Abstract
Ecdysis, or the shedding of the old cuticle, depends on coordinated stereotyped behaviors, regulated by a number of neuropeptides. In the hornworm, Manduca sexta, two neuropeptides interact, namely Ecdysis Triggering Hormone (ETH) and Eclosion Hormone. We looked at the effects of ETH in vivo and in vitro, on the brain and the ventral nerve cord to determine the roles played by these hormones. We monitored ecdysis onset and the presence of cGMP and Eclosion Hormone immunoreactivity. In vivo, only a fraction of larvae lacking the cell bodies containing Eclosion Hormone, and injected with ETH, were able to undergo ecdysis, with a delayed response. These animals showed strongest cGMP immunoreactivity in the subesophageal and thoracic ganglia, with concomitant reductions in Eclosion Hormone immunoreactivity in descending axons in comparison with animals not undergoing ecdysis. Animals lacking the brain showed reduced to no cGMP levels in all ganglia. In vitro, isolated CNS preparations lacking the brain initiated ecdysis motor programs after incubation in ETH, with faster onset times than controls, and with reduced cGMP-immunoreactivity. If ETH was applied only to the brain of the isolated CNS, cGMP-immunoreactivity was noted primarily in the subesophageal and thoracic ganglia, with a decrease in Eclosion Hormone immunoreactivity in descending axons. ETH addition to the rest of the nerve cord showed reduced Eclosion Hormone immunoreactivity but little to no cGMP-immunoreactivity in any ganglion. Controls showed strong cGMP-immunoreactivity in all ganglia, and even greater reductions in Eclosion Hormone staining after ETH application. These results support previous suggestions that Eclosion Hormone is required for a positive feedback loop with ETH as well as onset of an inhibitory component, but also suggest that ETH stimulates Eclosion Hormone release at multiple spike initiation zones. The resultant up regulation of cGMP does not appear to be required for onset of ecdysis. A new model for ecdysis regulation is considered.
Keywords: Ecdysis, ETH, Eclosion Hormone (EH), cGMP, inhibition, spike initiation zones
Introduction
Innate behaviors are noted in all animals, and are typically highly stereotyped, requiring coordination within the nervous system for their activation. Such behaviors include nest building, courtship, egg laying, escape and feeding. Similarly, shedding of the old cuticle in insects, at the end of each developmental stage, involves a series of innate behaviors collectively termed ecdysis. This shedding allows the insect to continue growth and development, to accommodate larger and/or different body forms. These behaviors depend on interactions between a number of neuropeptides, and provide a strong model for studying the coordinated regulation and modulation of innate behaviors by the neuroendocrine system. Based primarily on data collected from the tobacco hornworm, Manduca sexta, and the fruit fly, Drosophila melanogaster, a model has been proposed that describes the peptide interactions that help order and time the various behavioral phases that comprise this sequence of events. These peptides likely play a role in the ecdysis behaviors of many, if not all, insects (Truman et al., 1981; Ewer and Truman, 1996; Adams and Zitnan, 1997; O’Brien and Taghert, 1998; Zitnan et al., 1999).
The specific behaviors and motor programs leading to the shedding of the old cuticle are coordinated and maintained by the release of neuropeptides from the central nervous system and the periphery at the end of each molt cycle. In larval M. sexta, the ecdysis sequence consists of three behavioral phases to loosen and eventually slough off the old cuticle. These are pre-ecdysis I, pre-ecdysis II and ecdysis (Zitnan et al., 1999), followed by a post-ecdysial phase. A model has been suggested for this peptidergic control (see Fig. 1). In the model, Corazonin from the brain initially stimulates co-release of Pre-Ecdysis Triggering Hormone (PETH) and Ecdysis-Triggering Hormone (ETH) from the peripherally located Inka cells of the segmentally-repeated epitracheal glands lining the body wall (Kim et al., 2004). This is followed by the sequential activation of the behaviors required for the shedding of the old cuticle. These are depicted in Fig. 1 and outlined below:
Fig. 1.
Model for the regulation of ecdysis in the larval CNS of M. sexta. A description of the model is provided in the introduction. Abbreviations: (Br) brain; (SEG/TG) subesophageal and thoracic ganglia; (AG) abdominal ganglia; (PETH) Pre-Ecdysis Triggering Hormone; (ETH) Ecdysis Triggering Hormone; (EH) Eclosion Hormone; (?) unknown cell phenotype; (CCAP) Crustacean Cardioactive Peptide; (MIP) MyoInhibitory Peptide; (DH) Diuretic Hormone; (cGMPir) cGMP immunoreactivity; (CPG) central pattern generator; (Pre I) pre-ecdysis I; (Pre II) pre-ecdysis II; (E) ecdysis; (post) post-ecdysis. Dashed and solid arrows show excitatory effects. Capped line shows an inhibitory effect. Dashed line depicts separation of ganglia. dark cells have been shown to have increases in cGMPir. Clear cells have not. Numbers are discussed in the introduction.
PETH acts directly on the CNS to activate a central pattern generator (CPG1) driving pre-ecdysis I behaviors (Zitnan et al., 1999), putatively through release of a combination of diuretic hormones and kinins from lateral neurosecretory cells (L3,4) in each abdominal ganglion (Kim et al., 2006a).
At low concentrations, ETH activates a second central pattern generator (CPG2) driving pre-ecdysis II behaviors, although modulators of this behavior have not been identified (Zitnan et al., 1996; Zitnan et al., 1999).
ETH targets release of Eclosion Hormone (EH) from the ventromedial neurons of the brain. Both PETH and ETH appear to readily permeate the CNS sheath, while EH does not, and is thus released centrally as well as into the hemolymph from the posterior proctodeal nerves (Ewer et al., 1997a; Gammie and Truman, 1999; Novicki and Weeks, 1996). EH released from the proctodeal nerves stimulates the Inka cells to release more ETH. This results in a strong positive feedback loop, with the massive release of both hormones from their respective cells prior to the onset of ecdysis (Ewer et al., 1997a; Kingan et al., 1997). This is noted by reduced ETH and EH immunoreactivity in the Inka cells and proctodeal nerves of the CNS, respectively (Novicki and Weeks, 1996; Zitnan et al., 1999).
The higher concentration of ETH is required for activation of ecdysis, putatively via release of EH (Gammie and Truman, 1997a; Zitnan et al., 1999) as well as via activation of unknown components from the subesophageal ganglion (Zitnan and Adams, 2000). This is suggested to be via a cGMP-mediated mechanism (Ewer et al., 1994; Zitnan and Adams, 2000).
Centrally-released EH is believed to increase excitability of two pairs of homologous cells distributed in each ganglion of the ventral nerve cord through a cGMP-mediated mechanism (Ewer et al., 1994; Gammie and Truman, 1997a; Gammie and Truman, 1999). One cell is the interneuron “IN704” and the other is the lateral neurosecretory cell “NS27” (Gammie and Truman, 1999; Gammie and Truman, 1997b). The IN704 neurons of the abdominal ganglia have clearly identified co-localization of Crustacean Cardioactive Peptide (CCAP) and a combination of Myoinhibitory Peptides (MIPs) (Kim et al., 2006a). The subsequent co-release of CCAP and MIPs from the IN704 cells of each abdominal ganglion are suggested to modulate the ecdysis central pattern generator (CPG3) for the onset and coordination of ecdysis behaviors (Kim et al., 2006a).
The IN704 neurons of the subesophageal and thoracic ganglia show only sporadic CCAP immunoreactivity, and have different time courses of cGMP immunoreactivity (Ewer et al., 1994; Fuse unpublished results). They may be the source of inhibitory factors, which regulate timing of onset of ecdysis (Zitnan and Adams, 2000; Fuse and Truman, 2002), again via a cGMP-mediated mechanism (Fuse and Truman, 2002). Similar inhibition is noted in D. melanogaster (Baker et al., 1999).
The NS27 cells of the abdominal ganglia contain CCAP (Davis et al., 1993; Ewer et al., 1994) and bursicon (Taghert and Truman, 1982; Kostron et al., 1996; Honneger et al., 2002), which are likely released during or after ecdysis, to stimulate post-ecdysis behaviors such as cuticle stretching, tanning and hardening (Fraenkel et al., 1966; Dai et al., 2008).
