Abstract
The cortisol awakening response (CAR) is a burst of cortisol in response to awakening from sleep that is superimposed on the circadian rhythm of cortisol. Determination of the CAR is contingent on the timing of sample collection: A delay between waking and collection of the first sample may affect the rise of the CAR, and could explain equivocal findings reported in the literature. We evaluated the impact of a delay between wake time and collection of waking cortisol samples on the CAR. Two methods were used to identify wake time: polysomnography (PSG) and self-report (S-R). Participants (total n = 207, mean age 74.0 ± 7.2 yrs) included bereaved older adults (n = 35), caregivers (n =50), patients with insomnia and co-morbid medical disorders (n = 68), and the healthy older adults (n = 54). We used ANOVA to test if a delay > 15 minutes affected the CAR. We also fitted cubic spline models to assess expected cortisol levels, the expected CAR, and the expected decrease in CAR. Wake times measured by PSG and S-R did not differ significantly. Large delays were observed (for both PSG and S-R) between wake time and collection of the waking cortisol sample (24.8 ± 32.2 min for PSG and 28.3 ± 49.2 min for S-R). Both statistical methods indicated that a delay > 15 minutes between wake time and first cortisol sample collection significantly affected the CAR (p's < .005); later collection times were associated with smaller CAR values. Later collection times and reduced CAR values may affect the interpretation of clinical associations. Our data also show that S-R assessments of wake time perform equally well to PSG for evaluating adherence with CAR sampling procedures.
Keywords: sleep, cortisol awakening response, delay, PSG, self-report, older adults
1. Introduction
A sharp rise in circulating cortisol occurs in response to awakening from sleep (Carlsson F et al., 2006;Clow et al., 2004;Pruessner et al., 1997;Wilhelm et al., 2007). The cortisol awakening response (CAR) is characterized by a 50-75% increase in cortisol levels within the first 30 minutes after awakening eventually achieving morning acrophase (Hucklebridge et al., 1998;Pruessner et al., 1997). The CAR is considered a useful and easy to measure marker of hypothalamic-pituitary-adrenal (HPA) axis activity (Kirschbaum and Hellhammer, 1994;Pruessner et al., 1997). For example, several studies report that individuals with high levels of work stress or depressive symptoms show a greater CAR (Ehlert et al., 2005;Pruessner et al., 2003;Steptoe et al., 2005). In contrast, others have shown a blunted CAR in individuals with depressive symptoms (Kuehner et al., 2007;Stetler and Miller, 2005) or high levels of burnout (de Vente W. et al., 2003;Kudielka et al., 2006). Women tend to show larger waking values compared to men, but by 30 minutes after awakening the CAR is similar between men and women (Clow et al., 2004;Wust et al., 2000). Older adults with high positive well-being and low negative well-being have a lower CAR than older adults who score lower on positive well-being and higher on negative well-being suggesting a better adapted stress response (Evans et al., 2007). Why and how these individuals differ clinically remains unclear.
The timing of sample collection is important for determination of the CAR, and in naturalistic studies difficult to control (Kunz-Ebrecht et al., 2004). Participants often do not or can not obtain samples when requested (Kudielka et al., 2003) for a variety of reasons. A delay in taking the first sample may result in a blunted CAR as a result of the higher waking cortisol value. As a result, delays of 10 minutes or more are considered “non-compliant” (Kunz-Ebrecht et al., 2004) and often these samples are excluded from analyses. (Because there are numerous reasons for late samples, including factors both within and outside the participant's control, we prefer the more neutral term “delayed” to “non-compliant”). The variable findings with respect to observed CARs (i.e. increased and blunted CARs and depressive symptoms) (Edwards et al., 2001;Pruessner et al., 1997;Wust et al., 2000) suggest that a methodological concern actually lies within the reported versus actual wake time with respect to the saliva sample collection (Dockray et al., 2008;Wright and Steptoe, 2005). Presently, the reliability of using self-reported wake times with respect to the start of the morning rise is unclear.
The majority of studies examining the CAR rely on self-report wake times. However, to date only one report has examined the discrepancy between objectively-assessed and self-reported wake time in relation to the CAR. Dockray and colleagues used actigraphy (a watch-like device that measure rest/activity cycles which are subsequently analyzed for determination of wake/sleep patterns) and self-report to determine wake time. They found that delays (>15min) (according to both methods) between waking and cortisol sampling resulted in higher values for the waking sample, but had no effect on the CAR (Dockray et al., 2008). However, actigraphy has a low sensitivity to detect wakefulness (Paquet et al., 2007), and therefore may not be a reliable comparator to self-reported wake time.
No study has compared polysomnography (PSG)-assessed and self-report wake times in relation to the CAR. Furthermore, no study has evaluated the CAR in elders. This is important since aging is associated with alterations in the HPA axis and cortisol secretion (Deuschle et al., 1997) which have consequences for hippocampal function (Buckley and Schatzberg, 2005) and lymphocyte sensitivity (Bauer et al., 2000). Thus, the aim of our study was twofold. First, we evaluated the impact of the delay between wake time and collection of wake time cortisol samples on subsequent CAR values in a cohort of elders. We hypothesized that individuals with smaller delays between wake time and sampling time would have lower waking cortisol values and a larger CAR. Second, we sought to evaluate the discrepancy between self-reported wake time and PSG-assessed wake time and its effect on the CAR. This would help determine whether self-reported wake time is a reliable measure for use in cortisol awakening response studies conducted at home.
