. Trends Neurosci. 2008;31:243–250. doi: 10.1016/j.tins.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flickinger RA, Coward SJ. The induction of cephalic differentiation in regenerating Dugesia dorotocephala in the presence of the normal head and in unwounded tails. Dev Biol. 1962;5:179–204. doi: 10.1016/0012-1606(62)90009-x. [DOI] [PubMed] [Google Scholar]
- French V, Bryant PJ, Bryant SV. Pattern regulation in epimorphic fields. Science. 1976;193:969–981. doi: 10.1126/science.948762. [DOI] [PubMed] [Google Scholar]
- Garcia-Dorado D, Inserte J, Ruiz-Meana M, Gonzalez MA, Solares J, Julia M, Barrabes JA, Soler-Soler J. Gap junction uncoupler heptanol prevents cell-to-cell progression of hypercontracture and limits necrosis during myocardial reperfusion. Circulation. 1997;96:3579–3586. doi: 10.1161/01.cir.96.10.3579. [DOI] [PubMed] [Google Scholar]
- Gurley KA, Rink JC, Sánchez Alvarado A. Beta-catenin defines head versus tail identity during planarian regeneration and homeostasis. Science. 2008;319:323–327. doi: 10.1126/science.1150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptak-Solga AD, Nielsen S, Jain I, Thummel R, Hyde DR, Iovine MK. Connexin43 (GJA1) is required in the population of dividing cells during fin regeneration. Dev Biol. 2008;317:541–548. doi: 10.1016/j.ydbio.2008.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HJ, Drummond-Barbosa D. Insulin levels control female germline stem cell maintenance via the niche in Drosophila. Proc Natl Acad Sci U S A. 2009;106:1117–1121. doi: 10.1073/pnas.0809144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias M, Gomez-Skarmeta JL, Salo E, Adell T. Silencing of Smedbetacatenin1 generates radial-like hypercephalized planarians. Development. 2008;135:1215–1221. doi: 10.1242/dev.020289. [DOI] [PubMed] [Google Scholar]
- Jin EJ, Lee SY, Jung JC, Bang OS, Kang SS. TGF-beta3 inhibits chondrogenesis of cultured chick leg bud mesenchymal cells via downregulation of connexin 43 and integrin beta4. J Cell Physiol. 2008;214:345–353. doi: 10.1002/jcp.21202. [DOI] [PubMed] [Google Scholar]
- Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- Kobayashi C, Nogi T, Watanabe K, Agata K. Ectopic pharynxes arise by regional reorganization after anterior/posterior chimera in planarians. Mech. Dev. 1999;89:25–34. doi: 10.1016/s0925-4773(99)00192-6. [DOI] [PubMed] [Google Scholar]
- Kobayashi C, Saito Y, Ogawa K, Agata K. Wnt signaling is required for antero-posterior patterning of the planarian brain. Dev Biol. 2007;306:714–724. doi: 10.1016/j.ydbio.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Kumar A, Godwin JW, Gates PB, Garza-Garcia AA, Brockes JP. Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science. 2007;318:772–777. doi: 10.1126/science.1147710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird DW. The life cycle of a connexin: gap junction formation, removal, and degradation. J Bioenerg Biomembr. 1996;28:311–318. doi: 10.1007/BF02110107. [DOI] [PubMed] [Google Scholar]
- Lender T. L’inhibition spécifique de la différentiation du cervau des planaries d’eau douce en régénération. J Embr Exp Morph. 1960;8:291–300. [PubMed] [Google Scholar]
- Levin M. Gap junctional communication in morphogenesis. Progress in Biophysics and Molecular Biology. 2007a;94:186–206. doi: 10.1016/j.pbiomolbio.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. Large-scale biophysics: ion flows and regeneration. Trends in Cell Biology. 2007b;17:262–271. doi: 10.1016/j.tcb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. Gap junctions are involved in the early generation of left-right asymmetry. Dev Biol. 1998;203:90–105. doi: 10.1006/dbio.1998.9024. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. Gap junction-mediated transfer of left-right patterning signals in the early chick blastoderm is upstream of Shh asymmetry in the node. Development. 1999;126:4703–4714. doi: 10.1242/dev.126.21.4703. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. Expression of connexin 30 in Xenopus embryos and its involvement in hatching gland function. Developmental Dynamics. 2000;219:96–101. doi: 10.1002/1097-0177(200009)219:1<96::AID-DVDY1034>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Meinhardt H. Beta-catenin and axis formation in planarians. Bioessays. 2009;31:5–9. doi: 10.1002/bies.080193. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- Min J, Kim EJ, LaRossa RA, Gu MB. Distinct responses of a recA::luxCDABE Escherichia coli strain to direct and indirect DNA damaging agents. Mutat Res. 1999;442:61–68. doi: 10.1016/s1383-5718(99)00059-5. [DOI] [PubMed] [Google Scholar]