It should also be noted in this model that while ETH stimulates release of EH from the brain (Ewer et al., 1997a), ETH is capable of initiating ecdysis motor patterns in the absence of the brain in vitro (Zitnan and Adams, 2000). ETH, for instance, can induce ecdysis motor patterns in isolated CNS preparations lacking a brain, and these preparations show rapid increases in cGMP staining in the IN704 and NS27 network (Zitnan and Adams, 2000). Drosophila lacking EH (EH knock-outs) are likewise able to eclose as adults, although with markedly reduced success, and with disrupted post-eclosion phenotypes such as wing expansion (McNabb et al., 1997). Injection of ETH into brainless, but otherwise intact M. sexta larvae, on the other hand, is only sufficient to induce pre-ecdysis behaviors but not ecdysis behaviors (Novicki and Weeks, 1996), although removal of the source of EH, the ventromedial cell bodies, through targeted ablation (Gammie and Truman, 1999) or gross dissections of the brain (Zitnan and Adams, 2000) does not hinder the onset of ecdysis in all cases. Thus it is clear that the interactions between ETH and EH remain elusive.
Measurements of changes in intracellular levels of cGMP have also been instrumental in determining the timing of many events associated with EH and ETH activity in the subesophageal, thoracic and abdominal ganglia (eg. See Ewer et al., 1994), although the exact role of these large surges in cGMP is still unclear. This paper examines the differential effects of ETH on the brain and the ventral nerve cord (VNC) both in vivo and in vitro, in terms of ecdysis activity, cGMP immunoreactivity and release of EH. ETH appears to have very different effects in the activation of the central IN704/NS27 network when acting via the brain or directly on the VNC. Our evidence suggests that ETH induces EH release, but we suggest that there are multiple spike initiation zones for this release, some relatively distant from the brain. Moreover, the ability of ETH to activate ecdysis motor programs in vitro does not appear to depend on high levels of cGMP, while the activation of the inhibitory element regulating timing of ecdysis does.
Materials and methods
Animals
Larvae of the tobacco hornworm, Manduca sexta (Sphingidae, Lepidoptera), were raised on an artificial diet (MP Biomedical, Irvine, CA, USA) and maintained under a long day (17L:7D) photoperiod regimen with a 27°C:25°C superimposed thermoperiod and 50–60% relative humidity. Pharate 5th instar larvae were staged using external morphological markers (Copenhaver and Truman, 1982). Pharate 5th instar larvae were treated 1 hr after the mandibles became tanned and the old 4th instar head capsule became air-filled following reabsorption of the molting fluid (Air Filled Brown Mandible stage; “AFBM”). AFBM animals typically ecdysed within 4.5 ± 0.4 hrs (n=22).
Peptide Injections
ETH was synthesized at 95% purity (Peptron Inc., Daejeon, S. Korea) and stored in 10−2M aliquots in modified Miyazaki’s saline (Miyazaki, 1980; Trimmer and Weeks, 1989) at −20°C until needed for experiments. Experimental amounts were diluted in modified Weever’s saline (Weevers, 1966; Trimmer and Weeks, 1989) for in vivo injections. Larvae were injected in the terminal abdominal segment, on the side of body immediately posterior to the last spiracle with 100 pmoles ETH using a Hamilton syringe. The onset of pre-ecdysis and ecdysis behaviors, and the successful shedding of the old cuticle were monitored by eye, and time of onset of both behaviors was recorded.
Surgical procedures
Animals were anesthetized on ice for approximately 5 minutes then placed on a wax mount. A small triangular incision was made above the slipped head capsule, and the cuticle was peeled back to reveal the dorsal aspect of the brain. A triangular incision was made in the brain to remove the ventromedial cell bodies. Once experiments were completed, tissues were processed for EH immunoreactivity as described below, to confirm that the ventromedial cell bodies had been completely removed and to monitor EH immunoreactivity within the EH axons. Sham-operated animals had forceps inserted into the same opening without removing the brain. After the surgery, the incision was sealed with wax and the animals were left for 5 min at room temperature then injected with appropriate solutions. Animals were sacrificed 40 or 90 min later, as well as at ecdysis (variable timing), for processing of cGMP immunoreactivity.
Dissections
Animals were anesthetized on ice for approximately 10–15 minutes and their nerve cords dissected in cold Weever’s saline. In most preparations, the nerve cord was removed intact from brain to terminal abdominal ganglion. In “brainless” preparations, the connectives between the subesophageal ganglion and the brain were severed after dissection. Tissues were then fixed in 4% paraformaldehyde for immunohistochemistry or placed in modified Miyazaki’s saline for electrophysiological recordings, as described below.
In Vitro Silicone Barriers
To assess the actions of ETH on the brain or downstream on the ventral nerve cord (VNC), the brain and VNC (subesophageal, thoracic and abdominal ganglia; SEG, TG, AG, respectively) were separated by a water-proof barrier, or dam, using Vaseline petroleum jelly (Unilever) while leaving all nerve connections intact (see Fig. 2 for a schematic). The dam was constructed on a plastic Petri dish and drops of saline were placed on each side. The connectives between the brain and SEG were carefully laid within the dam, and the assay was tested for leaks by quickly dabbing one saline pool with a kimwipe (Fisher Scientific, Pittsburgh, PA, USA). If leaks existed, saline from both sides of the dam would become depleted, whereas if the dam was intact, only the saline being dabbed by the kimwipe would disappear. Saline was immediately replaced, so that tissues were never exposed to the air.
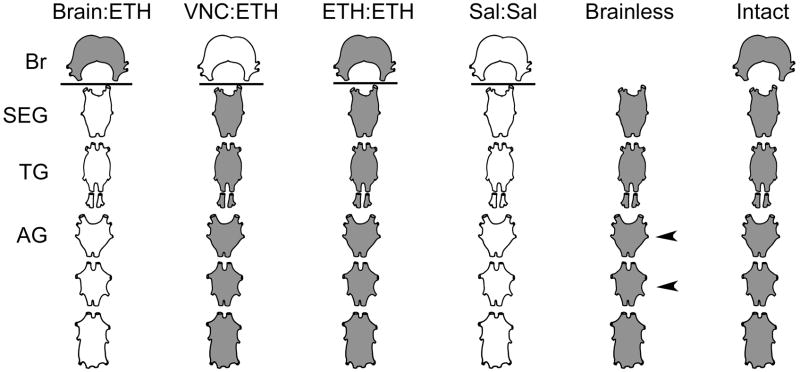
Fig. 2.
Schematic representation of the isolated CNS preparations used for in vitro Vaseline dam experiments (Brain:ETH, VNC:ETH, ETH:ETH; Sal:Sal) and electrophysiology (Brainless and Intact). The CNS is truncated to show the brain (Br), subesophageal ganglion (SEG), one thoracic ganglion (TG) and 3 abdominal ganglia (AG) with connectives intact. Grey shaded tissues represent sites of ETH application and white tissues represent sites of saline application. The lines show the sites of the Vaseline dam. The arrowheads identify the sites of suction electrode placement for electrophysiological recordings. (VNC): Ventral nerve cord; (Sal): Saline.
The brain and/or the VNC were incubated in either Miyazaki’s saline or 1 μM ETH. The brain was incubated in 80 μl of the appropriate solution, while the VNC was incubated in 120 μl of solution to accommodate a larger amount of tissue. Tissues were incubated for 15, 30, 40, 60 or 75 minutes, then quickly removed and placed in 4% paraformaldehyde fixative for cGMP-immunoreactive processing, as described above. ETH was placed on the brain alone (Br:ETH), on the VNC alone (VNC:ETH) or on both the brain and VNC (ETH:ETH; “control”), with a dam. Saline was also added to a dam preparation to ensure that no cGMP was noted during this same time period (Sal:Sal; “saline control”). “Intact” CNS preparations and those lacking a brain (“brainless”) were also treated with ETH in a single drop of saline in another set of experiments.
Immunohistochemistry
Freshly dissected tissues were fixed in 4% buffered paraformaldehyde for 1–2 hours at room temperature, then rinsed in PBS containing 1% triton-X100 (Sigma Chemical Co., St. Louis, MO, USA) (PBST) for at least 5 washes, with 30-minute intervals for each.