2. Methods
2.1 Participants
Participants were drawn from component projects that comprise the “Aging Well, Sleeping Efficiently: Intervention Studies” (AgeWise) program project (P01 AG 20677, PI Monk). Details on the program project and the specifics of each study including inclusion/exclusion criteria, recruitment, and procedures have been previously published (Hall et al., 2008). Briefly, eligible adults, aged 60 and older, completed a standardized data collection protocol in addition to the specific methods of each protocol. This included similar methods for saliva collection and assessments of sleep, health, functioning, and major mediators and moderators thought to significantly impact relationships between sleep and health in older adults.
AgeWise participants (total n = 207) included bereaved older adults (n = 35: 8M, 27F), caregivers (n = 50: 11M, 39F), individuals with insomnia and co-morbid medical disorders (n = 68: 24M, 44F), and the healthy older adults (n = 54: 31M, 23F). Forty-one participants were excluded from an initial sample of 248 for this analysis. Reasons for exclusion included currently taking corticosteroids (N = 12), or failing to complete cortisol sampling (N = 29). There were no differences in demographics, covariates, or waking cortisol values between those included in analyses and those excluded. The study was approved by the University of Pittsburgh Institutional Review Board. Written informed consent was obtained from all participants prior to collection of data.
2.2. Procedure
2.2.1. Cortisol assessment and analysis
Ten saliva samples were collected over 2 to 3 consecutive days (depending on the project) using Salivettes (saliva sampling devices, Sarstedt, Rommelsdorf, Germany). Collection times were immediately after awakening, 30 minutes post awakening, 3pm, 8pm, and bed time. Cortisol data for waking and waking+30min samples from a single day are reported here. Participants were also asked to note their time of awakening and the time of each saliva collection on a log. For participants who slept in the lab samples were collected by nursing staff; for participants who slept at home samples were collected and stored in their freezer until collected by a staff member. Participants were instructed to collect the waking and waking +30 min. sample before brushing their teeth, before eating or drinking (except water and medications), and before any exercise. Participants were instructed to collect saliva samples by chewing on the cotton roll included in the Salivette tube for 60 seconds so that the roll was saturated. Salivettes were stored at −80°C until assay. Salivettes were centrifuged at 3000rpm for 5 min, resulting in a clear supernatant of low viscosity. Free salivary cortisol levels were measured in duplicate using a commercial available chemiluminescence assay (CLIA) (IBL Hamburg, Germany). The lower concentration limit of this assay is 0.44 nmol/liter; intrassay coefficients of variance were less than 8%. Any sample > 50 nmol/L was repeated.
2.2.2. Polysomonography
Polysomonographic sleep studies included a screening night as well as one to two nights of basic PSG for sleep scoring. Sleep studies were conducted in the N-CTRC or participants' home using ambulatory monitors (Compumedics Siesta). In both settings, participants were prepared for sleep studies by trained sleep technicians. Saliva samples were collected concurrently with PSG studies.
Sleep studies were conducted at participants' usual sleep-wake times, as determined by sleep diaries (Monk et al., 1994) completed 2-weeks prior to PSG sleep studies. The basic PSG montage included bilateral central and occipital electroencephalogram (EEG) channels, electro-oculogram (EOG), submentalis electromyogram (EMG) and a V2 electrocardiogram (EKG) lead. On the screening nights, participants were monitored for sleep disordered breathing and periodic limb movements. Trained PSG technicians used Stellate Harmonie software to visually score sleep records in 20-s epochs. Polysomnogrpahic-assessed wake time was defined as the first epoch of wakefulness followed by sustained wakefulness.
2.2.3. Self-report wake times
Participants were asked to indicate their time of awakening on a sleep diary (in-home) or a post-sleep evaluation questionnaire (lab) completed upon awakening from PSG studies.
2.2.4. Covariates
Several covariates associated with the CAR were examined, including age, sex, BMI, sleep quality, depression, stress scores, and medication use. Categorical variables were used to evaluate the possible influence of individual projects and study setting (laboratory or ambulatory), and whether samples were collected on a weekday or weekend. We evaluated weekday versus weekend since an effect on the CAR has been shown previously (Kunz-Ebrecht et al., 2004;Wilhelm et al., 2007). Given the evidence of association with the CAR we assessed the 19-item Pittsburgh Sleep Quality Index (PSQI, (Buysse et al., 1989)), (Backhaus et al., 2004), the Hamilton Rating Scale for Depression (HRSD25) (Hamilton, 1967;Stetler and Miller, 2005), and the Perceived Stress Scale-4 item (Cohen et al., 1983;de Vente W. et al., 2003).
