Abstract
We have been studying the requirement for the aryl hydrocarbon receptor nuclear translocator (Arnt)-dependent DNA complex formation, which precede the activation of gene transcription. Using DEAE chromatography, we have obtained a Sf9 insect fraction F5 that is highly enriched with β-tubulin. F5 inhibits the formation of the AhR gel shift complex and this inhibition is sensitive to protease, suggesting that proteins that are present in this F5 fraction are responsible for the inhibition. Additional experiments have revealed that this inhibition is less pronounced in the presence of anti-β-tubulin IgG and β-tubulin enriched fraction from pig brain also inhibits the AhR gel shift formation. Sf9 β-tubulin interacts with Arnt and suppresses the binding of the AhR/Arnt heterodimer to its corresponding enhancer. Human β4 tubulin, which shares high sequence identity with Sf9 β-tubulin, suppresses the AhR-dependent luciferase expression by reducing the nuclear Arnt content and retaining Arnt in the cytoplasm. Fluorescence studies using the GFP fusion of human β4-tubulin has revealed that β4-tubulin prevents the localization of Arnt in Sf9 cells. Here we have provided evidence suggesting that β-tubulin may regulate the physiological content of Arnt.
Keywords: Arnt, β-tubulin, AhR
1. Introduction
Ah receptor nuclear translocator (Arnt) is a nuclear protein that is required for at least four signaling pathways, namely AhR [1], HIF-α [2], ER [3], and NFkB [4]. Arnt heterodimerizes with AhR to form a functional transcription factor which up-regulates xenobiotic metabolizing enzymes such as CYP1A1, CYP1A2, and CYP1B1 [5, 6]. Dimerization with Arnt is absolutely required for binding to the corresponding enhancer element DRE, followed by the activation of gene transcription. We are interested in determining the requirement for the formation of the Arnt-containing enhancer complexes.
Tubulin, a heterodimer of α- and β-tubulin, is the structural subunit of microtubules. Microtubules are dynamic cytoplasmic components which are important for cell proliferation; hence, microtubules are excellent cellular targets for cancer therapy [7]. There is good evidence supporting that alteration of the microtubule structure affects two of the Arnt-dependent pathways, namely the AhR and HIF1α signaling pathways. Inhibition of the AhR mediated CYP1A1 induction by microtubule destabilizing agents has been observed and the mechanism appears to be mediated through G2/M arrest triggered by these agents [8]. Short term treatment with microtubule destabilizing agents appears to stimulate NFκB-dependent transcription of the HIF-1α gene [9]; on the contrary, long term treatment with microtubule interfering agents inhibits HIF-1α protein accumulation [10].
In this report, we presented evidence supporting that β-tubulin suppresses the formation of AhR gel shift complex in vitro and inhibits Arnt-dependent DRE-driven luciferase expression. We showed that β-tubulin interacts with Arnt, retains Arnt in the cytoplasm, and reduces the nuclear Arnt content. Thus, alteration of the cellular β-tubulin content may affect the Arnt-dependent signaling pathways.
2. Materials and methods
2.1. Reagents
Anti-β-tubulin monoclonal IgG T4026 and goat anti-rabbit IgG-HRP were purchased from Sigma, St. Louis, MO. Anti-V5 monoclonal IgG (R960) was purchased from Invitrogen, Carlsbad, CA. Anti-Arnt IgG H-172 and goat anti-mouse IgG-HRP were purchased from Santa Cruz Biotechnology, Santa Cruz, CA. Sf9 and Hep3B cells were purchased from ATCC, Manassas, VA. Sf9 cells were grown in SF-900 II (Invitrogen, Carlsbad, CA) in the presence of 50 µg/ml of gentamycin and Hep 3B cells in Advanced MEM media (Invitrogen, Carlsbad, CA) supplemented with 5% FBS, L-glutamate (0.2 mM), penicillin (100 U/ml) and streptomycin (0.1 mg/ml). DRE oligos (OL5: 5’-TCGAGTAGATCACGCAATGGGCCCAGC-3; OL6: 5’-TCGAGCTGGGCCCATTGCGTGATCTAC-3’) were purchased from Invitrogen, Carlsbad, CA. 32P-gamma ATP was purchased from MP Biochemicals, Solon, OH. Other unspecified reagents were purchased from VWR, West Chester, PA.
2.2. Fractionation of Sf9 cytosol using DEAE chromatography
Sf9 cells were harvested at 1,000g for 10 min at 4 °C from Sf9 cell suspension. The pellet was resuspended in HEDG buffer (25 mM HEPES, pH 7.4, 1 mM EDTA, 1 mM DTT and 10% glycerol) containing 1 mM PMSF and 2 µg/ml of leupeptin. After three freeze-thaw cycles, the homogenate was centrifuged at 16,000g for 30 min at 4 °C. The supernatant was further centrifuged at 100,000g for 1 h at 4 °C to obtain the supernatant as Sf9 cytosol. Sf9 cytosol (150 mg) was used for DEAE ion-exchange chromatography using a linear salt gradient (25 to 500 mM KCl) over a period of 210 min at about 0.5 ml/min. The eluted fractions were analyzed by SDS-PAGE and fractions were arbitrarily pooled together to obtain Fractions 1 to 5 (F1 to F5).
2.3. Gel shift assay
Gel shift assay was performed as described previously using TALON-purified human AhR and Arnt [11]. In brief, during the “activation” step, Arnt was incubated with either AhR for 10 min at 30 °C in HEDG buffer in the presence or absence of protein factors and the ligand βNF (7 µM). Additon of poly(dI-dC) (200 ng, 10 min at room temperature) was performed, followed by addition of 32P-DRE (100,000 cpm, 10 min at room temperature). In some cases, protein factors were added after the addition of 32P-labelled probe. All gel shift samples were loaded onto a 4% native polyacrylamide gel and electrophoresis was conducted with 0.5× TBE at 4 °C.
2.4. Western blot analysis
To prepare the cytoplasmic extract, cells were lysed with HEDG buffer containing 1 mM PMSF and 2 µg/ml of leupeptin. After three freeze-thaw cycles, the sample was centrifuged at 16,000g for 10 min at 4 °C and the supernatant was the cytoplasmic extract. The pellet was then resuspended with the same buffer containing 0.4 M KCl. After 30 min on ice, the cell suspension was centrifuged at 16,000g for 10 min at 4 °C and the supernatant was the nuclear extract. For the whole cell lysate preparation, the cell pellet was resuspended with HEDG buffer containing 0.4 M KCl, 1 mM PMSF and 2 µg/ml of leupeptin. After 30 min on ice, the sample was centrifuged at 16,000g for 10 min at 4 °C and the supernatant was considered as the whole cell lysate. BCA reagents (Pierce) were used to determine the protein concentration. Samples were resolved by SDS-PAGE and then transferred onto a nitrocellulose or PVDF membrane using a Bio-Rad Trans-Blot unit (30 min, room temperature) or a Bio-Rad mini trans-Blot cell (1.5 h at 4 °C). After transfer, the membrane was blocked with 5% BSA in TBST buffer (25 mM Tris, pH 7.6, 150 mM NaCl, 0.05% Tween-20) for 1 h at room temperature and then incubated overnight with primary IgG at 4 °C, followed by secondary IgG-HRP for 1 h at room temperature. Antibodies used: anti-Arnt IgG H-172 (1:500), anti-V5 IgG (1:2,500–5,000), goat anti-rabbit IgG-HRP (1:5,000) and goat anti-mouse IgG-HRP (1:5,000) and anti-β-tubulin monoclonal IgG (1:500). SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL) was used to generate the results.
2.5. Preparation of PBT
PBT was isolated from pork brain by one cycle of assembly and disassembly of microtubules as described [12]. In brief, pork brain (100 g, obtained from local slaughterhouse) were homogenized in a pre-cooled blender in PM-4M buffer (100 mM Pipes, pH 6.9, 2 mM EGTA, 1 mM Magnesium Sulfate, 2 mM DTT, 4 M glycerol) at a ratio of 1 ml/g of tissue before being centrifuged at 6,500g for 15 min at 4 °C. The supernatant was further centrifuged at 96,000g for 75 min at 4 °C. GTP was added to the supernatant to make a final concentration of 0.5 mM. The GTP containing supernatant was incubated at 34 °C for 45 min in a water bath followed by centrifugation at 96,000g for 60 min at 27 °C. The pellets were resuspended in PM buffer (100 mM Pipes, pH 6.9, 2 mM EGTA, 1 mM magnesium sulfate, 2 mM DTT) before being incubated on ice for 30 min to depolymerize microtubules. The suspension was again centrifuged at 96,000g for 60 min at 4 °C and the supernatant was considered as PBT after a buffer exchange into HEDG buffer.