- Mineta K, Nakazawa M, Cebria F, Ikeo K, Agata K, Gojobori T. Origin and evolutionary process of the CNS elucidated by comparative genomics analysis of planarian ESTs. Proc Natl Acad Sci U S A. 2003;100:7666–7671. doi: 10.1073/pnas.1332513100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mire P, Nasse J, Venable-Thibodeaux S. Gap junctional communication in the vibration-sensitive response of sea anemones. Hearing Research. 2000;144:109–123. doi: 10.1016/s0378-5955(00)00047-2. [DOI] [PubMed] [Google Scholar]
- Momose-Sato Y, Honda Y, Sasaki H, Sato K. Optical imaging of large-scale correlated wave activity in the developing rat CNS. J Neurophysiol. 2005;94:1606–1622. doi: 10.1152/jn.00044.2005. [DOI] [PubMed] [Google Scholar]
- Morgan TH. Experimental studies of the regeneration of Planaria maculata. Arch. Entw. Mech. Org. 1898;7:364–397. [Google Scholar]
- Newmark PA, Sanchez Alvarado A. Bromodeoxyuridine specifically labels the regenerative stem cells of planarians. Developmental Biology. 2000;220:142–153. doi: 10.1006/dbio.2000.9645. [DOI] [PubMed] [Google Scholar]
- Nogi T, Levin M. Characterization of innexin gene expression and functional roles of gap-junctional communication in planarian regeneration. Dev Biol. 2005;287:314–335. doi: 10.1016/j.ydbio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Ishihara S, Saito Y, Mineta K, Nakazawa M, Ikeo K, Gojobori T, Watanabe K, Agata K. Induction of a noggin-like gene by ectopic DV interaction during planarian regeneration. Dev Biol. 2002;250:59–70. doi: 10.1006/dbio.2002.0790. [DOI] [PubMed] [Google Scholar]
- Oviedo NJ, Levin M. Gap junctions provide new links in left-right patterning. Cell. 2007a;129:645–647. doi: 10.1016/j.cell.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Oviedo NJ, Levin M. smedinx-11 is a planarian stem cell gap junction gene required for regeneration and homeostasis. Development. 2007b;134:3121–3131. doi: 10.1242/dev.006635. [DOI] [PubMed] [Google Scholar]
- Oviedo NJ, Nicolas CL, Adams DS, Levin M. Establishing and maintaining a colony of planarians. Cold Spring Harb Protoc. 2008a doi: 10.1101/pdb.prot5053. doi:10.1101/pbd.prot5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo NJ, Pearson BJ, Levin M, Sánchez Alvarado A. Planarian PTEN homologs regulate stem cells and regeneration through TOR signaling. Dis Model Mech. 2008b;1:131–143. doi: 10.1242/dmm.000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CP, Reddien PW. Smed-betacatenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science. 2008;319:327–330. doi: 10.1126/science.1149943. [DOI] [PubMed] [Google Scholar]
- Phelan P. Innexins: members of an evolutionarily conserved family of gap-junction proteins. Biochim Biophys Acta. 2005;1711:225–245. doi: 10.1016/j.bbamem.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Reddien PW, Sánchez Alvarado A. Fundamentals of planarian regeneration. Annu Rev Cell Dev Biol. 2004;20:725–757. doi: 10.1146/annurev.cellbio.20.010403.095114. [DOI] [PubMed] [Google Scholar]
- Rodriguez LV, Flickinger RA. Bipolar head regeneration in Planaria induced by chick embryo extracts. Biol Bull. 1971;140:117–124. doi: 10.2307/1540031. [DOI] [PubMed] [Google Scholar]
- Schilling AF, Filke S, Lange T, Gebauer M, Brink S, Baranowsky A, Zustin J, Amling M. Gap junctional communication in human osteoclasts in vitro and in vivo. J Cell Mol Med. 2008;12:2497–2504. doi: 10.1111/j.1582-4934.2008.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. The influence of the nerve in regeneration of the amphibian extremity. Q Rev Biol. 1952;27:169–200. doi: 10.1086/398873. [DOI] [PubMed] [Google Scholar]