2.2.4. Statistical analysis
Demographic and clinical descriptive variables for the whole group were examined. Prior to analyses, transformations were used to normalize the variables. Non-parametric tests were used if transformations did not improve normality. The relationship between the covariates and cortisol levels and the CAR were examined using Spearman correlation coefficients or ANOVAs as appropriate. The difference between PSG and self-reported wake time was tested using a Wilcoxon signed rank test. We first tested the effect of delay on the CAR using the same categories as reported by Dockray et al. (Dockray et al., 2008) (time between wake and collection of waking sample: < 1 min, 1-15min, and >15min). ANOVAs were used to test if there were differences in waking cortisol levels and CAR between groups using both PSG and self-reported wake times. All significant ANOVA's were followed-up with pairwise comparisons using the Tukey procedure. Results were considered significant with 2-tailed p < .05.
We also employed advanced statistical modeling techniques to identify a threshold time for determination of the CAR. A cubic smoothing spline model was fit using MATLAB 7.1 (MathWorks, 2005) to assess expected cortisol levels as a continuous function of elapsed time from wake time, the expected CAR as a continuous function of the delay from wake time to the taking of first sample, and the expected difference between actual CAR and predicted CAR as a function of the delay from wake time to taking of the first sample. A random effect for subject was included to account for varying baseline cortisol levels among subjects, the generalized maximum likelihood (GML) was used to estimate variance components and smoothing parameters, and 95% “Bayesian confidence intervals” are reported to draw inference on the curves (Gu Chong, 2002). All reported wake times were referenced to PSG since there was no difference in waking cortisol values when S-R wake time was used instead.
3. Results
The analyses included 207 elders aged 60-93 years, including 133 (64%) females and 74 (36%) males. The majority were Caucasian (94%). Table 1 describes the demographic and clinical characteristics of the sample. A majority were taking medications, particularly cardiac medicines (37.2%), antilipemics (41.6%), anti-hypotensive agents (21.7%), as well as OTC analgesics (71.0%) and vitamins (79.7%). PSG-assessed mean sleep latency was 32.0 ± 35.9 min and sleep efficiency was 72.5 ± 12.9% indicating some difficulty with sleep continuity in the sample as a whole. There was no effect on waking cortisol or the CAR for group (F(3,203) = .45, p =0.72; F(3,203) = .45, p = .72), whether the sample was taken on a weekday vs weekend (Wilcoxon exact p = .57; p = .97), or if the participant slept in the lab or at home (F(1,205) = 1.47, p = .23; F(1.205) = .72, p = .40).. There were also no significant effects of age (stratified by < 75 or ≥ 75 years) (F(1,205) = .08, p = .77; F(1.205) = 2.51, p = .12) or gender (F(1,205) = 1.36, p =.25; F(1,205) = 3.62, p = .06) on waking cortisol or the CAR. Lastly, waking values, but not CAR levels, were significantly associated with PSQI (r=−0.15, n=180, p=0.047) and HAMD (r=−0.20, n=182, p=0.007); higher waking cortisol values were associated with poorer sleep quality and greater depressive symptoms. The PSS was not associated with waking cortisol or CAR levels.
Table 1.
Demographic, sleep and cortisol characteristics
| N | % or Mean (STD) |
Range | |
|---|---|---|---|
| Demographic variables | |||
| Age | 207 | 74.0 (7.1) | 60.1-93.3 |
| Patient Type | |||
| % Bereaved | 35 | 16.9% | |
| % Caregiver | 50 | 24.1% | |
| % Insomnia | 68 | 32.9% | |
| % Healthy Controls | 54 | 26.1% | |
| %Female | 133 | 64.3% | |
| %Caucasian | 195 | 94.2% | |
| BMI | 198 | 26.6 (4.1) | 17.6-40.3 |
| Hamilton Rating Scale-25 item | 182 | 7.0 (5.2) | 0-25 |
| Pittsburgh Sleep Quality Index | 180 | 6.6 (4.0) | 1-16 |
| Perceived Stress Scale- 4 item | 180 | 3.3 (2.8) | 0-11 |
| Medications | |||
| Antihistamines | 22 | 10.6% | |
| Cardiac | 77 | 37.2% | |
| Antilipemics | 86 | 41.6% | |
| Hypotensive agents | 45 | 21.7% | |
| Analgesics | 147 | 71.0% | |
| Benzodiazepines | 18 | 8.7% | |
| Antidepressants | 21 | 10.1% | |
| Gastrointestinal | 72 | 34.8% | |
| Hormones | 61 | 29.5% | |
| Vitamins | 165 | 79.7% | |
| Diuretics | 38 | 18.4% | |
| Sleep Variables | |||