2.6. TALON co-precipitation assay
Co-precipitation of β-tubulin with baculovirus 6×His-tagged Arnt: Baculovirus Arnt (20 µl) was incubated with F5 (50 µg) and BSA (100 µg) at 30 °C for 10 min in a final volume of 100 µl of the co-precipitation buffer (50 mM HEPES, pH 7.4, 150 mM KCl, 10 % glycerol, 10 mM β-mercaptoethanol and 5 mM imidazole). After that, 900 µl of the co-precipitation buffer containing BSA (10 mg) and TALON resin (30 µl) was added, followed by rotation at 4 °C for 2 h. The pellet was washed five times with the co-precipitation buffer and the final pellet was suspended into 25 µl of SDS-PAGE treatment buffer (50 mM Tris, pH 6.8, 2 % SDS, 0.025 % bromophenol blue, 10 % glycerol and 5 % βME) and the samples were analyzed by Western blot using anti-β-tubulin IgG. Co-precipitation of 35S-labelled human AhR with baculovirus 6×His-tagged Arnt: Baculovirus Arnt (20 µl) was incubated with 35S-AhR (10 µl), different amount of F5, BSA (100 µg) and 10 µM βNF in a final volume of 100 µl of the co-precipitation buffer at 30 °C for 10 min. After that, 900 µl of the co-precipitation buffer containing BSA (10 mg) and TALON resin (30 µl) was added, followed by rotation at 4 °C for 2 h. The pellet was washed five times with the co-precipitation buffer and the final pellet was suspended into 25 µl of SDS-PAGE treatment buffer and analyzed by autoradiography of the dried SDS-PAGE gel.
2.7. Co-elution of β-tubulin with Arnt
Five 75 cm2 flasks containing about 80 % confluent Sf9 cells were infected with Arnt viral stock. After four days, cells were harvested and resuspended into 1 ml of the homogenization buffer (25 mM HEPES, pH 7.4, 0.4 M KCl, 10 % glycerol, 1 mM PMSF and 2 µg/ml of leupeptin). After three cycles of freeze/thaw and on ice incubation for 30 min, the homogenates were centrifuged at 16,000g for 30 min at 4 °C. The KCl concentration of the supernatant was adjusted to 80 mM before loading onto a column containing 125 µl of pre-equilibrated TALON resin. After the loading sample was passed through the resin for two times, the column was washed with a step gradient of 200 µl each of 25 mM HEPES, pH 7.4, 10 % glycerol containing various concentrations of KCl in the presence or absence of 10 mM imidazole (W1-W4). Then the bound proteins were eluted into three fractions (E1-E3) of 200 µl each of 25 mM HEPES, pH 7.4, 10 % glycerol containing 500 mM imidazole. As a negative control, the wild type Sf9 supernatant was used as the loading sample.
2.8. Baculovirus expression of human β4-tubulin and GFP2-Arnt
Human β4-tubulin cDNA, which was engineered with a C-terminal 6xHis tag, was cloned into BglII/EcoRI sites of pVL1392. Full length human Arnt cDNA was cloned into BglII/BamHI sites of pGFP2-C1 vector (Perkin Elmer) to generate GFP2-Arnt fusion cDNA. The GFP2-Arnt cDNA was then amplified and cloned into XbaI/BamHI sites of pVL1392. BaculoGold kit (BD Biosciences Pharmingen, San Diego, CA) was used to generate high titer viral stock, according to the manufacturer’s recommendation. Typically we performed TALON affinity purification to obtain the baculovirus expressed protein from five 150 mm plates of Sf9 cells 3 days after baculoviral infection using our published protocol [13].
2.9. Transient transfection studies
Hep3B cells were transfected with either the empty plasmid pcDNA or β4-tubulin expression plasmid (250 or 500 ng) once the cells reached 90% confluence in a 24-well plate. Each sample also contained 250 ng of the cyp1A1 promoter-driven luciferase reporter gene pGudluc1.1, 50 ng of the β-galactosidase plasmid as an internal control, and 1.6 µl of Fugene HD (Roche, Indianapolis, IN) and enough OPTI-MEM I media to make up a total volume of 25 µl per well. The transfection mix was added to 1 ml fresh complete media and incubated for 24 hours. Then 0.5 µl of 1 mM 3-MC ligand was added directly to the media containing the transfection mix to a final concentration of 0.5 µM. After 6 hours of induction, the cells were harvested and the luciferase and β-galactosidase assays using the Dual-Light reagents (Applied Biosystems, Foster City, CA) were performed using a TriStar LB941 microplate reader (Berthold, Oak Ridge, TN).
2.10. Cloning of Sf9 β-tubulin cDNA