Tissues being processed for cGMP immunoreactivity were immediately transferred to sheep anti-cGMP antibody (diluted to 1:10,000 in PBST; a generous gift of Drs. J. de Vente and J. W. Truman) for at least 48 hours on a shaker at 4°C. The other tissues were treated with collagenase (Type IV; Sigma) in PBST at a concentration of 0.5 mg/ml for 60 minutes at room temperature, then rinsed in PBST and blocked with 5% normal donkey serum (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) for 15 minutes at room temperature. Tissues being subsequently processed for EH immunoreactivity were incubated with rabbit anti-EH primary antibody (diluted 1:500; two separate batches generously donated by Drs. M. E. Adams and J. W. Truman) for at least 48 hours on a shaker at 4°C. Removal of the primary antibody consisted of at least 5 washes of PBS-TX, at 30-minute intervals.
For cGMP staining alone, tissues were incubated in peroxidase-conjugated donkey anti-sheep IgG secondary antibody (1:1000; Jackson), for 18 hours on a shaker at 4°C. After rinsing, the antibody complex was visualized using the chromagenic diaminobenzedene (DAB) reaction. Tissues were incubated with ammonium chloride (0.4 mg/ml), beta-D-glucose (2 mg/ml), DAB (50 μl/ml) and glucose oxidase (6 μl/ml) in PBST. Reactions generally took between 3–5 minutes, and were stopped by several rinses in PBST followed by PBS. The tissues were then dehydrated through an ethanol series (30%, 50%, 75%, 95%, 100%), cleared in xylenes for 15 minutes, and mounted in DPX (Fluka, Buchs, Switzerland) on poly-L lysine coated coverslips.
For EH staining, tissues were incubated in fluorescene isothiocyanate (FITC)-conjugated donkey anti-rabbit IgG secondary antibody (1:1000; Jackson), for 18 hours on a shaker at 4°C. After rinsing, the tissues were dehydrated and mounted as described above. The samples were viewed on a confocal microscope (BioRad MRC 600; BioRad, Hercules, CA, USA).
Control tissues were treated as described above, with the omission of either the primary or the secondary antibodies. No staining was noted in these preparations.
Signal Quantification
The intensity of immunoreactivity for the color reactions was quantified with digital image processing from captured images using Scion Image (http://rsb.info.nih.gov/ij/). Images were captured on a light microscope (Olympus, Pittsburgh, PA, USA), and quantified using Scion Image. The microscope was adjusted for Kohler illumination. A slide with a black dot was placed under the microscope and viewed on the computer monitor. The camera gain and black levels were adjusted so that the maximum gray level was at 255 pixel intensity, while the white portion was between 0–10 pixel intensity. Once the microscope was calibrated, it remained calibrated throughout the examination period. Average pixel intensity was determined by averaging the mean pixel density and standard deviation for the area to be analyzed. The data was similar when the color reactions were assessed subjectively using the scoring system from 0 (no stain) to 3 (maximal stain of cell bodies and axons) previously described by Ewer and Truman (1997b).
It should be noted that cGMP immunoreactivity is always detected in a select set of cells in the terminal abdominal ganglion throughout development (see asterisks, Fig. 5b, c), and this immunoreactivity does not appear to be associated with ecdysis (Zitnan and Adams, 2000). This stain was used as a positive control to assess the quality of the immunohistochemistry protocol when no other cGMP immunoreactivity was noted throughout the CNS. Thus a score of “no immunoreactivity” did not include the terminal staining.
Fig. 5.
Representative photomicrographs of cGMP immunoreactivity in isolated CNS preparations with a Vaseline dam between the brain and VNC, after incubated in 1 μM ETH for 40 min. (A) Entire CNS incubated in ETH (ETH:ETH); (B) Brain incubated in ETH and VNC incubated in saline (Brain:ETH); (C) Brain incubated in saline and VNC incubated in ETH (VNC:ETH). Subesophageal ganglion (SEG); Thoracic ganglion (TG); Abdominal ganglia (AG); Terminal abdominal ganglion (TAG). Asterisks indicate cells that were not scored for cGMP immunoreactivity. Scale bar = 150 μm.
The intensity of immunoreactivity for the fluorescent preparations was quantified digitally from captured and inverted confocal images using NIH Image (http://rsb.info.nih.gov/ij/). Images were captured at the point of largest cell diameter, or by z-series stacking, on the confocal microscope, prior to quantification with NIH Image. Images were inverted, and a set area surrounding the ventromedial axons of the terminal abdominal ganglion was highlighted. The intensity of this area for both left and right axons was averaged per tissue. A similar area next to the axons was highlighted to determine background levels in each tissue and these levels were subtracted from axonal measurements.
Electrophysiology
Pharate 5th instar larvae 1 hour into the AFBM stage were dissected as described above, and extracellular burst patterns of the dorsolateral anterior nerve branches of abdominal ganglia 5 and 6 (arrows in Fig. 2) were recorded after application of 1 μM ETH. This concentration has been previously shown to produce clear fictive pre-ecdysis and ecdysis motor patterns in these nerve branches (Gammie and Truman, 1999; Wells et al., 2006). Burst patterns triggered by peptide addition were detected by extracellular recordings using suction electrodes, formed from micropipettes whose tips were broken to a tip diameter of approximately 100 μm. Signals were amplified and filtered by a differential AC amplifier (Model 1700, A-M Systems, Carlsborg, WA, USA) and recorded on a PC using a CED Systems Micro 1401 analogue-digital converter with Spike 5 software (CED Systems; Cambridge, England). The presence of pre-ecdysis and ecdysis bursts was determined 1) by the presence or absence of synchrony of bursting between nerve recordings from two consecutive ganglia, respectively (Gammie and Truman, 1999), and 2) higher and lower frequencies of bursting, respectively. Burst frequencies were determined using CED spike sorting software.
Strict criteria were used when recording electrophysiologically. Animals were placed on ice within 1–1.25 hours after AFBM, and ETH was applied to the dissected nervous system no more than 1.5 hrs after this. To avoid spontaneous pre-ecdysis behaviors, any frayed or pinched nerve preparations were discarded. Moreover, basal nerve activity was recorded for 13–15 minutes prior to application of ETH. If spontaneous burst patterning was noted, if the signal to noise ratio was too low, or if there was an extremely high rate of tonic firing, the preparation was not used, and a fresh nerve cord was dissected.
Some preparations were also processed for cGMP immunoreactivity as described above, immediately after the onset of ecdysis burst patterns.
Statistical Analysis
All statistical analyses were performed using SigmaStat statistical software (Systat Software Inc., San Jose, CA, USA). For immunohistochemical studies, one or two-way analysis of variance (ANOVA) was used, with transformations of data that was not initially normal. Data were considered significantly different when p<0.05 unless other p values were noted, after use of Tukey’s HSD test. Percentage data for the cGMP immunohistochemistry studies was analyzed by a modified chi-square test for multiple groups. For electrophysiological data, either the Mann-Whitney Rank Sum Test (t-test) or two-way repeated measures ANOVA were used. If normality failed, the Holm-Sidak Method was used to determine statistical differences at varying time points within a group.
Results
Analysis of in vivo ecdysis responses after midbrain removal
It has been shown that in contrast to larvae lacking brains, ecdysis may still commence after EH-containing ventromedial cell bodies have been removed through targeted ablation (Gammie and Truman, 1999) or through grosser brain dissections (Zitnan and Adams, 2000). In order to characterize the responses in the absence of the ventromedial cell bodies, intact animals had their entire brains removed (no Br) or had the ventromedial neurons removed through surgical removal of the midbrain region (no MBr). Groups were then injected with ETH to induce precocious onset of pre-ecdysis and ecdysis (compared to the normal ecdysis onset time of 3.5 ± 0.4 hrs later for saline-injected controls). Thus, within a 90 min period, intact and sham-operated controls as well as “no MBr” animals injected with saline did not undergo ecdysis (Table 1). As expected, intact and sham-operated controls injected with ETH displayed pre-ecdysis behaviors within approximately 5 min (data not shown), and ecdysis behaviors within approximately 40 min (Table 1). In general, animals lacking the brain (no Br) only showed pre-ecdysis behaviors when injected with 100 pmoles ETH, within the 90 min period (Table 1). In contrast, pre-ecdysis and ecdysis behaviors were still induced in approximately 45% of animals lacking the midbrain (no MBr) when injected with ETH within approximately 47 min. Thus, removal of the ventromedial cell bodies was not sufficient to eliminate pre-ecdysis and ecdysis behaviors in all larvae, although ecdysis onset was significantly delayed in “no MBr” animals compared to sham-operated animals (p<0.05) and intact controls (p=0.07). It should be noted that animals were left at most 90 min after ETH injection, to assess cGMP levels, thus natural onset of ecdysis was never measured.