- Spiegel A, Kalinkovich A, Shivtiel S, Kollet O, Lapidot T. Stem cell regulation via dynamic interactions of the nervous and immune systems with the microenvironment. Cell Stem Cell. 2008;3:484–492. doi: 10.1016/j.stem.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Sundelacruz S, Levin M, Kaplan DL. Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PLoS ONE. 2008;3:e3737. doi: 10.1371/journal.pone.0003737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksmonski SL, Woodruff RI. For uptake of yolk precursors, epithelial cell-oocyte gap junctional communication is required by insects representing six different orders. J Insect Physiol. 2002;48:667–675. doi: 10.1016/s0022-1910(02)00095-1. [DOI] [PubMed] [Google Scholar]
- Weingart R, Bukauskas FF. Long-chain n-alkanols and arachidonic acid interfere with the Vm-sensitive gating mechanism of gap junction channels. Pflugers Arch. 1998;435:310–319. doi: 10.1007/s004240050517. [DOI] [PubMed] [Google Scholar]
- Wong RC, Pera MF, Pebay A. Role of gap junctions in embryonic and somatic stem cells. Stem Cell Rev. 2008;4:283–292. doi: 10.1007/s12015-008-9038-9. [DOI] [PubMed] [Google Scholar]
- Zada A, Soroker V, Harel M, Nakache J, Dunkelblum E. Quantitative GC analysis of secondary alcohol pheromones: determination of release rate of red palm weevil, Rhynchophorus ferrugineus, pheromone from lures. J Chem Ecol. 2002;28:2299–2306. doi: 10.1023/a:1021057501459. [DOI] [PubMed] [Google Scholar]
- Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, Penninger JM. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–460. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1. Summary of screen of RNAi innexin genes and phenotypes during tissue maintenance and regeneration.
dsRNA microinjections of different DJ-Inx genes were administered individually or in combination of up to four with an scheme similar to the one shown in Figures 3A and 3C. In all cases but one [Dj-Inx-5+12, 2+4(RNAi)], multiple RNAi led to behavioral changes in intact animals; however, in most cases this was not followed by abnormalities during regeneration. These behavioral signs were mostly observed about 30 days after first dsRNA microinjection, when intact planarians began to show behavioral changes characterized by slow movements and random contractions. The strongest behavioral changes were observed in worms subjected to Dj-Inx-5+13, −12(RNAi) (~95%, n=137). Behavioral changes in this group were mostly followed by fissioning in pre or post-pharyngeal areas (~43%, n=137), mortality (~ 37% mostly from small fissioning fragments. i.e.: generalized lyses), or development of ectopic photoreceptor-like pigmentation and pharynxes (~ 50%, n=30) (Figure 3B and supplemental Movie M1). Note: RNAi was performed in all cases by microinjection in the pre-pharyngeal area of dsRNA that consisted of single dsRNA or combination of dsRNA of different genes up to four different genes.
FIGURE S1. Planarian CNS, schedule of gap junction inhibitors treatment and the effect of n-alcohols on polarity during regeneration.
(A) Planarian CNS and pharynx are illustrated (left); representative image of immunostaining with anti-synapsin antibody (right). (B) Schedule of gap junction inhibitor (octanol) treatment (green line). (C) Different n-alcohols used to treat regenerative fragments and their effects on polarity. Note that abnormalities in anteroposterior (AP) polarity during regeneration were observed only after exposure to heptanol and octanol but not hexanol, which, is consistent with effective closing of GJ observed in diverse in vitro and in vivo models for heptanol and octanol but not hexanol. Further, among n-alcohols octanol is recognized as the most potent and effective gap junction blocker in different invertebrate and vertebrate animals; this is consistent with its effects during regeneration. Together this strongly supports the notion that treatment with these drugs specifically target gap junction proteins properties. In intact animals these drugs have transitory behavioral effects, which the animals survive without other noticeable changes.
FIGURE S2. Different planes of dissection, with or without disruption of VNC integrity, and confirmation of cut by marker analysis.