Total RNA was extracted from Sf9 cells using the Epicentre MasterPure RNA purification kit. Reverse transcription followed by 3′-RACE were performed using the FirstChoice™ RLM-RACE Kit (Ambion, Austin, TX) according to the manufacturer's recommendation. A degenerate primer (OL160, 5’-ATGMGNGARATHGTNCAY-3’) was used with the 3’-RACE adapter primer to generate the cDNA product, which was cloned into pGEM-T (Promega, Madison, WI) and sequenced.
2.11. Fluorescence studies of GFP2-Arnt in Sf9 cells
In a 6 well plate, Sf9 cells were grown to about 70 % confluence before infection. Sf9 cells were infected with the high titer baculoviral stock of GFP2-Arnt (50 µl) in the presence or absence of the high titer β4-tubulin viral stock (150 µl). Cells were analyzed 3 days post-infection using a Nikon Eclipse TE200 fluorescence microscope and Image-Pro Plus and ImageJ softwares.
2.12. Statistical analysis
Unpaired two-tailed t test was performed using the Prism 5 software to show statistical significant results.
3. Results
3.1. Proteins present in F5 fraction inhibit the AhR/Arnt/DRE complex formation
In an effort to identify protein factors that are necessary to form the AhR/Arnt/DRE complex, we fractionated proteins in Sf9 cytosol, realizing that this cytosol is capable of restoring the AhR gel shift complex. We used DEAE chromatography to obtain pooled fractions F1 to F5 (Fig. 1A). The F5 fraction was highly enriched with a protein of about 55-kDa in size. When we performed a gel shift experiment with different amounts of F5, we observed that the addition of F5 caused inhibition of the gel shift complex formation in the presence of Sf9 cytosol in a dose-dependent manner (Fig. 1B, lanes 2–4). Further enrichment of the 55-kDa protein by gel filtration using FPLC showed that the potency of suppression increased (Fig. 1A, right panel and 1B, lanes 8–11). It was not a nonspecific protein factor since BSA caused no inhibition (Fig. 1B, lanes 5–7). In order to rule out the possibility that the inhibitory factor is not a protein, we performed gel shift assay using F5 that was treated with bovine pancreatic protease. The inhibitory effect of F5 was reversed by protease in a time-, temperature- and concentration-dependent manner (Fig. 1C). Thus we concluded that the inhibition is caused by functional proteins present in F5, possibly the enriched 55-KDa protein.
Fig. 1. F5 fraction from Sf9 cells inhibited the formation of the AhR/Arnt/DRE gel shift complex.
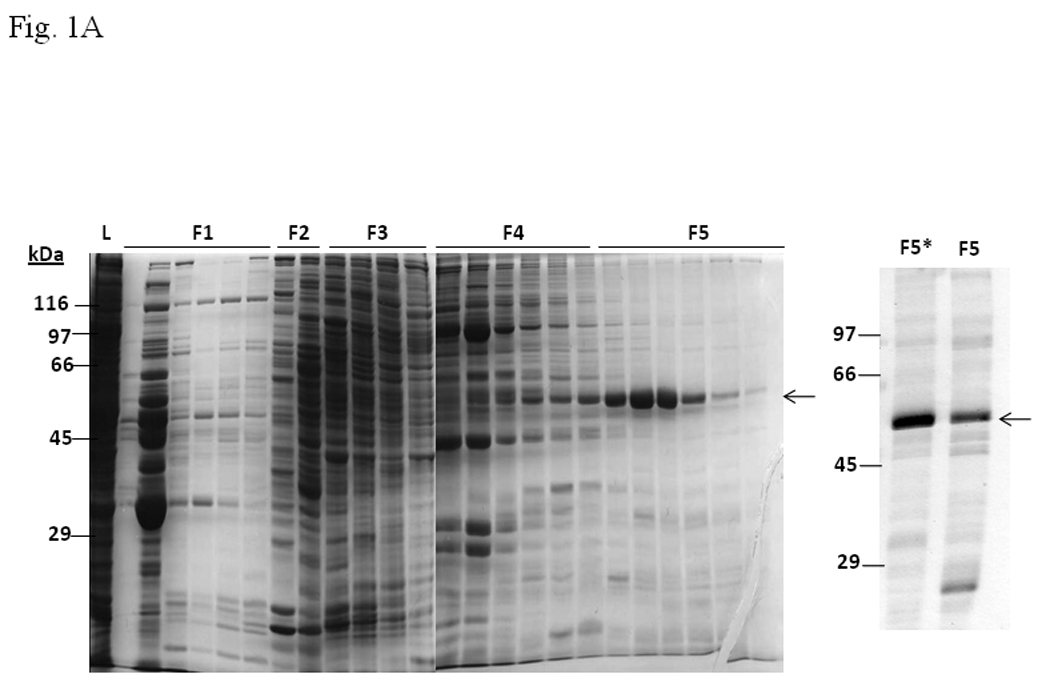

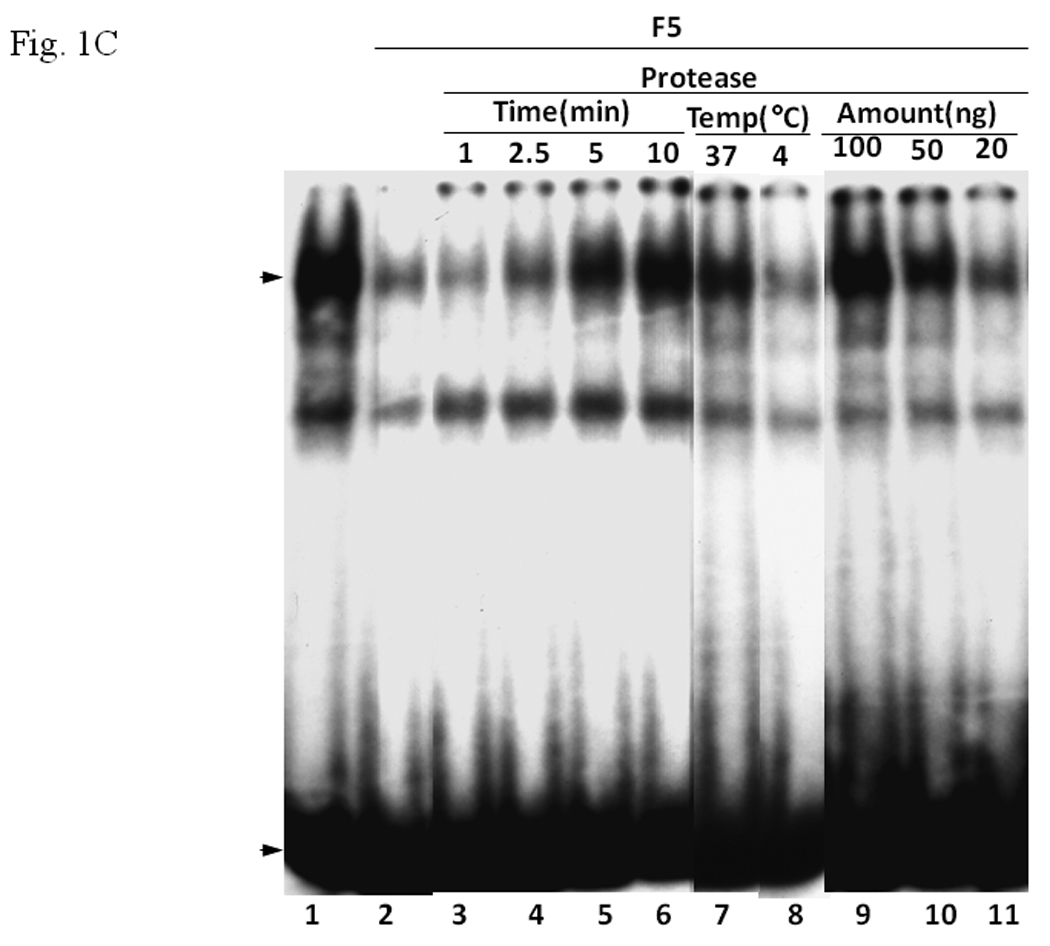
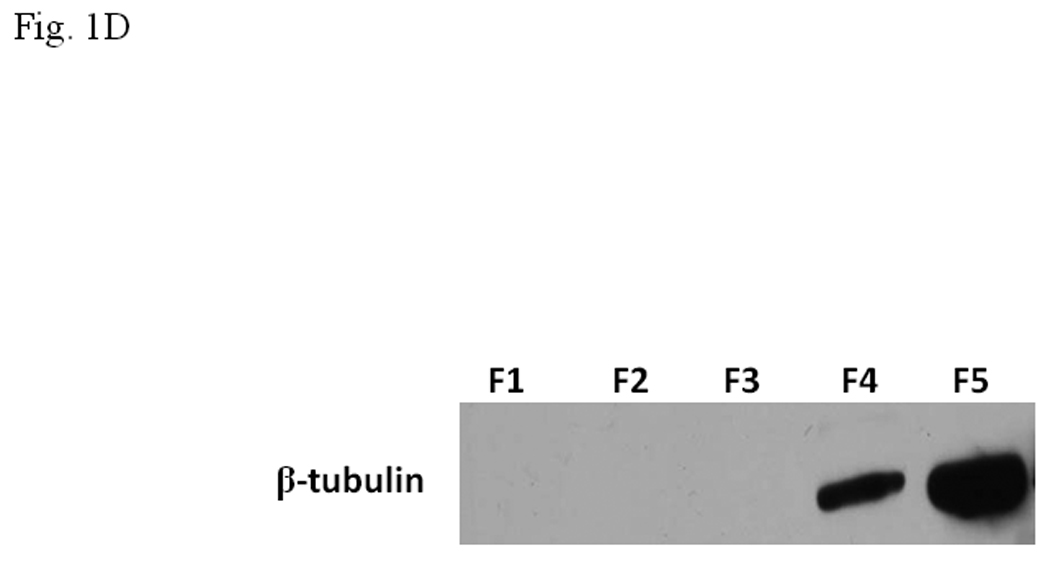
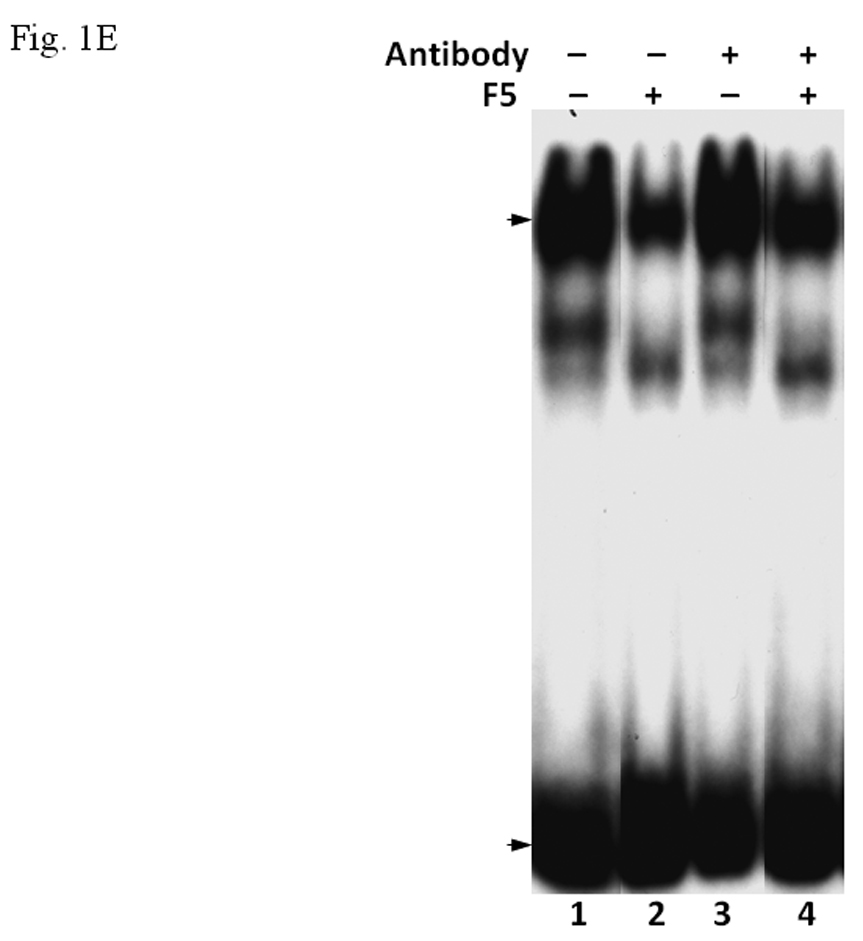
A. Coomassie blue staining of fractions from DEAE chromatography showing load (L) and fractions 1–5 (F1–F5). The arrow indicates the β-tubulin band. Right panel shows F5 and F5* (F5 subjected to gel filtration using FPLC to further enrich β-tubulin) of 5µg each. B. Gel shift assay showing the effect of F5 on the AhR/Arnt/DRE complex formation. All lanes contained baculovirus expressed human AhR, Arnt, Sf9 cytosol (2.5 µg) and 7 µM βNF. Concentration-dependent inhibition of the AhR/Arnt/DRE complex by F5 (lanes 2–4, 5–15 µg), BSA (lanes 5–7, 5–15 µg, negative controls) or F5 subjected to gel filtration to further enrich β-tubulin (F5*, lanes 9–11, 0.1–1 µg) was shown. Lanes 1 and 8 contained no F5, BSA or F5*. The upper arrow indicates the AhR/Arnt/DRE complex whereas the lower arrow indicates the free probe. Data are representative of two independent experiments. Lanes 8–11 contained 1µg of dIdC instead of the normal amount of 200 ng. C. Effect of protease (Sigma P4630, St. Louis, MO) treatment on F5 prior to gel shift assay. Protease inhibited the F5 effect in a time (lanes 3–6), temperature (lanes 7 and 8), and concentration (lanes 9–11) dependent manner. All lanes contained baculovirus expressed human AhR, Arnt, Sf9 cytosol (5 µg) and 7 µM βNF. Protease stock (1 µl) was used for each lane and the amount was listed in lanes 9–11. 100 ng of protease was used for lanes 3–8. The upper arrow indicates the AhR/Arnt/DRE complex whereas the lower arrow indicates the free probe. This gel shift assay was repeated twice with similar results. D. Western blot analysis of the F1–F5 fractions (10 µg) using anti-β-tubulin IgG T4026. E. Gel shift assay showing the effect of anti-β-tubulin IgG T4026 on the F5 inhibition of the AhR/Arnt/DRE complex. Gel shift assay showing that anti-β-tubulin IgG reversed the F5 inhibition (lanes 2 and 4) on the AhR gel shift complex. The upper arrow indicates the AhR/Arnt/DRE complex whereas the lower arrow indicates the free probe.