| Apnea-hypopnea Index (#/hr) | 205 | 15.2 (15.7) | 0-81.7 |
| Periodic Limb Movement Index (#/hr) |
203 | 5.0 (5.3) | 0-27.1 |
| Total Sleep Duration (PSG) | 207 | 429.0 (73.0) | 242-622 |
| Sleep Latency (PSG) | 207 | 32.0 (35.9) | 0.3-234 |
| Sleep Efficiency (PSG) | 207 | 72.5 (12.9) | 28.8-96.4 |
| Final Wake Time (PSG) | 207 | 6:26 (1:11) | 2:40 - 10:54 |
| Final Wake Time (Self-report) | 194 | 6:22 (1:18) | 1:00 - 10:30 |
|
Cortisol (Awakening Response Variables) |
|||
| Waking Cortisol Sample Time | 207 | 6:51 (1:02) | 4:35-11:00 |
| Waking + 30 Cortisol Sample Time | 207 | 7:27 (1:03) | 5:01-11:30 |
| Difference between PSG and Self- report wake time (minutes) |
194 | −3.3 (53.1) | −297.3 to 225.7 |
| Difference between PSG wake time and first cortisol sample (minutes) |
207 | 24.8 (32.2) | −44 to 228.7 |
| Difference between self-report wake time and first cortisol sample (minutes) |
194 | 28.3 (49.2) | −44 to 315.3 |
| Mean cortisol (nmol/L) (wake up) | 207 | 16.7 (11.9) | 1.6-92.9 |
| Mean cortisol (nmol.L) (wake +30) | 207 | 24.6 (15.5) | 1.3-140 |
| CAR | 207 | 7.9 (11.1) | −16.6 to 51.6 |
3.1 Differences in PSG and self-reported wake times and sampling times
Mean wake time defined by PSG (6:26AM ± 1:11h) did not differ significantly from self-reported wake time (6:22AM ± 1:18h). The difference between the two was 3.3 ± 53.1 minutes (Wilcoxon signed rank test, p = .22) and the correlation between the two was r = 0.81, p<0.0001. Subjects who slept at home did not differ from those who slept in the lab in their self-reported wake time versus PSG wake time (Wilcoxon exact, p=0.43), waking cortisol levels (lab =15.8 ± 11.0 nmol/L vs. at-home = 17.7 ±12.8 nmol/L, t=−1.21, df=205, p=0.23), or CAR (in-lab = 8.5 ±11.4 nmol/L vs. at-home = 7.2 ± 10.9 nmol/L, t=0.85, df=205, p=0.40).
On average, waking samples were collected at 6:51 AM ± 1:02h and the wake+30min sample at 7:27AM ± 1:03h. The average difference between collection of the waking and wake +30 sample was 36.2 ± 6.3 min with a range of 20-55min. The mean reported delay between PSG assessed wake time and taking the waking cortisol sample was 24.8 ± 32.2 min, while the delay for S-R wake time was 28.3 ± 49.2 min. Out of the 207 participants, we identified 38 who were considered adherent based on S-R (≤ 15 min), but who were considered non-adherent based on PSG. Table 2a shows that 17 participants (8%) took their waking sample with no delay after awakening (<1min), whereas 101 participants (49%) had a delay of > 15 minutes according to PSG. There were differences in the waking cortisol value between these groups (F(2,204) = 7.19, p = .001) where subjects with a discrepancy of > 15 minutes had higher cortisol levels than subjects with discrepancies of < 15 min. Table 2b shows that according to S-R, 34 participants (18%) took their sample with no delay after awakening (< 1min) whereas 80 participants (41%) had a discrepancy of > 15 minutes. The difference in waking cortisol was not significant (F(2,191) = 1.87, p = .16). The delay between wake time (PSG or S-R) and first cortisol sample also significantly influenced the CAR (F(2,204) = 7.18, p = .001 (PSG) and F(2, 191) = 5.47, p = .005 (S-R)). Those who had a delay > 15min had a smaller rise than both groups (< 1min and 1-15min) for PSG-assessed sleep and a smaller rise than the 1-15min delay in S-R assessed sleep (Figures 1a and 1b). Lastly, we separately examined those without a delay (≤ 15 min) and those with a delay (> 15 min) to determine whether associations between the CAR and other factors, including PSQI, HAMD and PSS scores, differed. There were no significant relationships in either subgroup (p's > .30)
Table 2a.
Based on PSG wake times
| < 1 minute difference n=17 (a) |
1-15 minute difference n=89 (b) |
> 15 minute difference n=101 (c) |
F (df), p-value | |
|---|---|---|---|---|
| Wake up Cortisol (nmol/L) |
13.3 (9.5) | 14.6 (11.8) | 19.2 (12.0) | F(2,204)=4.36, p=0.014 b < c |
| CAR (nmol/L) | 12.4 (11.2) | 10.3 (11.6) | 5.0 (10.0) | F(2,204)=7.19, p=0.001 a, b > c |
Table 2b.
Based on self-report wake times
| < 1 minute difference n=34 (a) |
1-15 minute difference n=80 (b) |
> 15 minute difference n=80 (c) |
F (df), p-value | |
|---|---|---|---|---|
| Wake up Cortisol (nmol/L) |
14.1 (8.4) | 16.1 (11.1) | 18.4 (13.9) | F(2,191)=1.87, p=0.16 |
| CAR (nmol/L) | 8.9 (9.2) | 10.4 (13.1) | 4.8 (8.9) | F(2,191)=5.47, p=0.005 b > c |
Figure 1.