Table 1.
Responses of pharate 5th instar larvae to injection of 100 pmoles ETH after brain surgeries. Surgical manipulations are described in the Materials and Methods in greater detail.
| Surgical group | ETH injection | % Ecdysis within 90′ | Onset time (min) | cGMPir at 40′ | cGMPir at 90′ | ||||
|---|---|---|---|---|---|---|---|---|---|
| SEG | TG | AG | SEG | TG | AG | ||||
| Intact | no | 4 (24) | − | − | − | − | − | − | − |
| Sham | no | 0 (12) | − | − | − | − | − | − | − |
| No MBr | no | 9 (22) | − | − | − | − | − | − | − |
| Intact | yes | 100 (27) | 41.9 ± 1.3 * | + | + | + | + | + | + |
| Sham | yes | 95 (19) | 39.1 ± 1.1 # | + | + | + | + | + | + |
| No MBr | yes | 45 (29) | 46.5 ± 2.6 | + | (+) | (+) | + | (+) | (−) |
| No Br | yes | 3 (29) | (45) | (+) | (+) | (+) | − | − | − |
MBr: midbrain; Br: brain
Onset times are means ± S.E.M.
Significant difference from no MBr with ETH (*p<0.1/#p<0.05)
cGMPir: cyclic GMP immunoreactivity
“−” to “+” represents extent of stain
Intact and sham-operated control groups injected with ETH displayed strong cGMP immunoreactivity in all parts of the VNC, including the subesophageal ganglion (SEG), thoracic ganglia (TG) and abdominal ganglia (AG), when dissected at 40 min after ETH injection (when pre-ecdysis behaviors were still apparent; Table 1), and at ecdysis approximately 1–10 min later (data not shown). This included the presence of stain within the NS27 and IN704 neurons, as well as ipsilateral and contralateral axons running the length of the CNS (eg. See Fig. 5a and b). Animals lacking the brain (no Br) did not undergo ecdysis, but did show increases in cGMP immunoreactivity in some preparations 40 min after ETH injection (n=4/6). This immunoreactivity was limited mostly to the axons running the length of the CNS, with only occasional faint stain noted in cell bodies (data not shown). Some animals did not show stain (n=2/6). Pre-ecdysis behaviors were still apparent when the animals were sacrificed. The stain was completely absent by 90 min after ETH injection, and no regular ecdysis-like behaviors were noted. Animals lacking the midbrain region (no MBr) showed variable cGMP immunoreactivity by 40 min after ETH injection. Some animals showed strong cGMP immunoreactivity in the SEG similar to controls (cell bodies and axons; n=3/6), with others showing mostly only axonal stain (n=2/6) or no stain (n=1/6) in the TG or AG. Animals undergoing ecdysis all showed strong cGMP immunoreactivity, but still with the abundance of stain in the SEG (n=6/6). By 90 min after ETH injection, “no MBr” only showed moderate to strong stain in the SEG, with less stain in the TG. Staining in the AG was always extremely faint, if present at all.
To determine whether EH was released from the axons of the ventromedial cells in the absence of the cell bodies (no MBr), EH immunoreactivity in the axons of the terminal abdominal ganglia was quantified. EH immunoreactivity has been shown to decline dramatically in proctodeal nerves and axons of the terminal abdominal ganglia of the CNS after successful ecdysis, concomitant with an increase in hemolymph concentrations of EH in M. sexta and D. melanogaster (Ewer et al., 1997a; Clark et al., 2004). EH immunoreactivity decreased significantly (p<0.05) in the terminal abdominal ganglia of ETH-injected sham-operated controls (“sham(ETH)”) after successful ecdysis (Fig. 3). In contrast, EH immunoreactivity remained higher in non-ecdysing, saline-injected sham-operated controls (“sham(saline)”). When animals had their midbrains surgically removed (“no MBr”), EH immunoreactivity was significantly decreased in animals undergoing successful ecdysis (“no MBr (E)”; p<0.05). Animals not undergoing ecdysis after 90 minutes did not show significant decreases in EH immunoreactivity (“no MBr (no E)”; p>0.5). The data from Table 1 and Fig. 3 suggest that EH is released in animals undergoing ecdysis, whether the ventromedial cell bodies are present or not, but that cGMP levels are not always elevated to detectable levels in the thoracic or abdominal ganglia under these conditions.
Fig. 3.
EH immunoreactivity in axons of the terminal abdominal ganglia from ecdysing and non-ecdysing animals. Examples of confocal stacked images of EH-immunoreactive axons are depicted from (A) sham-operated saline-injected “sham (saline)” and (B) sham-operated ETH-injected “sham (ETH)” animals. Sham (saline) animals did not go through ecdysis, while sham (ETH) animals did. Scale bar = 0.01 mm. (C) Relative pixel intensities of EH-immunoreactive axons from control and experimental groups. Sham: sham brain surgery; no MBr (E): removal of midbrain, and animals still ecdysed; no MBr (no E): removal of midbrain, and animals did not ecdyse after 90 min. Asterisks denote significant differences (P<0.05), using Tukey’s HSD on transformed data after One-way Analysis of Variance.
Analysis of in vitro ecdysis motor patterns in brainless nerve cords
To determine the effects of ETH application to nerve cords lacking EH from the brain, we isolated nerve cords from pharate 5th stage larvae, removed the brains (“brainless”), and incubated in 1 μM ETH. We compared them to intact preparations incubated in ETH. 100% of intact preparations showed robust fictive pre-ecdysis and ecdysis motor patterns after ETH application (Tables 2). Over 80% of brainless preparations also displayed pre-ecdysis and ecdysis motor patterns. There was no significant difference in the onset times of pre-ecdysis in these preparations as assessed by a t-test for unequal variance (p>0.5). However, onset of ecdysis was significantly faster when the brain was missing (p<0.1), and the delay from start of pre-ecdysis to start of ecdysis was shorter in these preparations (p<0.05). That is, ecdysis began significantly sooner after the onset of pre-ecdysis when the brain was absent.
Table 2.
Onset of fictive pre-ecdysis and ecdysis motor patterns of CNS preparations with (Intact) and without (Brainless) the brain, incubated in ETH.
| Surgical group | % showing Pre-ecdysis | % showing Ecdysis | Pre-ecdysis onset (min) | Ecdysis onset (min) | Delay between motor programs (min) |
|---|---|---|---|---|---|
| Intact | 100 (13) | 100 (13) | 6.8 ± 0.7 | 67.6 ± 6.6 | 66.9 ± 7.4 |
| Brainless | 91 (11) | 82 (11) | 9.4 ± 2.1 | 57.9 ± 2.1* | 52.5 ± 2.6* |
Values are means + S.E.M.
cGMPir: cyclicGMP immunoreactivity
Pre-E, pre-ecdysis; E, ecdysis
Significant difference from intact (p<0.07)
Cyclic GMP immunoreactivity was assessed around the expected time of onset of fictive ecdysis in intact and brainless nerve cord preparations incubated in 1 μM ETH. Staining was noted in significantly fewer preparations when the brain was missing, in both the IN704 (Fig. 4a) and NS27 neurons (Fig. 4b). Brainless nerve cords that were recorded from electrophysiologically, and had begun ecdysis burst patterns were also quickly removed from the apparatus and processed for cGMP immunoreactivity. Only 1/10 brainless preparations showed minimal if any signs of cGMP immunoreactivity (data not shown).
Fig. 4.
Percentage of tissues stained for cGMP immunoreactivity in the (A) IN704 interneurons or (B) NS27 neurons of the subesophageal ganglion (SEG), thoracic ganglia (TG) or abdominal ganglia (AG) when incubated with ETH for 40 minutes. Intact or brainless preparations were incubated with 1 μM ETH. Letters are significantly different from controls (p<0.05). Sample sizes ranged from 26–28 tissues for each group.
To determine if the burst characteristics were similar in intact and brainless preparations, the burst frequencies during the course of fictive pre-ecdysis and ecdysis motor patterns were analyzed (Table 3). Intact CNS preparations fired at significantly higher burst frequencies at the beginning of pre-ecdysis (0–3 min) compared to brainless preparations (p<0.05), indicating that the burst cycle durations were lengthened when the brain was removed. But by 8 minutes into pre-ecdysis bursting, burst patterns were not significantly different (p>0.5). In contrast, ecdysis burst frequencies were never significantly different between intact and brainless preparations.