3.2. Sf9 β-tubulin inhibits the formation of the AhR/Arnt/DRE complex
The enriched 55-kDa protein in F5 fraction was subsequently purified by 2D gel electrophoresis (IEF/SDS-PAGE) followed by Edman degradation (Midwest Analytical, St. Louis, MO). Based upon the N-terminus sequence MREIVHIQAGQCGN, the identity of this purified protein in F5 was determined as β-tubulin. This finding was confirmed by Western blot using monoclonal IgG against β-tubulin (Fig. 1D). We examined whether anti-β-tubulin IgG would abolish the inhibitory effect of the F5 fraction. Upon incubation with anti-β-tubulin IgG with the gel shift samples containing F5, the inhibitory effect of F5 was partially reversed when compared to the controls, suggesting that β-tubulin is likely to cause this inhibition (Fig. 1E).
3.3. Pork brain tubulin inhibits the AhR/Arnt/DRE complex formation
Since our data suggested that β-tubulin inhibited the formation of the AhR/Arnt/DRE complex, we predicted that tubulin proteins from another source should have the same effect. Therefore, we enriched pork brain tubulin (PBT) according to published protocol [12] to test whether PBT would have similar inhibitory effect as F5. Coomassie blue staining and Western blot analysis showed that PBT contained an enriched amount of tubulin (Fig. 2A, top). After normalization of the tubulin amount by the Coomassie blue staining, F5 and PBT showed a different β-tubulin content on Western blot (Fig. 2A, bottom). This difference is likely caused by the specificity of the antibody against different species. We observed that the addition of PBT caused inhibition of the gel shift complex formation in a dose-dependent manner and the pattern was similar to F5 (Fig. 2B). F5 did not appear to affect the heterodimerization of AhR and Arnt since addition of F5 to the gel shift sample at the “activation” (heterodimerization) step was not required (Fig. 2C). F5 could equally suppress the gel shift formation when added after the radiolabelled probe, suggesting that F5 might interfere with the binding of the AhR/Arnt heterodimer to the DRE.
Fig. 2. PBT suppressed the AhR gel shift complex similarly as F5.
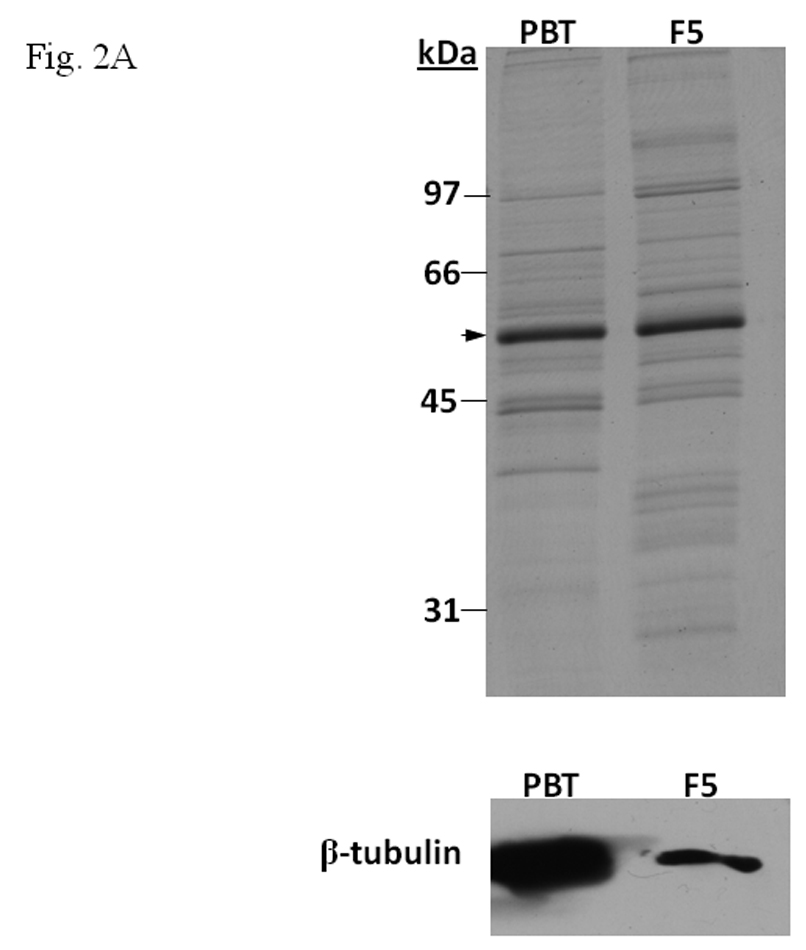
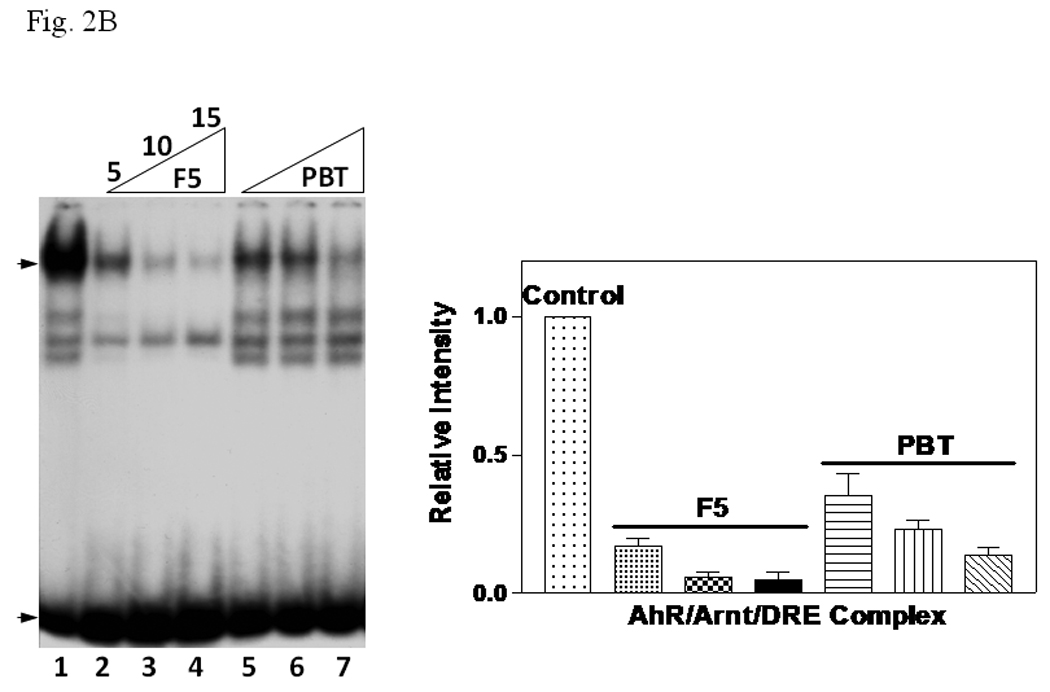
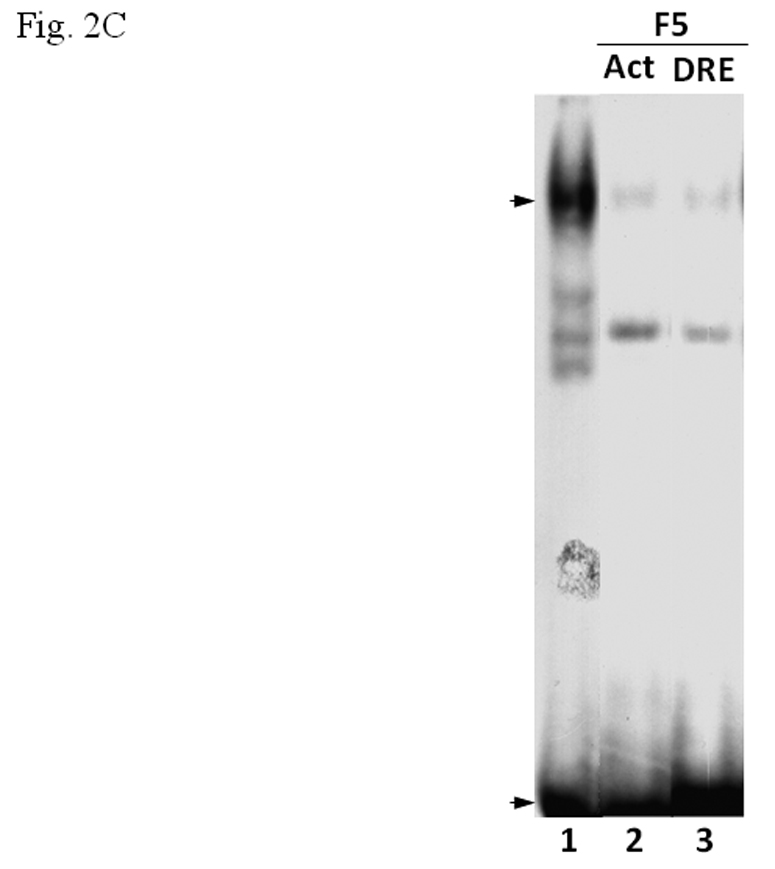
A. β-tubulin content in PBT and F5. Coomassie blue staining (top) of 2.5 µg each and Western blot analysis (below) of 2.5 µg of F5 and normalized amount of PBT based on Coomassie blue staining. The arrow indicates the β-tubulin band. B. Gel shift assay (left) showing the suppression of the AhR/Arnt/DRE gel shift complex formation by F5 and PBT. All lanes contained baculovirus expressed human AhR, Arnt, Sf9 cytosol (2.5 µg) and 7 µM βNF. B. Concentration-dependent inhibition of the AhR/Arnt/DRE complex by F5 (lanes 2–4, 5–15 µg) or PBT (lanes 5–7, equivalent to the β-tubulin content in lanes 2–4). The upper arrow indicates the AhR/Arnt/DRE complex whereas the lower arrow indicates the free probe. Graph (right) shows the quantification of the AhR/Arnt/DRE complex band intensity. The gel shift assay (left) was repeated three times and the intensity of the AhR/Arnt/DRE complex bands was measured by UN-SCAN-IT software with lane 1 arbitrarily set as 1 in each experiment to determine relative intensity. Error bars show means ± SD (n = 3). C. Gel shift assay showing that F5 suppressed the AhR gel shift complex formation when F5 was added before or after the activation step. All lanes contained baculovirus expressed human AhR, Arnt, Sf9 cytosol (2.5 µg) and 7 µM βNF. Lane 1, no F5; Lane 2, F5 was added before the activation step; lane 3, F5 was added after the activation step (added after 32P-DRE addition). The upper arrow indicates the AhR/Arnt/DRE complex whereas the lower arrow indicates the free probe.