Figure 1a and 1b display the waking and waking+30min mean values for participants who took their waking sample < 1 minute, 1-15 minutes and > 15 minutes after waking according to PSG-assessed wake (1a) and SR-assessed wake )1b).
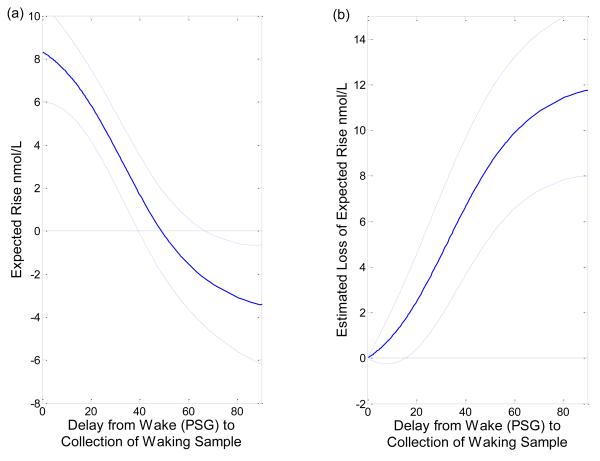
Figure 2 displays raw wake and wake+30min cortisol samples from each subject. Figure 3 displays the smoothing spline estimate of the expected cortisol level as a function of minutes from wake along with 95% confidence interval. The continuous effect of delay upon taking the waking sample on CAR is quantified through smoothing spline estimates in Figure 3. Figure 4a shows the expected CAR as a function of delay in the taking of the waking sample from wake time and the 95% confidence interval. The CAR decreases as the delay in taking the first sample increases. The provided 95% “Bayesian” confidence intervals have the across the curve interpretation that, if this experiment were repeated a large number of times, on average the confidence intervals would contain 95% of the true curve. The 95% confidence interval for the expected CAR contains zero, and the expected CAR is not significantly different from zero, when the wake+30 min sample is taken between 39.3 minutes, and 70.7 minutes after PSG-assessed wake time. The expected CAR is statistically less than zero if that sample is taken more than 70 minutes after PSG-assessed wake time. The expected difference in CAR when the waking sample is taken at wake time versus if there is a delay is displayed in Figure 4b along with its 95% confidence interval. A delay of more than 15.7 minutes, from wake time to taking the waking sample, will result in a significantly smaller CAR.
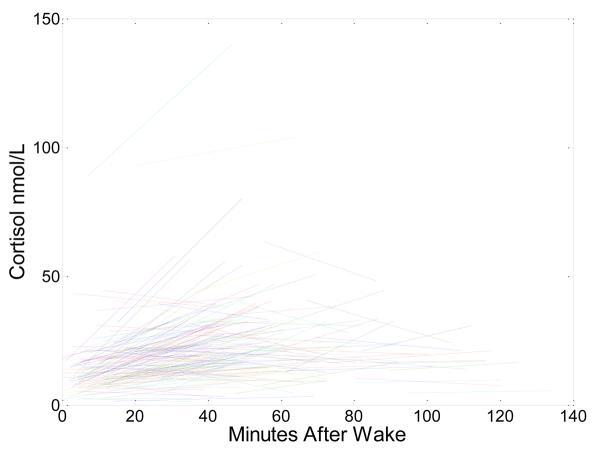
Figure 2.
is the raw data plot of waking and waking+30 min samples for all participants
Figure 3.
displays the smoothing spline estimate of the expected cortisol level as a function of minutes from wake along with 95% confidence interval. The continuous effect of delay upon taking the waking sample on CAR is quantified through smoothing spline estimates.
Figure 4.
Figure 4a shows the expected CAR as a function of delay in the taking of the waking sample from wake time and the 95% confidence interval. The CAR decreases as the delay in taking the first sample increases.
Figure 4b shows the expected difference in CAR when the waking sample is taken at wake time versus if there is a delay.
4. Discussion
The results indicate that delays > 15 min between wake time and collection of the waking cortisol sample significantly affect CAR values. These findings corroborate concerns expressed by other investigators regarding reliability of sample collection in the home environment (Kunz-Ebrecht et al., 2004) given that the delay between wake and collection of the first saliva sample can dramatically affect the measured level of cortisol, and therefore the CAR (Kunz-Ebrecht et al., 2004;Wright and Steptoe, 2005). Delays of < or >10 minutes are used to define non-adherence by various research groups. Non-adherence rates using this 10 min cutoff have been reported at 7.3% (Kunz-Ebrecht et al., 2004), 20% - 38% (Broderick et al., 2004), and 26% and 39% (Kudielka et al., 2003) (Kudielka et al., 2007). Three of these studies used electronic tracking caps that time stamp the saliva collections via bottle openings (Broderick et al., 2004;Kudielka et al., 2003;Kudielka et al., 2007). Our findings are comparable even though we used a different cutoff time: 49% and 41% were not adherent based on PSG and S-R wake time. A growing number of reports associate various psychiatric and psychosomatic disorders with an altered CAR. Thus, significant delays in sampling may have implications for the interpretations of the CAR and its clinical relevance (Dockray et al., 2008;Kudielka et al., 2003;Wright and Steptoe, 2005).