To confirm the predicted cut targeted the region of interest, a group of planarians were processed shortly after amputation with an antibody that recognizes planarian CNS structures (Cebrià, 2008). Top row, representation of the planarian CNS and pharynx showing eleven different dissection planes (Roman numerals) intended to disrupt the VNC or leave intact. Bottom row, representative images from immunostaining performed with anti-synapsin antibody (CNS marker, green signal) in planarians that were dissected as shown above. Worms (n>10 per condition) were fixed approximately 3 hours post dissection for later processing with immunostaining. Note that analysis of actual animals confirms the targeting of each class of surgery. Scale bar = 200 μm.
FIGURE S3. Schematic representation of gene expression of innexin 5, 12 and 13.
Illustrative design of the spatial expression of innexin genes is based upon data previously published (Nogi and Levin 2005). In intact animals expression of Dj-Inx-5, −12 and −13 is mostly found associated with CNS structures (color coded for each gene). Different expression domains for each gene along the CNS can be recognized being Dj-Inx-5, and −13 present in both brain and VNC while Dj-Inx-12 is mostly restricted to VNC in post-pharyngeal areas. For simplicity Dj-Inx-5 expression associated with sub-epithelial cells is not represented here. Interestingly, simultaneous RNAi of these three genes led to strong phenotypes associated with A/P polarity determination and ectopic organ formation.
FIGURE S4. Different versions of multiple headed worms obtained by blocking GJ proteins and disrupting VNC integrity in post-pharyngeal fragments.
(A) Dissection of post-pharyngeal fragments was performed to additionally disrupt VNC structural integrity in presence or absence of octanol. Top row shows schematics of different amputations performed. Bottom row shows representative pictures of fragments fixed three hours post-amputation that were processed for immunostaining with anti-synapsin antibody (green signal over a pseudocolored red background) for each respective dissection (n>10 per condition). Notice that immunostaining experiments with CNS marker revealed a close resemblance between the two rows, suggesting that the intended dissection specifically targeted the tissue of interest. Scale bar = 500μm. (B) Representative images of phenotypes obtained after regeneration of post-pharyngeal fragments with lateral dissections in presence of octanol. Three phenotypes were characterized by ectopic heads and pharynx formation and A/P polarity problems. These animals were classified into 3 categories (i.e.: bipolar head plus, triple head, and quadruple head). CNS abnormalities are indicated with arrows. Animals considered within the category of “bipolar head plus” effectively developed polarity problems (bipolar head) but formation of additional head was aborted leading to VNC with abnormal morphology. In all conditions, CNS abnormalities developed; in only one case this led to formation of ectopic heads in dorsal ventral axis (triple head animal at the bottom row); this was associated with the type of dissection that originated such changes (see window cut in post-pharyngeal fragment −right most in A). Scale bar = 500μm. (C) Spatial gene expression profile of single and quadruple headed worms (n≥5 each case) with markers of digestive system (Dj-Inx-7), CNS (Dj-PC2), posterior marker (Dj-Abd-BA), and neoblasts (Dj-Inx-11). Notice that in all cases, dramatic morphological changes were induced in quadruple headed worms. This suggests that each head is followed by new A/P axis that organizes new distribution of organ and system irrespective of the original polarity within the fragment. Scale bar = 200μm. Interestingly, all heads resulting from decapitations always regenerated as normal worms. Altogether these results suggest that recovery to original A/P axis information can be induced if amputation of multi-headed worms involves post-pharyngeal areas, which is consistent with not fully differentiation of post-pharyngeal areas within bipolar-headed planarians. As for the head fragments that regenerated normally, this is consistent with reversion of octanol effects and that these changes are unlikely due to alterations in DNA sequence.
FIGURE S5. Different planes of amputation in multiheaded worms
(A) Representative images of decapitation in multiheaded worms. Removal of one, two, three and even four head simultaneously always lead to regeneration of the missing head. (B) Different amputation along the A/P axis in bipolar-headed planarians led to variable results. All amputations of multiheaded worms were performed in absence of octanol (i.e.: no gap junction inhibitor in the media and after 3-4 weeks of first amputation). In all cases (3/3) that amputation plane traverse post-pharyngeal area a number of animals regenerated either as normal worm or bipolar-headed animal and the percentage for each case were variable (numbers within parenthesis are amputations).
FIGURE S6. Octanol incorporation within tissue of regenerating planarian.
Worms were treated with octanol (as indicated at the bottom of the graph) and every 24 hours for seven consecutive days a pool of animals (n>10) were subjected to rapid-freeze and processed for GC-MS. Concentration of incorporated octanol was calculated per gram of planarian tissue (nmol/g) and was observed to steadily decrease within the first three days of treatment, reaching low levels that persisted subsequently.