Table 3.
Frequencies of fictive pre-ecdysis and ecdysis motor patterns in isolated CNS preparations incubated in ETH. Values were collected at the onset (0 min) and at periods after pre-ecdysis or ecdysis behaviors.
| Pre-ecdysis bursts (Hz × 10) | Ecdysis bursts (Hz × 10) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Surgical group | N | 0 min | 3 min | 8 min | 25 min | N | 0 min | 3 min | 6 min |
| Intact | 10 | 1.17 ± 0.07 | 1.15 ± 0.04 | 1.11 ± 0.01 | 0.86 ± 0.02 | 8 | 0.54 ± 0.02 | 0.46 ± 0.02 | 0.36 ± 0.01 |
| Brainless | 10 | 0.91 ± 0.05* | 1.01 ± 0.09* | 1.02 ± 0.07 | 0.88 ± 0.05 | 8 | 0.54 ± 0.03 | 0.40 ± 0.02 | 0.36 ± 0.03 |
Values are means + S.E.M.
Times (min) are noted after onset of either Pre-ecdysis or Ecdysis
Significant difference from intact (p<0.05)
Analysis of cGMP immunoreactivity after ETH application in vitro
To determine if ETH had a differential effect on the brain or VNC in M. sexta, portions of the isolated nerve cord were incubated with 1 μM ETH by using a silicone dam to separate the tissues non-invasively (see Fig. 2 for experimental details). The brain was isolated from the VNC by a silicone dam such that ETH could be applied directly to both the brain and VNC (ETH:ETH; “control”), to the brain alone (“Brain:ETH”), or to the VNC alone (“VNC:ETH”), without eliminating neural inputs between these regions. Saline was applied to adjoining tissues. Tissues were compared to saline treated controls (Sal:Sal). ETH was also applied for a variety of times, and tissues were assessed for cGMP immunoreactivity in the IN704 and NS27 neurons.
The NS27 cells are homologous, paired lateral neurosecretory cells found in the subesophageal ganglion (SEG), the thoracic ganglia (TG), and all abdominal ganglia (AG). The IN704 cells are smaller paired interneurons that typically lie next to the NS27 neurons, again in each SEG, TG and AG. In fact, two paired homologs of both NS27 and IN704 appear to exist in the SEG, in the fused maxillary and labial regions. A smaller anteriorly placed pair of cells in the mandibular region of the SEG also typically stained for cGMP, but this pair was not quantified in this study (see asterisk, Fig. 5a; SEG). Likewise, a posterior pair of lateral neurosecretory cells (L2,5) also stained for cGMP on occasion (see asterisk, Fig 5a; AG) but this pair was not quantified in this study, due to its highly variable staining patterns from preparation to preparation.
After 15–20 minutes of incubation of the entire CNS with ETH, low levels of cGMP immunoreactivity were apparent, primarily in the SEG and TG. In VNC:ETH preparations, faint stain was noted in the SEG and TG but not in the AG (data not shown; n=7). By 25 min after incubation in ETH, staining was strong in all ganglia of the controls (n=4) but still remained low in the SEG and TG of Br:ETH and VNC:ETH groups, with little to no stain in the AG (data not shown, n=4 and 3, respectively). Within intact preparations, staining was strong and consistent by 40 min of incubation (eg. Fig. 5a) and persisted for at least 60–75 minutes (data not shown; n=6). Staining was variable in Br:ETH and VNC:ETH groups during this time course. Therefore, 25–30 tissues were scored for each experimental group after 40 minutes of incubation in ETH or saline to determine trends in staining patterns (Fig. 6 and 7).
Fig. 6.
Percentage of tissues stained for cGMP immunoreactivity in the (A) IN704 interneurons or (B) NS27 neurons of the subesophageal ganglion (SEG), thoracic ganglia (TG) or abdominal ganglia (AG) when incubated with 1 μM ETH for 40 minutes. Tissues received ETH or saline on either side of a Vaseline dam between the brain and VNC. Controls received ETH on both sides of the dam (ETH:ETH). Other groups received ETH on the brain alone (Brain:ETH) or the VNC alone (VNC:ETH). Letters represent similarities between or within groups (p>0.05). Sample sizes ranged from 26–28 tissues for each group.
Fig. 7.
Average relative pixel intensity of all cells showing cGMP immunoreactivity in the (A) IN704 interneurons or (B) NS27 neurons of the subesophageal ganglion (SEG), thoracic ganglia (TG) or abdominal ganglia (AG) when incubated with 1 μM ETH for 40 minutes. Tissues received ETH or saline on either side of a Vaseline dam between the brain and VNC. Controls received ETH on both sides of the dam (ETH:ETH). Other groups received ETH on the brain alone (Brain:ETH) or the VNC alone (VNC:ETH). Letters represent similarities between or within groups (p>0.05). Sample sizes ranged from 26–28 tissues for each group.
In general, when ETH was applied for 40 min to the entire CNS (ETH:ETH), strong cGMP immunoreactivity was typically noted in the SEG, TG and AG of both the IN704 and NS27 cell groups (Fig. 5a). When ETH was applied to the brain alone (Br:ETH), cGMP immunoreactivity in the SEG and TG occurred most often, with fewer preparations showing stain in the AG (Fig. 5b), and if ETH was applied to the VNC alone (VNC:ETH), few preparations showed stain in any ganglia (Fig. 4c).
It should be pointed out that on occasion, full cGMP staining was noted in the NS27/IN704 neurons of the SEG, TG and AG even in treated groups, but the percentage of preparations responding this way was very low in “VNC:ETH” or even “brainless” groups. Moreover, if less than complete stain was noted, it was always reduced in the AG first. Under no circumstances was cGMP immunoreactivity noted in the AG when it was absent in the SEG or TG. Moreover, if ETH was applied to the VNC posterior to the SEG, cGMP immunoreactivity was never noted (0/6; data not shown). More specific analysis of variance of the staining revealed some broad differences in treatment groups for the IN704 and NS27 neurons.
Within the IN704 neurons (Fig. 6a) 60–80% of the controls (“ETH:ETH”; black bars) showed cGMP immunoreactivity in SEG, TG and AG after 40 min incubation in ETH. There were no significant differences in the percentage of stained ganglia across the entire CNS (p>0.5). cGMP immunoreactivity in the SEG of brain-treated preparations (“Brain:ETH”; grey bars) was not significantly different from controls, while staining occurred less often in the TG and least in the AG (p<0.05). Low rates of stain were noted equally in the SEG, TG and AG of the VNC-treated tissues (“VNC:ETH”; white bars), which were all significantly lower than controls. These values were significantly lower than Br:ETH preparations in the SEG and TG, but not in the AG. Staining was noted in fewer than 5% of preparations when tissues were incubated in saline (Sal:Sal; data not shown, n=18).
Within the NS27 neurons (Fig. 6b) 40–60% of control preparations showed strong stain by 40 min, with stain in the TG occurring most often. In brain-treated groups, staining was comparable to controls in the SEG, but less so in the TG, and was least abundant in the AG. As with the IN704 neurons, few preparations showed stain in the SEG, TG or AG of the VNC:ETH group, and all were significantly lower than controls. Stain was less abundant in the SEG and TG of VNC:ETH tissues than Br:ETH tissues as well.
When preparations showed any cGMP immunoreactivity, the intensity of stain in IN704 (Fig. 7a) and NS27 (Fig. 7b) neurons was monitored. The intensity of cGMP-immunoreactivity was only marginally reduced in the SEG of brain-treated tissues compared to controls (p=0.07), but was significantly reduced in the TG and AG (p<0.05). Stain intensity was reduced in all regions of the VNC-treated group compared to controls (p<0.05). Interestingly, because of the low proportion of VNC preparations showing cGMP stain, significant differences in pixel intensity were not noted when individual ganglia were compared (data not shown), but differences were noted when data was pooled within the SEG, thoracic or abdominal regions (Fig. 7).