3.4. β-Tubulin does not alter AhR-Arnt heterodimerization but interacts with Arnt
We performed TALON co-precipitation assay to address whether F5 affects the AhR-Arnt interaction. The baculovirus expressed 6His-Arnt was used as the bait to co-precipitate 35S-labelled AhR in the rabbit reticulocyte lysate in the presence or absence of F5. When F5 at the amount that exerted the inhibitory effect in the gel shift assay was added to the co-precipitation sample, we observed that the interaction between AhR and Arnt was unaffected (Fig. 3A, lanes 1–2). This observation remained unchanged even after addition of an excess amount of F5, suggesting that F5 does not interfere with the AhR/Arnt interaction (Fig. 3A, lane 3). When we performed TALON co-precipitation assay using baculovirus expressed 6His-Arnt as the bait to pull down β-tubulin in F5, we observed that Arnt clearly interacted with Sf9 β-tubulin (Fig. 3B). To further confirm our finding that Arnt interacts with Sf9 β-tubulin, we analyzed the affinity purified fractions of baculovirus expressed 6His-Arnt to examine whether β-tubulin could be co-eluted with Arnt. The results showed that β-tubulin was co-eluted with Arnt in the Arnt-infected Sf9 lysate; β-tubulin was found in the E1 and E2 fractions where Arnt was eluted (Fig. 3C). This elution of β-tubulin was not observed when the wild type Sf9 lysate was used as the loading sample, confirming that β-tubulin interacts with Arnt and does not bind directly to the TALON resin.
Fig. 3. β-Tubulin did not affect AhR-Arnt interaction but interacted with Arnt.

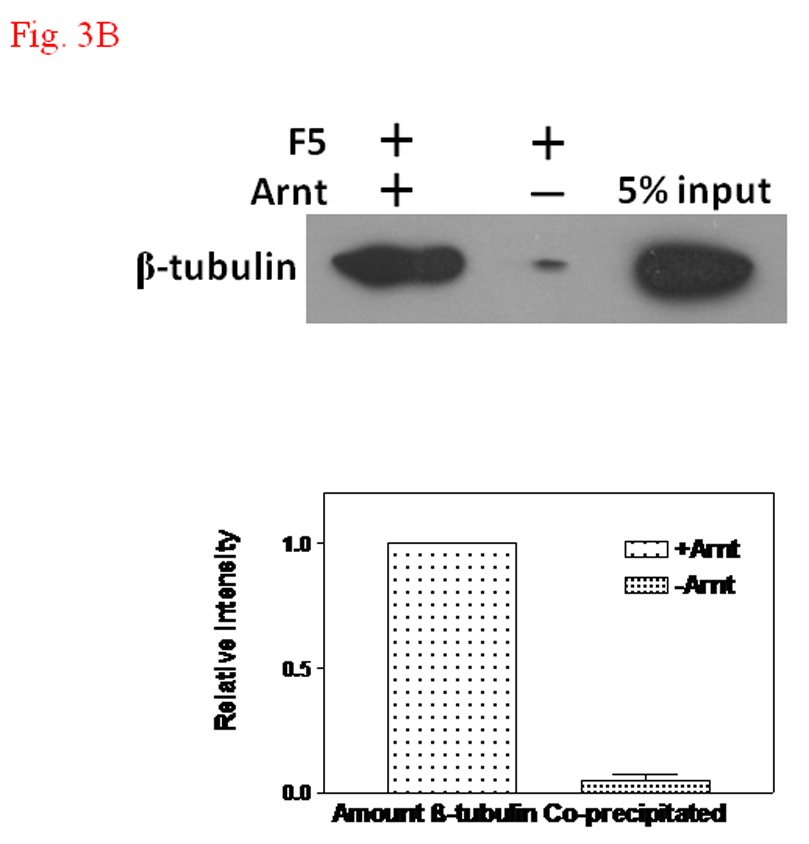
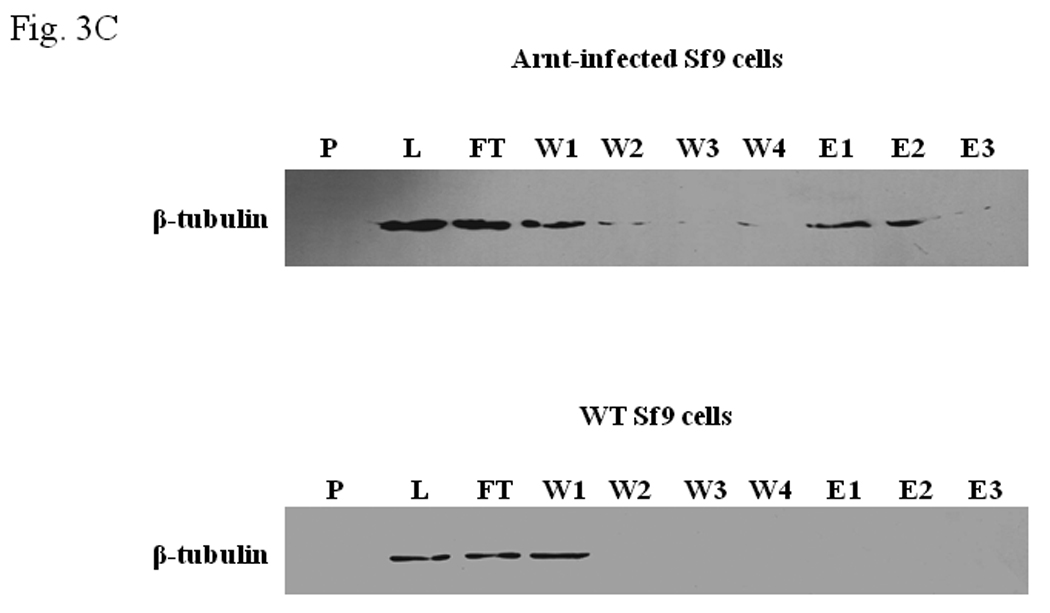
A. TALON co-precipitation assay showing that F5 did not inhibit the heterodimerization of AhR and Arnt. In vitro translated 35S-AhR and baculovirus expressed human Arnt were incubated with TALON-resin. The bound proteins were analyzed by autoradiography after SDS-PAGE. Arrow indicates 35S-AhR. Lane 1, 35S-AhR and Arnt, no F5; lane 2, 35S-AhR and Arnt plus F5 (100 µg); lane 3, 35S-AhR and Arnt plus F5 (300 µg); and lane 4, 35S-AhR only. Lane 5 shows the intensity of 5% of 35S-AhR used for the experiment. B. TALON co-precipitation assay showing that β-tubulin interacted with Arnt. Western blot analysis (top) using anti-β-tubulin IgG. Baculovirus expressed human Arnt was used as the bait to co-precipitate β-tubulin. 5% input shows the intensity of 5% of F5 used for the experiment. Graph (bottom) showing the quantification of the β-tubulin band intensity. The Western blot (top) was repeated three times and the intensity of the β-tubulin bands was measured by UN-SCAN-IT software. The co-precipitated β-tubulin by Arnt was arbitrarily set as 1 (n = 3, means ± SD). C. Western blot analysis showing the β-tubulin content in different Sf9 fractions in the presence (top) or absence (bottom) of baculovirus expressed human Arnt. P, pellet; L, load; FT, flow through; W1-4, wash with the purification buffer with a step gradient of 80 mM KCl (W1), 300 mM KCl (W2), 600 mM KCl (W3), 600 mM KCl and 10 mM imidazole (W4); E1–E3, fractions eluted with the purification buffer plus 500 mM imidazole. Anti-β-tubulin IgG (T4026) was used to detect β-tubulin. WT = wild type.