In this study, waking cortisol values were affected by the delay between wake time and first cortisol sampling according to PSG, but not according to self-report. PSG-determined wake, scored in 20-s epochs, was defined as first epoch of wakefulness followed by no further epochs of sleep. Self-report – determined wake was ascertained as what time the participant stated they woke up. Unfortunately the definition of waking varies by modality used (Tryon, 2004). In a previous report using PSG-assessed sleep, Wilhelm and colleagues clearly identified the CAR as a response to awakening (Wilhelm et al., 2007), which is similar to our findings. In contrast to our study, participants had waking samples taken in the dark and in a supine position, and they were all woken at 7:00am. What is not known is how the duration of waking influences the CAR. Our findings suggest that for a large percentage of participants wake defined by PSG or S-R might capture differential aspects of waking. If PSG-defined wake is taken as “true” or “physiological” waking, we contend that data from participants who collect the sample within 16 minutes of PSG or S-R wake time are reliable and usable.
There are various reasons for a delay to occur between wake and collection of the first sample, such as participant characteristics (Pruessner et al., 1997). For instance, some older adults, particularly men, may require additional time to urinate after awakening. Another consideration is that participants in this study may have wanted to remove the PSG electrodes and wires before taking the first cortisol sample. In addition to participant characteristics that may influence the delay, there are other sources of variance which may also directly affect cortisol secretion and CAR values, including stress (Pruessner et al., 2003), sleep quality (Backhaus et al., 2004), the process of awakening and dissociations with other physiological systems (e.g. adrenocorticotropic hormone (ACTH)) (Hellhammer et al., 2009). Wilhelm and colleagues showed that cortisol and ACTH rose more steeply in the 30 min after awakening than in the hour prior to awakening; and that ACTH rose more steeply than cortisol in the last hour of sleep (Wilhelm et al., 2007). Given the multiple sources of variance on cortisol and the CAR, and to minimize any delay associated with adherence, it is incumbent upon investigators to provide clear and understandable instructions so that CAR interpretations are not compromised (Kudielka et al., 2003). For instance, as done in the study by Kunz-Ebrecht et al (Kunz-Ebrecht et al., 2004) physical demonstration of exactly how samples should be taken and stored, as well as how they should be taken (i.e. take the first sample while lying in bed) would be beneficial.
Interestingly, CAR values were significantly affected by delay according to both PSG and S-R sleep measures. Our findings are partially consistent to those observed by Dockray et al (Dockray et al., 2008) and Wright and Steptoe (Wright and Steptoe, 2005) who found that those who delayed > 10 minutes between waking and taking the first sample had higher waking values. With regards to the CAR, our findings remain in contrast to those of Dockray et al (Dockray et al., 2008), but are consistent with those of Wright and Steptoe (Wright and Steptoe, 2005) in which a steeper rise was observed among those considered adherent. Interestingly, the application of spline modeling confirmed the results of Dockray et al that a delay > 15 minutes may introduce problems when assessing the CAR. Our data suggest that a small delay (< 16 minutes) is acceptable between waking and the collection of the waking cortisol sample thereby providing support for the reliability of assessing the CAR in participants' homes rather than coming to the laboratory. It also encourages the use of more samples since the acceptable window is extended beyond 10 minutes. Still, the responsibility rests on the ability of investigators to maintain consistent methodologies and provide clear instructions for obtaining cortisol samples at home so that better comparisons across studies can be made.
Psychoneuroendocrinology studies increasingly rely on collection of data in the field (Broderick et al., 2004;Clow et al., 2004). With the introduction of salivary assays, the number of these studies has increased due to their non-invasive nature and simplicity of sample collection (Kirschbaum and Hellhammer, 1994). However, relying on participant self-report may be difficult if the participants are not effectively instructed or are unable to adhere to instructions. Our results indicate that self-reported wake time is as reliable as PSG-assessed wake time. Given the growing evidence that the CAR is a response to awakening (Born et al., 1999;Dockray et al., 2008;Wilhelm et al., 2007), investigators should have confidence in self-report with regards to the integrity and interpretation of the CAR.