Analysis of EH immunoreactivity after ETH application in vitro
It has been shown that EH immunoreactivity is significantly reduced in proctodeal nerves and axons of the terminal abdominal ganglia of the CNS when animals undergo ecdysis (Ewer et al., 1997a). We likewise saw reduced EH immunoreactivity in axons of the terminal abdominal ganglia when animals were injected with ETH (Fig. 3), suggesting that EH was released under these conditions. EH immunoreactivity was likewise diminished in axons of the terminal abdominal ganglia after isolated CNS preparations were incubated with 1 μM ETH for approximately 45 minutes compared to controls incubated in saline (Fig. 8), suggesting that EH release could be monitored in vitro as well. In order to assess whether ETH induced release of EH in the CNS via activity on the brain or VNC, the brain or the VNC alone were incubated with ETH using the silicone dam method, and EH immunoreactivity was quantified. ETH application to the entire CNS (ETH:ETH), the brain alone (Br:ETH) or to the VNC alone (VNC:ETH) resulted in reduced EH immunoreactivity in the axons compared to saline-incubated controls (Sal:Sal). Levels were significantly lower in whole CNS-treated preparations compared to both brain and VNC groups (p>0.5), but both brain and VNC groups did not show significantly different levels of staining from each other. These data suggest that ETH actions on the brain and VNC are sufficient to induce some level of EH release but that this is significantly reduced compared to the effects when ETH is applied to the entire CNS.
Fig. 8.
EH immunoreactivity in axons of the terminal abdominal ganglia of isolated CNS preparations with various saline or ETH treatments. (A, B) Representative confocal stacked images of EH-immunoreactive axons after incubation in saline or ETH, respectively. Arrows show stained axons. Scale bar = 0.01 mm. (C) Relative pixel intensities of axons of the terminal abdominal ganglia of isolated CNS preparations. Treatments are noted below each bar. [Sal:Sal] saline applied to the entire CNS (brain and VNC); [ETH:ETH] ETH applied to the entire CNS (brain and VNC); [BR:ETH] ETH applied to the brain alone, with saline on the VNC; [VNC:ETH] ETH applied to the entire VNC, but not the brain, with saline applied to the brain. Letters denote significant differences (P<0.05), using Tukey’s HSD on transformed data after One-way Analysis of Variance. Sample sizes are noted in white.
Discussion
Our data supports the suggestion that EH is essential for the elevation of ETH in the hemolymph in vivo, via a positive feedback loop, but that the rise in cGMP is not a requirement for onset of ecdysis. That is, up regulation of cGMP in the IN704/NS27 neurons of the abdominal ganglia is not required for release of the ecdysis motor program. We propose that EH (via cGMP up regulation) is, however, important for activation of inhibitory neurons of the SEG/TG. Finally, our data suggests that ETH triggers EH release from the ventromedial neurons via multiple spike initiation zones, one site being dendritic arbors near the cell bodies in the brain, and the other at a remote site from the brain.
EH is essential for the positive feedback loop
It has been clearly established that the interaction between ETH from Inka cells and EH originating from the brain results in a strong positive feedback loop, leading to the release of large concentrations of both ETH and EH into the hemolymph (Ewer et al., 1997a, Kingan et al., 1997). This allows ETH to differentially activate pre-ecdysis and ecdysis behaviors sequentially depending on its concentration in the hemolymph (Zitnan et al., 1999). Injected ETH appears to have a relatively short half-life in the hemolymph, where it merely acts as a trigger for release of endogenous ETH through the feedback loop. This is supported by the fact that brainless larvae are typically unable to undergo ecdysis in vivo (Novicki and Weeks, 1996), even when injected with ETH (Table 1).
If only the EH cell bodies are removed from the brain, through targeted ablation (Gammie and Truman, 1999) or by grosser brain dissections (Zitnan and Adams, 2000 and Table 1), ecdysis still proceeds for approximately half of the animals after ETH injection. The region of the brain left after dissection shows EH-immunoreactive dendritic arbors without the associated cell bodies (Fuse, personal observation). Under these conditions, animals only undergo ecdysis when EH is sufficiently depleted from the ventromedial axons (Fig. 3), and with a delayed onset time (Table 1). We propose that this delay may be due to loss of ETH-induced current flowing from the cell body that would summate with flow from the nearby dendrites in the spike initiation zone to drive spiking. Removal of the cell bodies would thus reduce part of the current in response to ETH, but dendritic current flow would likely provide enough depolarization to still drive firing. Thus a delay in EH release would result in a delayed elevation in ETH levels via the positive feedback loop. This is substantiated by the fact that cGMP elevation in cell bodies is low to non-existent in brainless animals, such that there may only be limited EH release via activation of a more posterior spike initiation zone. A fraction of “no MBr” animals show strong cGMP, and all controls show maximal staining at a time point just prior to ecdysis, indicative of full activation. This cGMP stain remains elevated in controls after ecdysis, over a 90 min interval, but is generally lost in brainless and “no MBr” animals, especially in the abdominal ganglia (Table 1). Ewer and Truman (1997) likewise showed that debrained animals injected with EH instead of ETH showed only a weak to non-existent cGMP response, which was elevated to a greater extent when the few brainless animals went through ecdysis.
We cannot exclude the possibility that other factors in the remaining tritocerebral region of the brain, that are not yet identified, are required for proper EH release. This is less likely, since ETH induces fictive ecdysis motor patterns in brainless tissues in vitro (Table 2). Nevertheless, EH-knock out flies undergo eclosion ~70% of the time, where EH neurons are lost but other brain neurons remain intact (McNabb et al., 1997). However, ETH receptors in Manduca brains are found not only on the ventromedial neurons but also on lateral MIP-containing cells as well as other less well-described cells, suggesting that multiple inputs could be activated for successful release of EH in vivo (Kim et al., 2006a). Other factors such as MasITP from lateral neurosecretory cells of the brain have also been implicated in ecdysis regulation (Drexler et al., 2007).
cGMP is not required for onset of the ecdysis motor program
While cGMP up regulation parallels the onset of ecdysis behaviors under normal conditions in vivo (Ewer et al., 1994 and 1997b; Fuse and Truman, 2002) our data suggests that ecdysis can occur without this up regulation. This is corroborated in vitro in a number of ways. When ETH is applied to brainless preparations, cGMP levels are transient or non-existent by 40 min in most preparations (Fig. 4). Under these conditions, ecdysis still occurs (Table 2), with relatively normal burst patterns (Table 3), and only marginally faster onset times (this is discussed further below). These data suggest that cGMP is uncoupled from ecdysis onset in this preparation, and should be useful for beginning to truly clarify the role of cGMP during ecdysis. This reduced accumulation of cGMP occurs in the intact CNS as well, if ETH is applied to the brain alone, or to the VNC posterior to the brain (Fig. 5, 6). Under these conditions, EH is still released, although to a lesser extent than in controls (Fig. 8), and cGMP levels show different levels of accumulation in the SEG, TG and AG if ETH is applied differentially to the brain or VNC. If applied to the brain, cGMP is elevated in the SEG to the same extent as controls, while application to the VNC results in reduced cGMP levels in all tissues. Thus, not only is cGMP uncoupled, but there appear to be multiple spike initiation zones for ETH in the CNS.
Multiple spike initiation zones on the VM neurons
Release of EH appears to be activated by a number of spike initiation zones, some downstream of the brain (Gammie and Truman, 1999). EH axons in both Manduca and Drosophila project the length of the CNS, and appear to make contact with the cell 27/704 neurons of the SEG, TG and AG (Ewer et al., 1997b), offering prime targets for locally-stimulated release of EH. When the ventromedial neurons are stimulated, cGMP immunoreactivity is only noted in the SEG and TG, but not in the AG. In contrast, when the proctodeal nerves are stimulated, only the abdominal ganglia show cGMP immunoreactivity, and at reduced levels (Gammie and Truman, 1999). Thus at least two different spike initiation zones for EH release may exist, one at the level of the brain (dendritic arbors) and the other in the VNC (possibly at the terminal axons or the proctodeal nerves). Multiple spike initiation zones have been shown to exist at far distances downstream of the cell bodies in a number of invertebrates, even localized to ganglia within neighboring segments (Calabrese, 1980; Vedel and Moulins, 1978; Meyrand et al., 1992; Killian et al., 2000).