3.5. Human β4-tubulin shares high sequence identity with Sf9 β-tubulin
We performed 3’-RACE using the Sf9 cDNA library to determine the partial cDNA sequence of Sf9 β-tubulin. The Sf9 cDNA library was generated using the FirstChoice RLM-RACE kit (Ambion, Austin, TX). The translated protein sequence of Sf9 β-tubulin shared significant sequence identity with β-tubulin in drosophila and human; among the human β-tubulins, Sf9 β-tubulin is most homologous to β2- and β4-tubulin but less to β1-tubulin (Fig. 4). However, we did not locate the poly-A sequence at the 3’ end of the raced cDNA. Based on the full length cDNA sequences of human β-tubulins and the molecular weights of human and Sf9 β-tubulins, we probably identified close to 95% of the full length sequence of Sf9 β-tubulin. To examine whether human β-tubulin might interfere with the AhR function, we obtained the full length human β4-tubulin cDNA from ATCC and cloned the cDNA into pCDNA6/V5 (Invitrogen, Carlsbad, CA) for transient transfection studies. Expression of human β4-tubulin in the rabbit reticulocyte lysate (Promega, Madison, WI) was confirmed by the expression of a V5-tagged protein that has a molecular weight of about 55 kDa (data not shown).
Fig. 4. Sequence alignment of different β-tubulins.

Drosophila beta 2 (droso b2, NP_524290), human beta 2 (h beta-2, NP_821133), human beta 4 (h beta-4, NP_006077), Sf9 and human beta 1 (h beta-1, NP_11040). The sequences of the drosophila and human β-tubulins were obtained from BLAST databases. The consensus regions are shaded.
3.6. Human β4-tubulin suppresses the AhR-dependent luciferase expression by reducing the nuclear Arnt content in Hep3B cells
We investigated whether human β4-tubulin would affect the AhR-dependent activation of gene transcription in Hep3B cells. 3MC induced the DRE-driven luciferase expression in Hep3B cells by 3.6 fold and this 3MC-dependent activity was suppressed by human β4-tubulin in a dose-dependent manner, with 40 % suppression when 500 ng of the β4-tubulin plasmid was co-transfected (Fig. 5A). Our Western results showed that the transfected β4-tubulin was exclusively found in the cytoplasm (Fig. 5B). The presence of the transfected cytoplasmic β4-tubulin reduced the nuclear Arnt content and retained the Arnt protein in the cytoplasm. In the absence of the transfected β4-tubulin, Arnt was nuclear and no Arnt was detectable in the cytoplasm. The transfected β4-tubulin allowed the detection of Arnt in the cytoplasm and reduced the nuclear Arnt content, although most of the Arnt protein remained nuclear.
Fig. 5. Human β4-tubulin suppressed AhR signaling in Hep3B cells.
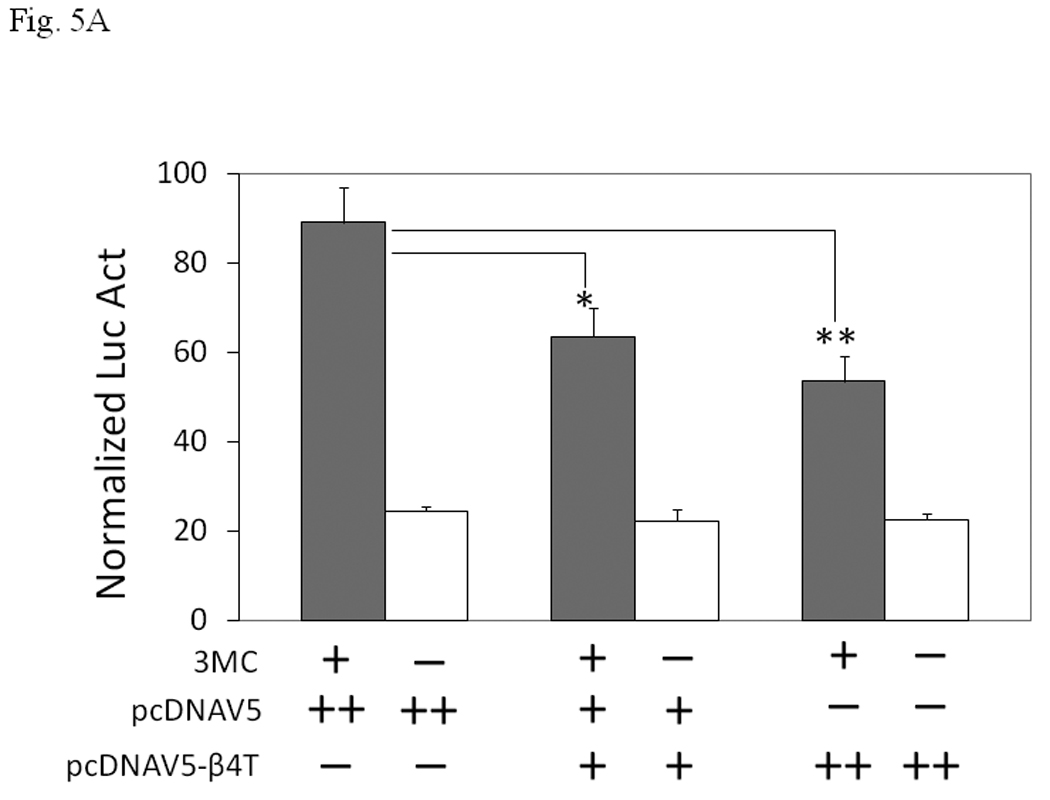
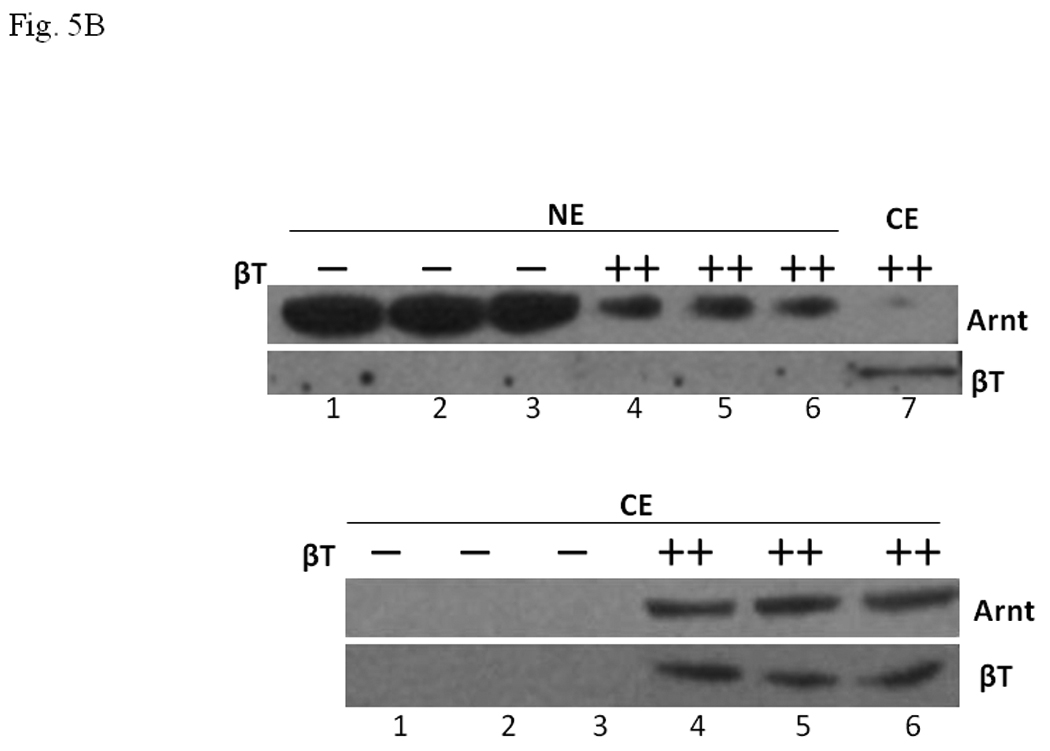
A. Transient transfection results showing suppression of the 3MC-driven luciferase activity by 250 ng (+) or 500 ng (++) of human β4-tubulin plasmid (pcDNAV5-β4T). Transfected DNA was normalized to 800 ng in all transfected samples with the empty plasmid pcDNAV5. Error bars show means ± SD (n = 3). *p < 0.01 whereas **p < 0.003. This experiment was repeated 4 times with similar results. B. Western blot analysis showing the effect of the transfected β4-tubulin on the nuclear and cytoplasmic Arnt content. Cells were transfected with 500 ng (−) of empty plasmid pcDNAV5 or 500 ng (++) of β4-tubulin plasmid pcDNAV5-β4T. Either 35 µg (top) or 50 µg (bottom) of proteins per lane was loaded. This experiment was performed in triplicate in the presence of 1 µM 3MC for 6 h: (Top) lanes 1–3, nuclear extract (NE) with empty plasmid; lanes 4–6, nuclear extract (NE) with β4 tubulin plasmid; lane 7, cytoplasmic extract (CE) with β4 tubulin plasmid. (Bottom) lanes 1–3, cytoplasmic extract (CE) with empty plasmid; lanes 4–6, cytoplasmic extract (CE) with β4 tubulin plasmid. Lane 7 (top) is the same sample as lane 4 (bottom), except that the film exposure time was different.