Several factors limit the generalizability of these findings. First, our sample consisted of older adults, and included primarily those with significant sleep complaints. Most were taking some medication, which may have affected cortisol values in unexpected ways, and there was considerable variability in sleep duration and wake times. Several investigators found similar findings among middle-aged adults 40-60 years (Broderick et al., 2004), 45-59 years (Kunz-Ebrecht et al., 2004) and 50-67 years of age (Kudielka et al., 2007). However, we cannot state if self-report wake time in young adults or in adults with no sleep complaints is similarly reliable with regards to assessment of the CAR. Moreover, we cannot confirm if adherence with a saliva collection protocol would differ significantly in younger cohorts. Presently, too few reports are available describing adherence. It appears however, as if the CAR remains stable across the age span (Pruessner et al., 1997). Second, the cohort consisted of four different groups (bereaved, caregivers, people with insomnia and healthy older adults) who were enrolled into intervention studies aimed at improving or maintaining good quality sleep. Thus, the protocol was not designed exclusively to answer the hypotheses posed in this report. Moreover, to be included bereaved, caregivers and people with insomnia had to have significant sleep complaints. Nonetheless, we found no effect by group on wake time or time of cortisol collection. Inherent differences between samples collected at-home or in the lab could affect waking levels and the CAR. The two settings resulted in different instructions on how to collect saliva samples. In the lab, nursing staff physically collected the samples, whereas at home participants were expected to be self-reliant. Additionally, our instructions could have been more specific and detailed. For example, they could have instructed the participant to collect the sample before getting out of bed, i.e. while still lying in bed. In this report we did not observe any differences between these two conditions. The diurnal rhythm of cortisol (Clow et al., 2004) and sleep patterns (Ohayon et al., 2004) vary with advancing age; therefore, the wide age range of the cohort is a limitation. Future work will further evaluate group differences on the diurnal rhythm and basal values. The accuracy of sampling times was limited by relying on self-report. It has been suggested that using actigraphy and electronic devices (e.g. MEMS caps) would be desirable for understanding the delay issue (Dockray et al., 2008). In the present study the cortisol sampling occurred over 2 or 3 days. Although this is consistent with other similar studies (Federenko et al., 2004;Kudielka and Kirschbaum, 2003;Pruessner et al., 2003;Steptoe et al., 2005), some investigators suggest that up to 6 days of monitoring is required to understand trait aspects of the CAR (Hellhammer et al., 2007).
In spite of these limitations, we confirmed that delays between wake time and obtaining waking cortisol samples can affect the resulting CAR, and subsequently the clinical interpretation. We also confirmed that self-report wake time is a reliable measure to use when assessing the cortisol awakening response. Further investigation is needed of how to improve adherence such that delays between waking and cortisol sampling are minimized allowing for accurate CAR interpretations. We suggest that one way this can be accomplished is through consistent and clear instructions provided by investigators to participants.
Acknowledgements
We are grateful to the laboratory of C. Kirschbaum for its assay of the cortisol samples.
Funding for this study was provided by NIA Grant AG20677; the NIA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
This is not an industry supported study. Dr. Buysse has consulted for Actelion, Arena, Cephalon, Eli Lilly, GlaxoSmithKline, Merck, Neurocrine, Neurogen, Pfizer, Respironics, Sanofi-Aventis, Sepracor, Servier, Stress Eraser, Takeda, and Transcept Pharmaceuticals, Inc. The other authors have indicated no financial conflicts of interest.
Reference List
- Backhaus J, Junghanns K, Hohagen F. Sleep disturbances are correlated with decreased morning awakening salivary cortisol. Psychoneuroendocrinology. 2004;29:1184–1191. doi: 10.1016/j.psyneuen.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Bauer ME, Vedhara K, Perks P, Wilcock GK, Lightman SL, Shanks N. Chronic stress in caregivers of dementia patients is associated with reduced lymphocyte sensitivity to glucocorticoids. J Neuroimmunol. 2000;103:84–92. doi: 10.1016/s0165-5728(99)00228-3. [DOI] [PubMed] [Google Scholar]
- Born J, Hansen K, Marshall L, Molle M, Fehm HL. Timing the end of nocturnal sleep. Nature. 1999;397:29–30. doi: 10.1038/16166. [DOI] [PubMed] [Google Scholar]
- Broderick JE, Arnold D, Kudielka BM, Kirschbaum C. Salivary cortisol sampling compliance: comparison of patients and healthy volunteers. Psychoneuroendocrinology. 2004;29:636–650. doi: 10.1016/S0306-4530(03)00093-3. [DOI] [PubMed] [Google Scholar]
- Buckley TM, Schatzberg AF. Aging and the role of the HPA axis and rhythm in sleep and memory-consolidation. Am J Geriatr Psychiatry. 2005;13:344–352. doi: 10.1176/appi.ajgp.13.5.344. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carlsson F, Garde AH, Hansen AM, Persson R, Orbaek P, Karlson B. The cortisol awakening response - an exploration of intraindividual stability and negative responses. SJWEH. 2006;(Suppl):15–21. [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- de Vente W, Olff M, Van Amsterdam JG, Kamphuis JH, Emmelkamp PM. Physiological differences between burnout patients and healthy controls: blood pressure, heart rate, and cortisol responses. Occup Environ Med. 2003;60(Suppl 1):i54–i61. doi: 10.1136/oem.60.suppl_1.i54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschle M, Gotthardt U, Schweiger U, Weber B, Korner A, Schmider J, Standhardt H, Lammers CH, Heuser I. With aging in humans the activity of the hypothalamus-pituitary-adrenal system increases and its diurnal amplitude flattens. Life Sci. 1997;61:2239–2246. doi: 10.1016/s0024-3205(97)00926-0. [DOI] [PubMed] [Google Scholar]
