What’s in a name? that which we call a rose By any other name would smell as sweet; William Shakespeare, Romeo and Juliet 1594
This issue of Prostaglandins, Leukotrienes & Essential Fatty Acids has two interesting papers concerning the topic of isoprostane nomenclature, This has been a difficult arena in which to be definitive since the discovery of these free radical products of arachidonate autoxidation over twenty years ago by Roberts(1). The story of how they were discovered and description of the mechanism by which they form is a fascinating one that speaks of hard work and cleaver scientific investigation. The problem has always been as to how to devise a meaningful name for each unique compound in light of the 64 isomers of arachidonate that can form in each specific family member, e.g.F2-isoprostanes. The current IUPAC approved nomenclature system is concisely presented in the paper of Mueller and in general is based on the systematic prostaglandin nomenclature which centers on the oxygen atom substituent of the cyclopentane ring structure common to all prostanoids.
It is of interest to recall how the names PGE2 and PGF2a were first proposed as being the ether extractible active molecule and another active principle extractable from ether with pH8 phosphate buffer that caused smooth muscle relaxation or contraction (2). While it is easy to see the use of “E” for the ether extractible activity, and one must recall that in Swedish phosphate is spelled “fosfat”, and hence the letter “F”. The total number of double bonds was eventually added as designated by the subscripted numbers when structures were elucidated. The other letters of the alphabet to follow the PG designation initially referred to “acid” treated product “A” and “base” treated product “B”. Again when structures of these PGs were determined, it was clear that these letter designations corresponded to unique oxygen substitution patters on the cyclopentane or cyclopentene ring and could be used in a structural based nomenclature to describe these important, biologically active compounds. Active prostaglandin PGD2 was found and filled in the alphabetic gap, then the endoperoxides PGG2 and PGH2 followed by prostacyclin (PGI2) were discovered. PGJ2 was a metabolite of PGD2 which is where we stand with unique oxygen atom substituents of the cyclic arachidonate structure.
The challenge for the isoprostane nomenclature has always been devising a rational system that would clearly and uniquely describe the multiple isomeric entities that differ at stereocenters and double bond characteristics. This was never a problem with enzymatic derived prostanoids since cyclooxygenase controlled stereochemistry. A rational system base on linolenic acid and termed “phytoprostane system” is proposed in one of the articles in this issue, to perhaps more closely link biology (biological activity) with structure and still retain the classic prostaglandin lettering system. Interestingly some of the “J” series become “A” series when going between the IUPAC and phytoprostane nomenclature systems. uses The phytoprostane system uses a somewhat larger structural unit to begin the naming. This appears to work well with the 18-carbon free radical derived products that contain this cyclopentane vinyl alcohol unit.
The question is whether there is enough compelling evidence to change from the approved systematic protocol. The nomenclature of isoprostanes has already been a contentious business with very different schools of thought as to how to deal with the various issues and challenges. As a famous lipid biochemist (who wishes to remain anonymous) once told me, it is easier to convince biochemists to share their toothbrushes than to share another’s nomenclature system.
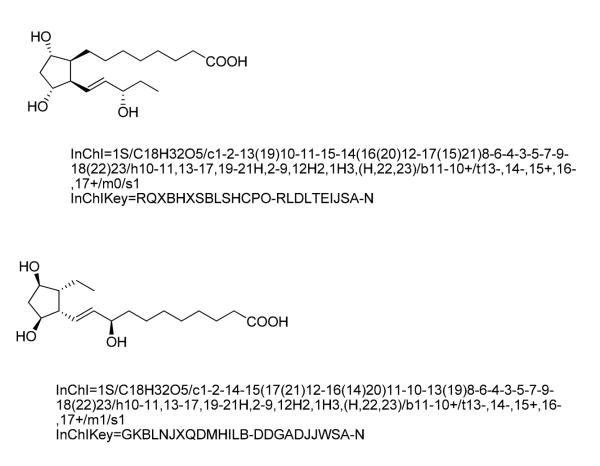
What does the future hold? As we are now well into the world of database searching of scientific information and published articles (PubMed), there is an alternative being promoted for complex organic molecule data retrieval. One emerging approach, the IUPAC International Chemical Identifier (InChI) (3)and its condensed format, the InChIKey (4,5), is based on a textual identifier that unambiguously defines the covalent arrangement of atoms as well as stereochemistry. Furthermore, the first 14 alphabetic characters of the InChIKey define the molecular connectivity of the molecule, whereas the next 10 alphabetic characters define stereochemistry, bond geometry and isotopic substitution. This separation of molecular connectivity from stereochemistry enables convenient searching for all stereoisomers of a particular prostane skeleton. For example, 8-iso-PGF2α (15-F2t-IsoP) has the following InChIKey: PXGPLTODNUVGFL-NAPLMKITSA-N and one can use “PXGPLTODNUVGFL” to perform a Google search or a LIPID MAPS “quick search” (http://www.lipidmaps.org) to identify compounds differing only in stereochemistry, bond geometry and isotopic substitution. An important point to note is that one cannot convert an InChIKey back to the full stereochemical structural representation, i.e. structure to InChIKey is a one-way transformation. However this becomes a moot point if the structures (and InChiKeys) have been deposited in a database (such as LIPID MAPS, PubChem, etc). In that case a search of the database with the InChiKey will retrieve the structure. Taking an example from two of the phytoprostanes described in the Figure 1 (Mueller) results in a corresponding InChI string and InChIKey for each. A question will be as to whether or not these are satisfactory replacements for a language-sensible nomenclature as opposed to a computer sensible nomenclature.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morrow JD, Harris TM, Roberts LJ., 2nd Noncyclooxygenase oxidative formation of a series of novel prostaglandins: analytical ramifications for measurement of eicosanoids. Anal Biochem. 1990;184:1–10. doi: 10.1016/0003-2697(90)90002-q. [DOI] [PubMed] [Google Scholar]
- 2.Bergstrom S, Shorvall J. The Isolation of Prostaglandin F from Sheep Prostate Glands. Acta Chem. Scand. 1960;14:1693–1700. [Google Scholar]
- 3.Official InChI (IUPAC) website: http://www.iupac.org/inchi/
- 4.Online conversion of Molfile to InChIKey. http://www.inchi.info/converter_en_html.
- 5.Example of a chemistry drawing tool for generating InChIKeys (Chemdraw only generates the InChI string not the InChIKey) http://www.chemaxon.com/marvin/examples/index.html.