Role of SEG/TG in inhibition
Our data supports the suggestion that EH activates inhibitory neurons of the SEG and TG, via a cGMP-mediated mechanism (Fuse and Truman, 2002). The IN704 neurons of the SEG have been implicated as the inhibitory neurons, via a cGMP-mediated mechanism, since zaprinast, a cGMP-specific phosphodiesterase inhibitor, causes a significant delay in onset of ecdysis in vivo (Fuse and Truman, 2002). Moreover, cGMP levels in descending axons have been shown to drop sooner in the SEG than in the AG neuropil, suggesting the possibility that inhibition is being lifted in the SEG prior to the onset of ecdysis (Ewer et al., 1994;1997b). Our data shows that when ventromedial cell bodies are removed from larvae in vivo, EH is still depleted from the ventromedial axons of ecdysing animals to the same extent as controls (Fig. 3), and cGMP up regulation is noted only in the SEG and occasionally in the TG, but not in the AG of these animals. Moreover, timing of onset of ecdysis never occurs more rapidly than controls (Table 1), suggesting that inhibition is still in place. In contrast, when brainless nerve cords are incubated in ETH, the fictive ecdysis motor program begins prematurely (Table 2), and cGMP levels are reduced in the SEG, TG and AG equally (Fig. 4). Thus, the loss of EH from the brain appears sufficient to eliminate activation of inhibition under these conditions.
It is important to remember that CCAP immunoreactivity, which is apparent consistently in the homologous IN704/NS27 cells of the AG, does not consistently show up in the SEG or TG (Ewer et al., 1994), suggesting that changes in cGMP levels in the different tissues serves different functions. It also leaves putative inhibitory factors unidentified. Clearly, identifying what the inhibitory neurons/factors are is essential to further clarifying the model for ecdysis regulation.
Thus, the role of cGMP may be more important for inhibition, as noted above, as well as for sustaining ecdysis motor programs, rather than initiating them. It may very well be that cGMP helps maintain motor activity, through increased cell excitability (Gammie and Truman, 1997a). Increases in cGMP levels found in Drosophila during ecdysis do not occur in the CCAP-containing neurons (Ewer and Truman, 1996), suggesting that cGMP elevation is not coupled to release of CCAP to release the ecdysis motor program. Moreover, if ecdysis in M. sexta is prolonged in vivo through removal of a small ring of cuticle at the onset of ecdysis (Zitnan and Adams, 2000), or by application of liquid band aid to limit cuticle removal (C. Ayala, personal communication), cGMP levels remain elevated for the duration of the behavior, especially in the thoracic ganglia. Ca+2 increases are noted in response to ETH application in both Manduca and Drosophila, specifically in cells associated with ecdysis behaviors, such as the CCAP neurons (Kim et al, 2006a; 2006b). Thus, this may be the second messenger required for activation of the abdominal ecdysis motor patterns instead.
Up regulation of cGMP may also be important for activating neurons associated with post-ecdysis activity, such as cuticle/wing expansion, tanning and hardening. McNabb and colleagues (1997) found that lack of EH in Drosophila did not limit all insects from undergoing successful eclosion, although post-eclosion behaviors such as wing spreading were significantly affected.
Updating the model for ecdysis regulation
Components of the model for ecdysis regulation are substantiated by our data, but additions are also postulated, where the roles of the SEG, TG and AG are quite different (Fig. 9). Given the abundance of information on ETH receptor expression, cGMP immunoreactivity and in vivo and in vitro responses, it is quite clear that the neuroendocrine regulation of ecdysis behaviors is much more complex than originally postulated. Thus we have broken down the model into the early and late phases of ecdysis (Fig. 9).
Fig. 9.
Updated model for the regulation of ecdysis in the larval CNS. See Fig. 1 caption for details on abbreviations. The behavioral repertoire has been divided into two parts: beginning sequence of behaviors (A), and end sequence of behaviors (B).
(1, 2) We have not addressed the role of PETH in onset of pre-ecdysis I behaviors, nor the role of low levels of ETH on pre-ecdysis II behaviors in this study.
Our data supports the suggestion that positive feedback between ETH and EH neurons is required to increase ETH and EH levels in the hemolymph sufficiently for the sequential onset of pre-ecdysis and ecdysis. However, we suggest that the spike initiation zone of the ventromedial neuron is not limited to the nearby dendritic arbors, but also exists within the VNC. We suggest it may be near or within the terminal abdominal ganglion. Thus at higher ETH concentrations, ventromedial cells continue to be stimulated at multiple spike initiation zones, including the neighboring dendritic arbors, and possibly axons of the terminal abdominal ganglion or the proctodeal nerves themselves. Activation of only one spike initiation zone is not sufficient for full EH release, resulting in lack of ecdysis behaviors in vivo. In vitro, high levels of ETH are supplied exogenously, making the feedback loop immaterial.
ETH action on unknown cells of the SEG appear to be required for successful release of abdominal CCAP and MIPs from the IN704 cells, to release the ecdysis motor sequence.
We propose that the activation of the SEG neurons by ETH does not result in up regulation of cGMP, and given the nature of ETH receptors (heptameric G-protein coupled receptors; Iversen et al., 2002; Park et al., 2003) the second messengers may be cAMP or Ca+2.
EH released centrally stimulates inhibitory neurons of the SEG/TG (IN704) to inhibit the downstream IN704/NS27 cells of the AG via an unknown inhibitor. This appears to be a cGMP-mediated mechanism.
EH also activates cGMP up regulation in the NS27/IN704 group of the AG, but this does not appear necessary for the onset of ecdysis, and may be more important for sustained activity in these cells, or even for activating cells for post-ecdysis activities.
Thus, the complexity of interactions between a wide variety of neuropeptides is clearly extensive, and suggests that this system should offer many new insights into the regulation and modulation of innate behaviors in invertebrates and vertebrates alike.
Acknowledgments
We also wish to thank Alex Vaughan for his technical assistance with the extracellular electrophysiology. This research was supported by funds from USDA NRI Grant to M. F. (MF80-2217); the National Institutes of Health, MBRS SCORE Program-NIGMS to M. F. (Grant #2S06 GM52588-09), The National Center on Minority Health and Health Disparities (grant #5P20-MD000262), NSF LS-AMP fellowship to M. A. U. (HRD-0350008).