- Dockray S, Bhattacharyya MR, Molloy GJ, Steptoe A. The cortisol awakening response in relation to objective and subjective measures of waking in the morning. Psychoneuroendocrinology. 2008;33:77–82. doi: 10.1016/j.psyneuen.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Edwards S, Evans P, Hucklebridge F, Clow A. Association between time of awakening and diurnal cortisol secretory activity. Psychoneuroendocrinology. 2001;26:613–622. doi: 10.1016/s0306-4530(01)00015-4. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Nater UM, Bohmelt A. High and low unstimulated salivary cortisol levels correspond to different symptoms of functional gastrointestinal disorders. J Psychosom Res. 2005;59:7–10. doi: 10.1016/j.jpsychores.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Evans P, Forte D, Jacobs C, Fredhoi C, Aitchison E, Hucklebridge F, Clow A. Cortisol secretory activity in older people in relation to positive and negative well-being. Psychoneuroendocrinology. 2007;32:922–930. doi: 10.1016/j.psyneuen.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Federenko I, Wust S, Hellhammer DH, Dechoux R, Kumsta R, Kirschbaum C. Free cortisol awakening responses are influenced by awakening time. Psychoneuroendocrinology. 2004;29:174–184. doi: 10.1016/s0306-4530(03)00021-0. [DOI] [PubMed] [Google Scholar]
- Gu Chong . Smoothing Spline ANOVA Models. Springer-Verlag; New York: 2002. [Google Scholar]
- Hall M, Buysse DJ, Nofzinger EA, Reynolds CF, III, Thompson W, Mazumdar S, Monk TH. Financial strain is a significant correlate of sleep continuity disturbances in late-life. Biol Psychol. 2008;77:217–222. doi: 10.1016/j.biopsycho.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hellhammer DH, Wust S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34:163–171. doi: 10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state- and trait components. Psychoneuroendocrinology. 2007;32:80–86. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Hucklebridge F, Clow A, Evans P. The relationship between salivary secretory immunoglobulin A and cortisol: neuroendocrine response to awakening and the diurnal cycle. Int J Psychophysiol. 1998;31:69–76. doi: 10.1016/s0167-8760(98)00042-7. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Bellingrath S, Hellhammer DH. Cortisol in burnout and vital exhaustion: an overview. G Ital Med Lav Ergon. 2006;28:34–42. [PubMed] [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosom Med. 2003;65:313–319. doi: 10.1097/01.psy.0000058374.50240.bf. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hawkley LC, Adam EK, Cacioppo JT. Compliance with ambulatory saliva sampling in the chicago health, aging, and social relations study and associations with social support. Ann Behav Med. 2007;34:209–216. doi: 10.1007/BF02872675. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology. 2003;28:35–47. doi: 10.1016/s0306-4530(02)00008-2. [DOI] [PubMed] [Google Scholar]
- Kuehner C, Holzhauer S, Huffziger S. Decreased cortisol response to awakening is associated with cognitive vulnerability to depression in a nonclinical sample of young adults. Psychoneuroendocrinology. 2007;32:199–209. doi: 10.1016/j.psyneuen.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004;29:516–528. doi: 10.1016/s0306-4530(03)00072-6. [DOI] [PubMed] [Google Scholar]
- Monk TH, Reynolds CF, Kupfer DJ, Buysse DJ, Coble PA, Hayes AJ, Machen MA, Petrie SR, Ritenour AM. The Pittsburgh Sleep Diary. J Sleep Res. 1994;3:111–120. [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Paquet J, Kawinska A, Carrier J. Wake detection capacity of actigraphy during sleep. Sleep. 2007;30:1362–1369. doi: 10.1093/sleep/30.10.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von AK, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Hellhammer DH, Pruessner JC, Lupien SJ. Self-reported depressive symptoms and stress levels in healthy young men: associations with the cortisol response to awakening. Psychosom Med. 2003;65:92–99. doi: 10.1097/01.psy.0000040950.22044.10. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Brydon L, Kunz-Ebrecht S. Changes in financial strain over three years, ambulatory blood pressure, and cortisol responses to awakening. Psychosom Med. 2005;67:281–287. doi: 10.1097/01.psy.0000156932.96261.d2. [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Blunted cortisol response to awakening in mild to moderate depression: regulatory influences of sleep patterns and social contacts. J Abnorm Psychol. 2005;114:697–705. doi: 10.1037/0021-843X.114.4.697. [DOI] [PubMed] [Google Scholar]
- Tryon WW. Issues of validity in actigraphic sleep assessment. Sleep. 2004;27:158–165. doi: 10.1093/sleep/27.1.158. [DOI] [PubMed] [Google Scholar]
- Wilhelm I, Born J, Kudielka BM, Schlotz W, Wust S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007;32:358–366. doi: 10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Wright CE, Steptoe A. Subjective socioeconomic position, gender and cortisol responses to waking in an elderly population. Psychoneuroendocrinology. 2005;30:582–590. doi: 10.1016/j.psyneuen.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Wust S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response - normal values and confounds. Noise Health. 2000;2:79–88. [PubMed] [Google Scholar]