3.7. Human β4-tubulin alters the localization of GFP2-Arnt in Sf9 cells
We examined whether β4-tubulin would change the subcellular localization of Arnt. Baculoviral stock containing human β4-tubulin and GFP2-Arnt cDNA were used to infect Sf9 cells. Expression of the GFP2-Arnt protein was highly localized and appeared to be nuclear in Sf9 cells 3 days after infection (Fig. 6, left). However, when cells were co-infected with baculovirus β4-tubulin and GFP2-Arnt, the fluorescence became diffuse throughout the cells (Fig. 6, right), suggesting that the interaction between β4-tubulin and Arnt interferes with the Arnt localization within cells.
Fig. 6. Human β4-tubulin suppressed the GFP2-Arnt localization in Sf9 cells.
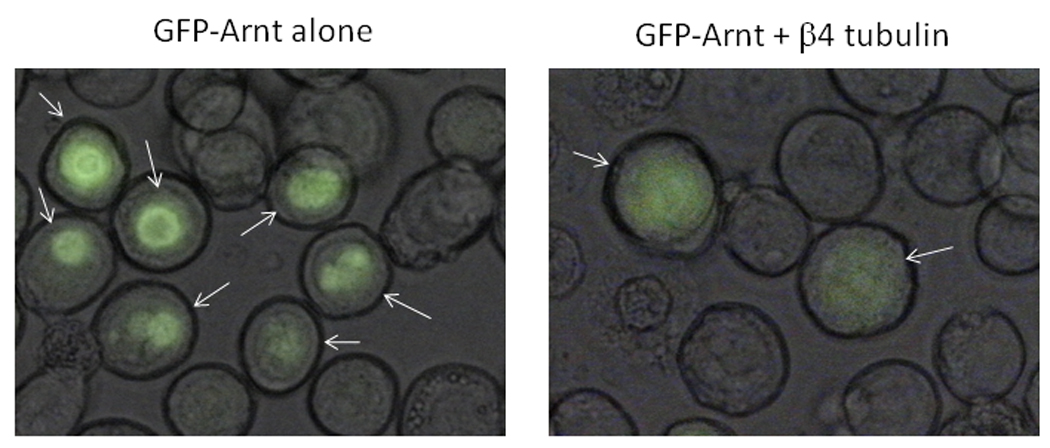
Sf9 cells were infected for 3 days with baculovirus GFP2-Arnt +/− baculovirus β4-tubulin in a 6-well plate. High titer baculovirus GFP2-Arnt (50 µl) +/− high titer baculovirus β4 tubulin (200 µl) were used for infection. Arrows indicate cells with GFP2-Arnt expression. GFP-Arnt was more diffuse in the presence of β4-tubulin.
4.0. Discussion
In an effort to better understand how AhR activates gene transcription, we are interested in identifying accessory proteins that are necessary for the formation of the AhR/Arnt/DRE complex. We have reported that p23 and CyP40 restore the AhR/Arnt/DRE gel shift complex [11, 14], but it appears that additional protein factors are involved. Sf9 cytosol causes the ligand-dependent AhR/Arnt/DRE gel shift complex formation using baculovirus expressed human AhR and Arnt [13]. Therefore, we examined whether this activity can be enriched using DEAE chromatography. When we performed gel shift assay to assess the involvement of F5 fraction in the AhR/Arnt/DRE complex formation, we observed that as little as 0.5 µg of F5 was able to cause some formation of the gel shift complex, suggesting that protein factors are present in this fraction. However, the intensity of the gel shift complex was surprisingly suppressed when an increased amount of F5 was added to the gel shift samples. This observation prompted us to explore whether an inhibitory factor was present in F5. We concluded that it is the β-tubulin protein in F5 that causes the suppression of the AhR/Arnt/DRE complex formation for a number of reasons: (1) β-tubulin was highly enriched in F5; (2) protease-treated F5 did not exhibit suppression, suggesting that protein was involved in this suppression; (3) a highly enriched β-tubulin fraction from pig brain also suppressed the AhR gel shift complex formation in a similar fashion; (4) anti-β-tubulin IgG partially reversed the gel shift suppression caused by F5 and (5) more enriched β-tubulin fraction from F5 showed noticeably more potent suppression of the AhR gel shift complex.
It has been reported that microtubule targeting agents such as colchicine and nocodazole inhibit CYP1A1 induction in HepG2 and primary rat hepatocytes via an unclear mechanism [8]. In mouse hepatoma 1c1c7 cells, long term treatment of nocodazole causes G2/M arrest and this cell cycle arrest is thought to be responsible for the suppression of the TCDD-induced CYP1A1 expression at both the mRNA and protein levels [15, 16]. During long term exposure to many microtubule interfering agents, there may be some indirect effect that reduces the amount of TCDD-dependent nuclear translocation of AhR and interestingly increases the AhR protein synthesis [17]. Nonetheless, all these data argued that the cell cycle status, which is influenced by the microtubule structure, affects the AhR-mediated CYP1A1 induction. But our data prompted us to explore whether the amount of β-tubulin, regardless of its structure, might influence the Arnt-dependent signaling through direct protein-protein interaction. β-tubulin appears to inhibit the binding of the AhR/Arnt heterodimer to the DRE rather than to inhibit the AhR-Arnt heterodimerization. We postulated that β-tubulin physically interferes with the binding of this heterodimer to the DRE in vitro and this interference is unlikely to be physiological since most of the β-tubulin actions are believed to be cytoplasmic and the formation of the AhR/Arnt/DRE complex is in the nucleus. Tubulin cytoskeleton appears to be not involved in the TCDD-driven nuclear translocation of AhR since disruption of microtubule structure by one-hour treatment of colchicine did not affect the translocation [18], suggesting that there may not be any direct physical contact between the cytoplasmic AhR and β-tubulin. In addition, we were not able to observe any interaction between β-tubulin and AhR (data not shown). However, we found that Sf9 β-tubulin interacts with Arnt. This β-tubulin-Arnt interaction appears to interfere with the formation of the Arnt-DNA complex in general since both the AhR and HIF-1α (data not shown) gel shift complexes were inhibited by β-tubulin.
We partially cloned the Sf9 β-tubulin cDNA and discovered that human β4-tubulin is highly homologous to Sf9 β-tubulin. β4-tubulin suppresses 3MC-induced luciferase activity that is mediated through AhR. Our data clearly showed that an increased amount of β4-tubulin retains Arnt in the cytoplasm, thereby decreasing the Arnt content in the nucleus for Arnt-dependent function. This finding is substantiated by our observations that Sf9 β-tubulin interacts with Arnt and β4-tubulin diffuses the GFP2-Arnt fluorescence in Sf9 cells. Thus it is conceivable that human β-tubulin may play a role in affecting the nuclear targeting of Arnt after protein synthesis (Fig. 7). This is unique as compared to the data from studies using microtubule interfering agents because the β-tubulin effect we observed appears to depend on the β-tubulin content rather than the microtubule structure. Neither colchicine nor taxol affected the β-tubulin inhibition of the AhR gel shift complex formation (our unpublished observation). The events which determine the nuclear localization of Arnt are unclear at present and alteration of these events might change the nuclear Arnt content which in turn should affect all Arnt-dependent functions in the nucleus. We propose that one of these events is related to the β-tubulin content: an increase in the β-tubulin content in the cytoplasm may reduce the endogenous Arnt content in the nucleus. Although an increase in the cytoplasmic β-tubulin content may affect normal cellular function, this hypothesis provides a means for rational drug design to modulate the Arnt-dependent signaling pathways by a β-tubulin-related mechanism. For example, by mapping out the interaction surface between Arnt and β-tubulin, one could design a small molecule that has a high affinity to Arnt and prevents Arnt from translocation into the nucleus after its synthesis in the cytoplasm. In addition, a β-tubulin analog, which associates with Arnt but does not involve in tubulin cytoskeleton formation, could be generated to limit the amount of the cellular Arnt content.
Fig. 7. Proposed mechanism on how β-tubulin plays a role in the AhR signaling.