FIGRUE S7. Octanol treatment used to block gap junction proteins does not alter DNA sequence.
Chemical-induced genotoxicity was detected by monitoring bioluminescent emission under different octanol dilutions. Experiment was run for about 48 minutes. Increases in biolumiscence are associated with DNA damage. (A) Dose-dependent response curves for different octanol dilutions; note that even high octanol concentrations (1000 ppm) did not induce notable increases in bioluminescent emission. Experiments described in this manuscript used octanol dilutions of 1/30000, shown to not be mutagenic by this standard test. (B) For comparison, dose-dependent response curves for different dilutions of Mitomycin C (which is known as a typical mutagen) from (Min et al., 1999). Note that even low dilutions of this compound induce dramatic changes of signal in the same assay by 60-100-fold (compare to the signal for octanol which is < 2). Measurements were run in triplicates and error bars represent standard deviation.
FIGURE S8. Summary schematic of early events during planarian regeneration involving GJ-mediated neural signals.
(A) An algorithm based on bimodal decisions was derived, representing in a simplified manner how information mediated by GJ proteins and VNC affect regenerative pattern. The key inputs provided to a wound at early stages of regeneration are schematized in a hypothetical case in which transverse amputations produce 3 fragments (*, “PW” = long fragment including pre-existing brain and a posterior wound, ** = post-pharyngeal fragment with anterior (AW) and posterior (PW) wounds, and *** = tail fragment with only AW. Time post-amputation is shown in the vertical left scale from 0 to >24 hours. As soon as the wound surface is minimized by mechanical contractions, instructive inputs from differentiated tissue are required at the wound to begin to establish polarity and identity of the regenerate. Key signals include GJ-mediated signals and VNC integrity functioning within the first 3-6 hours of regeneration. Information provided to the wounded area likely includes the presence of brain tissue in the animal’s body. Since anterior wounds regenerate heads despite GJ inhibition, additional mechanisms must also exist. In a long fragment (*), both GJ-mediated signals and VNC integrity inform the PW about the presence of brain and that no anterior blastema is needed; thus, neoblasts surrounding PW are specified by other means (e.g.: intercalary regeneration) to form a posterior blastema. If no brain is detected within the regenerating fragment, this narrows down the possibilities to fragments below the head region (e.g.: post-pharyngeal** or tail***). A critical step for the blastema is to identify whether there is only one wound within the pre-existing tissue. If so (it is a fragment only with an anterior wound *), then the neoblasts must be instructed to form an anterior blastema. Conversely, if there are two wounds in the fragment (e.g.: ** post-pharyngeal) the subsequent information instructing neoblasts at those wounds concerns the determination of wound location (i.e.: AW or PW). For AW, alternative cues independent of GJs are in place. Anterior blastemas form faster (Morgan, 1898); thus they can prevent (via neural/GJ-dependent signaling) the differentiation of additional anterior structures (black dotted line). Thus, inputs at this point allow sensing of anterior blastema formation and the PW is instructed to form a posterior blastema that integrates with pre-existing A/P axis. Fate decisions during this process can be altered by blocking neural GJC and disrupting VNC integrity (which affect the determination of head tissue within the fragment). It is also formally possible that other tissues (e.g.: mesenchyme, intestine) may influence polarity determination. Nevertheless, our model and the early timeline revealed by Fig. 2 are consistent with proposed anterior regenerative process based on gene expression (Agata and Umesono, 2008).
FIGURE S9. Molecular conservation of ß-catenin isoforms between planarians species, and down-regulation of Dj-ßcatenin-B leads to anteroposterior polarity defects.
(A) Alignment of deduced amino-acid sequences of novel clone Dj-ßcatenin-B (accession number BP190419) and known Smed-ßcatenin-1 (accession number EU082826). Similarity is 80% within this C-terminal region. Black: identical residues. Grey: conserved substitutions. (B) Dj-ßcatenin-B(RNAi) induces A/P polarity defects during regeneration. Notice that anterior regenerating fragments reach maximum polarity defects, suggesting that most anterior region in the worm are not structurally restricted to polarity defects. Animals subjected to Dj-ßcatenin-B(RNAi) and treated with octanol during regeneration also displayed AP polarity abnormalities but this treatment was toxic for (killed all) fragments. Interestingly, post-pharyngeal fragments exhibited increased incidence of AP polarity defects during regeneration, suggesting an additive effect between these two pathways.