Abbreviations
- PETH
Pre-ecdysis-Triggering Hormone
- ETH
Ecdysis-Triggering Hormone
- EH
Eclosion Hormone
- CCAP
Crustacean Cardioactive Peptide
- MIPs
Myoinhibitory Peptides
- IN704
Interneuron 704
- NS27
Neurosecretory Cell 27
- Br
Brain
- MBr
Midbrain
- VNC
Ventral Nerve Cord
- SEG
Subesophageal Ganglion
- TG
Thoracic Ganglia
- AG
Abdominal Ganglia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams ME, Zitnan D. Identification of ecdysis-triggering hormone in the silkworm Bombyx mori. Biochem Biophys Res Commun. 1997;230:188–191. doi: 10.1006/bbrc.1996.5915. [DOI] [PubMed] [Google Scholar]
- Calabrese RL. Control of multiple impulse-initiation sites in a leech intemeuron. J Neurophysiol. 1980;44:878–896. doi: 10.1152/jn.1980.44.5.878. [DOI] [PubMed] [Google Scholar]
- Clark A, del Campo M, Ewer J. Neuroendocrine Control of Larval Ecdysis Behavior in Drosophila: Complex Regulation by Partially Redundant Neuropeptides. J Neurosci. 2004;24(17):4283–4292. doi: 10.1523/JNEUROSCI.4938-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhaver PF, Truman JW. The role of eclosion hormone in the larval ecdyses of Manduca Sexta. J Insect Physiol. 1982;28:695–701. [Google Scholar]
- Davis NT, Homberg U, Dircksen H, Levine RB, Hildebrand JG. Crustacean cardioactive peptide-immunoreactive neurons in the hawkmoth Manduca sexta and changes in their immunoreactivity during postembryonic development. J Comp Neurol. 1993;338:612–627. doi: 10.1002/cne.903380410. [DOI] [PubMed] [Google Scholar]
- Drexler A, Harris CC, dela Pena MG, Asuncion-Uchi M, Chung S, Webster S, Fuse M. Molecular characterization and cell-specific expression of an Ion Transport Peptide in the tobacco hornworm, Manduca sexta. Tissue Cell Res. 2007;329(2):391–408. doi: 10.1007/s00441-007-0391-9. [DOI] [PubMed] [Google Scholar]
- Ewer J, De Vente J, Truman JW. Neuropeptide induction of cyclic GMP increases in the insect CNS: resolution at the level of single identifiable neurons. J Neurosci. 1994;14:7704–7712. doi: 10.1523/JNEUROSCI.14-12-07704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewer J, Truman JW. Increases in cyclic 3′, 5′-guanosine monophosphate (cGMP) occur at ecdysis in an evolutionarily conserved crustacean cardioactive peptide-immunoreactive insect neuronal network. J Comp Neurol. 1996;370(3):330–341. doi: 10.1002/(SICI)1096-9861(19960701)370:3<330::AID-CNE4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Ewer J, Gammie SC, Truman JW. Control of insect ecdysis by a positive-feedback endocrine system: roles of eclosion hormone and ecdysis triggering hormone. J Exp Biol. 1997a;200:869–881. doi: 10.1242/jeb.200.5.869. [DOI] [PubMed] [Google Scholar]
- Ewer J, Gammie SC, Truman JW. Invariant association of ecdysis with increases in cyclic 3′,5′-guanosine monophosphate immunoreactivity in a small network of peptidergic neurons in the hornworm, Manduca sexta. J Comp Physiol. 1997b;181:329–337. doi: 10.1007/s003590050118. [DOI] [PubMed] [Google Scholar]
- Fuse M, Truman JW. Modulation of ecdysis in the moth Manduca sexta: the roles of the suboesophageal and thoracic ganglia. J Exp Biol. 2002;205:1047–1058. doi: 10.1242/jeb.205.8.1047. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Truman JW. An endogenous elevation of cGMP increases the excitability of identified insect neurosecretory cells. J Comp Physiol [A] 1997a;180:329–337. doi: 10.1007/s003590050052. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Truman JW. Neuropeptide hierarchies and the activation of sequential motor behaviors in the hawkmoth, Manduca sexta. J Neurosci. 1997b;17:4389–4397. doi: 10.1523/JNEUROSCI.17-11-04389.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, Truman JW. Eclosion hormone provides a link between ecdysis-triggering hormone and crustacean cardioactive peptide in the neuroendocrine cascade that controls ecdysis behavior. J Exp Biol. 1999;202:343–352. doi: 10.1242/jeb.202.4.343. [DOI] [PubMed] [Google Scholar]
- Honegger HW, Market D, Pierce LA, Dewey EM, Kostron B, Wilson M, Choi D, Klukas KA, Mesce KA. Cellular localization of bursicon using antisera against partial peptide sequences of this insect cuticle-sclerotizing neurohormone. J Comp Neurol. 2002;452(2):163–177. doi: 10.1002/cne.10357. [DOI] [PubMed] [Google Scholar]
- Iversen A, Cazzamali G, Williamson M, Hauser F, Grimmelikhuijzen CJ. Molecular identification of the first insect ecdysis triggering hormone receptors. Biochem Biophys Res Commun. 2002;299(5):924–931. doi: 10.1016/s0006-291x(02)02798-5. [DOI] [PubMed] [Google Scholar]
- Killian KA, Bollins JP, Govind CK. Anatomy and physiology of neurons composing the commissural ring nerve of the cricket, Acheta domesticus. J Exp Zool. 2000;286(4):350–366. [PubMed] [Google Scholar]
- Kim YJ, Spalovska-Valachova I, Cho KH, Zitnanova I, Park Y, Adams ME, Zitnan D. Corazonin receptor signaling in ecdysis initiation. PNAS. 2004;17:6704–6709. doi: 10.1073/pnas.0305291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Zitnan D, Cho KH, Schooley DA, Mizoguchi A, Adams ME. Central peptidergic ensembles associated with organization of an innate behavior. PNAS. 2006a;103(38):14211–14216. doi: 10.1073/pnas.0603459103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Zitnan D, Galizia CG, Cho KH, Adams ME. A command chemical triggers an innate behavior by sequential activation of multiple peptidergic ensembles. Curr Biol. 2006b;16(14):1395–1407. doi: 10.1016/j.cub.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Kingan TG, Gray W, Zitnan D, Adams ME. Regulation of ecdysis-triggering hormone release by eclosion hormone. J Exp Biol. 1997;200:3245–32356. doi: 10.1242/jeb.200.24.3245. [DOI] [PubMed] [Google Scholar]
- Kostron B, Kaltenhauser U, Seibel B, Braunig P, Honegger H. Localization of bursicon in CCAP-immunoreactive cells in the thoracic ganglia of the cricket Gryllus bimaculatus. J Exp Biol. 1996;199(2):367–377. doi: 10.1242/jeb.199.2.367. [DOI] [PubMed] [Google Scholar]
- McNabb SL, Baker JD, Agapite J, Steller H, Riddiford LM, Truman JW. Disruption of a behavioral sequence by targeted death of peptidergic neurons in Drosophila. Neuron. 1997;19:813–823. doi: 10.1016/s0896-6273(00)80963-0. [DOI] [PubMed] [Google Scholar]
- Meyrand P, Weimann JM, Marder E. Multiple axonal spike initiation zones in a motor neuron: serotonin activation. J Neurosci. 1992;12(7):2803–2812. doi: 10.1523/JNEUROSCI.12-07-02803.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. The ionic mechanism of action potentials in neurosecretory cells and non-neurosecretory cells of the silkworm. J Comp Physiol. 1980;140:43–52. [Google Scholar]
- Novicki A, Weeks JC. The initiation of pre-ecdysis and ecdysis behaviors in larval Manduca sexta: the roles of the brain, terminal ganglion and eclosion hormone. J Exp Biol. 1996;199:1757–1769. doi: 10.1242/jeb.199.8.1757. [DOI] [PubMed] [Google Scholar]
- O’Brien MA, Taghert PH. A peritracheal neuropeptide system in insects: release of myomodulin-like peptides at ecdysis. J Exp Biol. 1998;201(2):193–209. doi: 10.1242/jeb.201.2.193. [DOI] [PubMed] [Google Scholar]
- Park Y, Kim YJ, Dupriez V, Adams ME. Two subtypes of ecdysis-triggering hormone receptor in Drosophila melanogaster. J Biol Chem. 2003;278(20):17710–17715. doi: 10.1074/jbc.M301119200. [DOI] [PubMed] [Google Scholar]
- Taghert PH, Truman JW. Identification of the bursicon-containing neurones in abdominal ganglia of the tobacco hornworm, Manduca sexta. J Exp Biol. 1982;98:385–401. [Google Scholar]
- Trimmer BA, Weeks JC. Effects of nicotinic and muscarinic agents on an identified motoneuron and its direct afferent inputs in larval Manduca sexta. J Exp Biol. 1989;144:303–337. [Google Scholar]
- Truman JW, Taghert PH, Copenhaver PF, Tublitz NJ, Schwartz LM. Eclosion hormone may control all ecdyses in insects. Nature. 1981;291:70–71. [Google Scholar]
- Vedel JP, Moulins M. A motor neuron involved in two centrally generated motor patterns by means of two different spike initiation sites. Brain Res. 1978;138:347–352. doi: 10.1016/0006-8993(77)90751-x. [DOI] [PubMed] [Google Scholar]
- Weevers RD. A lepidopteran saline: effects of inorganic cation concentrations on sensory, reflex, and motor responses in a herbivorous insect. J Exp Biol. 1966;44:163–175. doi: 10.1242/jeb.44.1.163. [DOI] [PubMed] [Google Scholar]
- Wells C, Aparicio K, Salmon A, Zadel A, Fuse M. Structure Activity Relationship of ETH during ecdysis in the tobacco hornworm, Manduca sexta. Peptides. 2006;27(4):698–709. doi: 10.1016/j.peptides.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Zitnan D, Adams ME. Excitatory and Inhibitory Roles of Central Ganglia in Initiation of the Insect Ecdysis Behavioural Sequence. J Exp Biol. 2000;203:1329–1340. doi: 10.1242/jeb.203.8.1329. [DOI] [PubMed] [Google Scholar]
- Zitnan D, Kingan TG, Hermesman JL, Adams ME. Identification of ecdysis-triggering hormone from an epitracheal endocrine system. Science. 1996;271:88–91. doi: 10.1126/science.271.5245.88. [DOI] [PubMed] [Google Scholar]
- Zitnan D, Ross LS, Zitnanova I, Hermesman JL, Gill SS, Adams ME. Steroid induction of a peptide hormone gene leads to orchestration of a defined behavioral sequence. Neuron. 1999;23:523–535. doi: 10.1016/s0896-6273(00)80805-3. [DOI] [PubMed] [Google Scholar]