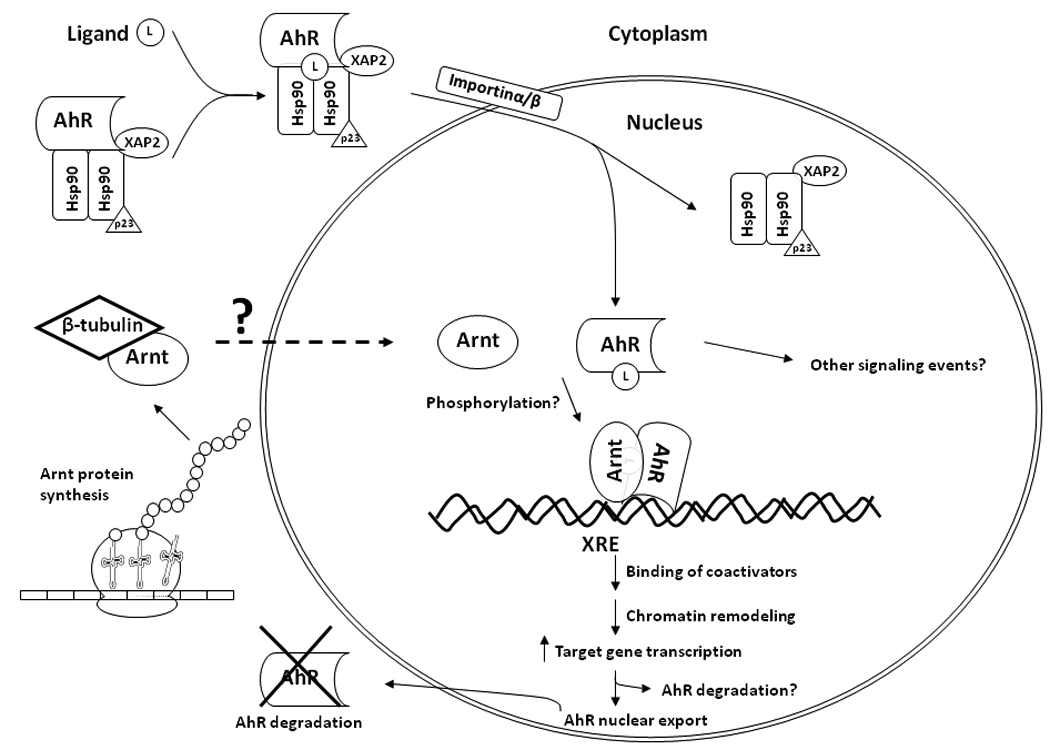
By interacting with Arnt in the cytoplasm, β-tubulin controls the Arnt content in the nucleus. Increased cytoplasmic β-tubulin content reduces the nuclear Arnt content, causing a reduction of the amount of the AhR/Arnt heterodimer formation upon ligand activation. Activation of the AhR-dependent gene transcription is compromised when the cytoplasmic β4-tubulin content increases.
The β-tubulin C-terminal sequence exhibits high level of heterogeneity among different isotypes. Human β4-tubulin shares 85% sequence identity to chicken class III β4-tubulin that is selectively expressed in brain [19]. However, β4-tubulin is overexpressed in some epithelial ovarian tumors that are resistant to taxol [20]. This overexpression alters the assembly dynamic of microtubules in a way that the tumors become resistant to the taxol effect [21]. It appears that the overall amount of β tubulin remains unchanged, except that there is an isotype difference in β-tubulin expression that causes the resistance to occur [20]. Although Sf9 β-tubulin is most homologous to human β4-tubulin, the C-terminal sequence of Sf9 β-tubulin is still unknown. It is therefore conceivable that another β-tubulin may interact with Arnt physiologically since β4-tubulin is not normally found in other cell types that express Arnt.
In summary, we have provided evidence that β-tubulin inhibits the AhR gel shift complex formation and the subsequent activation of gene transcription is also inhibited. Mechanistically, β-tubulin appears to inhibit the binding of the AhR/Arnt heterodimer to the DRE and this inhibition likely involves the interaction between β-tubulin and Arnt. Increased β4-tubulin content in the cytoplasm suppresses localization of Arnt, increases the cytoplasmic Arnt content, and decreases the nuclear Arnt content. The detailed mechanism for the physiological role of β-tubulin in Arnt disposition remains to be investigated.
Acknowledgements
We thank Marcia Foxx for her help in fluorescence microscopy and Dr. Mike Denison (UC Davis) for providing us the luciferase reporter plasmid pGudluc1.1. This work is supported by a grant from the National Institutes of Health (R01 ES014050).
Abbreviations
- AhR
aryl hydrocarbon receptor
- Arnt
Ah receptor nuclear translocator
- HIF-1α
hypoxia inducible factor 1 alpha
- βME
β-mercaptoethanol
- DRE
dioxin response element
- HRE
hypoxia response element
- βNF
β-napthoflavone
- HEDG
25 mM HEPES, pH 7.4, 1 mM EDTA, 1 mM DTT, 10 % glycerol
- PBT
pork brain tubulin
- 3MC
3-methylchloranthrene
- EPO
erythropoietin
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoffman EC, Reyes H, Chu FF, Sander F, Conley LH, Brooks BA, et al. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science. 1991;252:954–958. doi: 10.1126/science.1852076. [DOI] [PubMed] [Google Scholar]
- 2.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunnberg S, Pettersson K, Rydin E, Matthews J, Hanberg A, Pongratz I. The basic helix-loop-helix-PAS protein ARNT functions as a potent coactivator of estrogen receptor-dependent transcription. Proc Natl Acad Sci USA. 2003;100:6517–6522. doi: 10.1073/pnas.1136688100. Epub 2003 May 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright CW, Duckett CS. The aryl hydrocarbon nuclear translocator alters CD30-mediated NF-kappaB-dependent transcription. Science. 2009;323:251–255. doi: 10.1126/science.1162818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Ann Rev Cell Dev Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- 6.Puga A, Ma C, Marlowe JL. The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem Pharmacol. 2009;77:713–722. doi: 10.1016/j.bcp.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jordan A, Hadfield JA, Lawrence NJ, McGown AT. Tubulin as a target for anticancer drugs: agents which interact with the mitotic spindle. Med Res Rev. 1998;18:259–296. doi: 10.1002/(sici)1098-1128(199807)18:4<259::aid-med3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 8.Dvorak Z, Vrzal R, Ulrichova J, Pascussi JM, Maurel P, Modriansky M. Involvement of cytoskeleton in AhR-dependent CYP1A1 expression. Curr Drug Metab. 2006;7:301–313. doi: 10.2174/138920006776359310. [DOI] [PubMed] [Google Scholar]
- 9.Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. Microtubule disruption utilizes an NFkappa B-dependent pathway to stabilize HIF-1alpha protein. J Biol Chem. 2003;278:7445–7452. doi: 10.1074/jbc.M209804200. [DOI] [PubMed] [Google Scholar]
- 10.Escuin D, Kline ER, Giannakakou P. Both microtubule-stabilizing and microtubule-destabilizing drugs inhibit hypoxia-inducible factor-1alpha accumulation and activity by disrupting microtubule function. Cancer Res. 2005;65:9021–9028. doi: 10.1158/0008-5472.CAN-04-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shetty PV, Bhagwat BY, Chan WK. P23 enhances the formation of the aryl hydrocarbon receptor-DNA complex. Biochem Pharmacol. 2003;65:941–948. doi: 10.1016/s0006-2952(02)01650-7. [DOI] [PubMed] [Google Scholar]
- 12.Williams RC, Jr, Lee JC. Preparation of tubulin from brain. Methods Enzymol. 1982;85(Pt B):376–385. doi: 10.1016/0076-6879(82)85038-6. [DOI] [PubMed] [Google Scholar]
- 13.Chan WK, Chu R, Jain S, Reddy JK, Bradfield CA. Baculovirus expression of the Ah receptor and Ah receptor nuclear translocator. Evidence for additional dioxin responsive element-binding species and factors required for signaling. J Biol Chem. 1994;269:26464–26471. [PubMed] [Google Scholar]
- 14.Shetty PV, Wang X, Chan WK. CyP40, but not Hsp70, in rabbit reticulocyte lysate causes the aryl hydrocarbon receptor-DNA complex formation. Arch Biochem Biophys. 2004;429:42–49. doi: 10.1016/j.abb.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Scholler A, Hong NJ, Bischer P, Reiners JJ., Jr Short and long term effects of cytoskeleton-disrupting drugs on cytochrome P450 Cyp1a-1 induction in murine hepatoma 1c1c7 cells: suppression by the microtubule inhibitor nocodazole. Mol Pharmacol. 1994;45:944–954. [PubMed] [Google Scholar]
- 16.Santini RP, Myrand S, Elferink C, Reiners JJ., Jr Regulation of Cyp1a1 induction by dioxin as a function of cell cycle phase. J Pharmacol Exp Ther. 2001;299:718–728. [PubMed] [Google Scholar]
- 17.Vrzal R, Daujat-Chavanieu M, Pascussi JM, Ulrichova J, Maurel P, Dvorak Z. Microtubules-interfering agents restrict aryl hydrocarbon receptor-mediated CYP1A2 induction in primary cultures of human hepatocytes via c-jun-N-terminal kinase and glucocorticoid receptor. Eur J Pharmacol. 2008;581:244–254. doi: 10.1016/j.ejphar.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 18.Petrulis JR, Kusnadi A, Ramadoss P, Hollingshead B, Perdew GH. The hsp90 Co-chaperone XAP2 alters importin beta recognition of the bipartite nuclear localization signal of the Ah receptor and represses transcriptional activity. J Biol Chem. 2003;278:2677–2685. doi: 10.1074/jbc.M209331200. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan KF, Cleveland DW. Identification of conserved isotype-defining variable region sequences for four vertebrate β tubulin polypeptide classes. Proc Natl Acad Sci USA. 1986;83:4327–4331. doi: 10.1073/pnas.83.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kavallaris M, Kuo DY, Burkhart CA, Regl DL, Norris MD, Haber M. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific β-tubulin isotypes. J Clin Invest. 1997;100:1282–1293. doi: 10.1172/JCI119642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamath K, Wilson L, Cabral F, Jordan MA. βIII-tubulin induces paclitaxel resistance in association with reduced effects on microtubule dynamic instability. J Biol Chem. 2005;280:12902–12907. doi: 10.1074/jbc.M414477200. [DOI] [PubMed] [Google Scholar]


