Abstract
Polymer-based microparticles are in clinical use mainly for their ability to provide controlled release of peptides and compounds, but they are also being explored for their potential to deliver vaccines and drugs as suspensions directly into mucosal sites. It is generally assumed that uptake is mediated by epithelial M cells, but this is often not directly measured. To study the potential for optimizing M cell uptake of polymer microparticles in vivo, we produced sub-micron size PLGA particles incorporating a recombinant protein. This recombinant protein was produced with or without a c-terminal peptide previously shown to have high affinity binding to Claudin 4, a protein associated with M cell endocytosis. While the PLGA nanoparticles incorporate the protein throughout the matrix, much of the protein was also displayed on the surface, allowing us to take advantage of the binding activity of the targeting peptide. Accordingly, we found that instillation of these nanoparticles into the nasal passages or stomach of mice was found to significantly enhance their uptake by upper airway and intestinal M cells. Our results suggest that a reasonably simple nanoparticle manufacture method can provide insight into developing an effective needle-free delivery system.
Keywords: nanoparticle, mucosal vaccine, M cell
1. Introduction
One common therapeutic strategy to overcome infectious diseases such as influenza, SARS, salmonella, rotavirus and norovirus, is to develop effective vaccines that can induce potent mucosal immune response. The most efficient step towards the induction of mucosal immunity is the transport of vaccine antigens across the epithelial barrier by M cells. M cells are a specialized subset of cells expressed in mucosal epithelium, as in intestinal Peyer’s Patches and Nasal Associated Lymphoid Tissue (NALT) [1]. Thus, M cell targeted vaccines can be used in both oral and nasal routes of administration. M cells are characterized by poorly organized brush border membrane, basolateral lymphocyte-containing pocket and high endocytic activity [2, 3]. The development of M cell targeted vaccines is limited by the lack of information on M cell biology. In this regard, identification of receptor genes associated with Peyer’s Patch and NALT and development of technology that targets M cells through these receptors would be highly beneficial.
Claudin 4 is one such receptor that is highly expressed in colon, nasopharynx surface epithelium and Peyer’s Patch M cells [4–6]. Claudin 4 is a tight junction transmembrane protein that plays a role in establishing trans-epithelial electrical resistance in the mucosal epithelium [7–9]. In addition, Claudin 4 functions as a receptor for Clostridium perfringens enterotoxin (CPE). The second extracellular loop of Claudin 4 is known to bind to the C-terminal 30 amino acids of CPE (CPE30). The equilibrium affinity for CPE binding to Claudin 4, and the kinetics for different CPE peptides binding to Claudin 4 has been investigated [10]. In a previous study, Ling at el. [10] fused CPE30 to C terminus of influenza hemagglutinin (HA) and showed that CPE30 maintained its binding ability to Claudin 4, demonstrating the use of attaching targeting ligands to deliver antigens.
While antigens with targeting ligands can be used in conventional injectable vaccine formulations, encapsulation of vaccine antigens might provide an efficient vehicle for needle-free (intranasal or oral) M cell targeted vaccine delivery. Poly(lactide-co-glycolic acid) (PLGA) is a FDA approved copolymer of lactic and glycolic acids currently being used in a few drug delivery systems, though primarily in depot injection formulations [11]. Due to its excellent toxicological profile, PLGA is also being investigated for possible use in vaccine delivery systems [12–15]. Use of PLGA in a targeted sustained delivery system for vaccine delivery could provide optimized immune responses via selective targeted delivery of antigens, eliminating the need for booster doses by controlled release of antigens over a longer period and with sufficient safety to permit use in humans [16–19].
The particle size seems to be the most important characteristic for M cell uptake. It has been reported that particles less than 10 µm and 1 µm are suitable for oral and nasal delivery of vaccine antigens, respectively [3, 20]; however, recent studies have suggested efficient internalization of particles less than 1 µm in diameter [21, 22]. Additional physiochemical properties such as surface charge or inclusion of additional targeting may provide enhanced targeting and uptake by M cells [23, 24]. Thus, to study the potential for optimizing targeted M cell uptake of polymer nanoparticles in vivo, we produced sub-micron size PLGA particles incorporating recombinant proteins with the influenza HA with or without a c-terminal targeting peptide, CPE30. Our data shows increased uptake of targeted nanoparticles using in vitro and in vivo uptake studies, suggesting that this delivery technique can be used for targeted mucosal vaccines, or delivery of other bioactive molecules to mucosal immune tissue.
2. Materials and Methods
2.1. Materials
The PLGA (poly(DL-lactide-co-glycolide) 85:15, MW 50,000 – 75,000) and Poly(vinyl alcohol) (PVA, MW 30,000 – 70,000, 87 – 90% hydrolyzed) were obtained from Sigma-Aldrich. 4-(2-Hydroxyethyl)-1- Piperazineethanesulfonic Acid (HEPES, 1M), Phosphate Buffered Saline (PBS, 1X) and Sodium Dodecyl Sulfate solution (10% SDS), F-12 Kaighn’s medium, and geneticin were purchased from Invitrogen. Methylene Chloride optima®, PBS (10X ready concentrate pouches), HEPES (powder fine white crystals) and sodium hydroxide (certified A.S.C) were obtained from Fisher Scientific. Rhodamine 6G was obtained from Fluka® Analytical and 16% paraformaldehyde was obtained from Electron Microscopy Sciences. Prolong Gold antifade reagent with DAPI and 0.2 µm 505/515 (yellow-green) Neutravidin FluoSpheres® were purchased from Molecular Probes, and Pierce BCA™ Protein Assay Kit was obtained from Thermo Scientific.
2.2. Production and characterization of recombinant proteins and targeting peptides
2.2.1 Recombinant protein production
HA recombinant proteins were produced as described in Ling et al. [10] with minor changes. HA from influenza virus A (A/Puerto Rico/8/34/Mount Sinai, AF389118 (Hemagglutinin aa 1–528; truncated before the transmembrane domain) was used and subcloned into pENTR3C vector and recombined into BaculoDirect Linear DNA (BaculoDirect™ Baculovirus Expression Systems; Invitrogen). The resulting expression virus was used to express protein in an insect cell line, SF-9 cells. A trimerization sequence (ts, from Fibritin-C) was inserted to facilitate trimerization of HA, and His-tag (HT) was inserted for purification purposes. CPE30 (the terminal 30 amino acids of CPE) was introduced to the C terminus. The recombinant proteins were purified using ammonium sulfate precipitation followed by HT based cobalt resin binding and collection of purified protein via imidazole gradient elution.
2.2.2. Surface Plasmon Resonance
The Biacore X100 was used in this study using a GST-Claudin 4 fusion protein (“GST-R4”) as described in [10]. CM5 sensor chips were used to immoblize the ligands through an amine-coupling reaction - GST-R4 on the assay channel, and GST on the control channel. The ligands and analytes (C-CPE, a recombinant peptide from the terminal 135 amino acids of the CPE; HA-HT; and HA-HT-CPE) were all buffer exchanged to HBS-EP buffer (10 mM HEPES, 0.15 M NaCl, 3 mM EDTA, 0.005% Surfactant P20, pH 7.4) immediately before use. The binding was carried out at 25°C. All of the analytes were tested at a concentration of 5 uM, with a flow rate of 10ul/min, 120 second association and 120 second dissociation. Between analyte samples, the chip was regenerated with 50 mM NaOH. To analyze the data, the assay channel was subtracted from the control channel to remove any nonspecific binding of the analyte from the sensorgram curve.
2.2.3. Toxicity of CPE
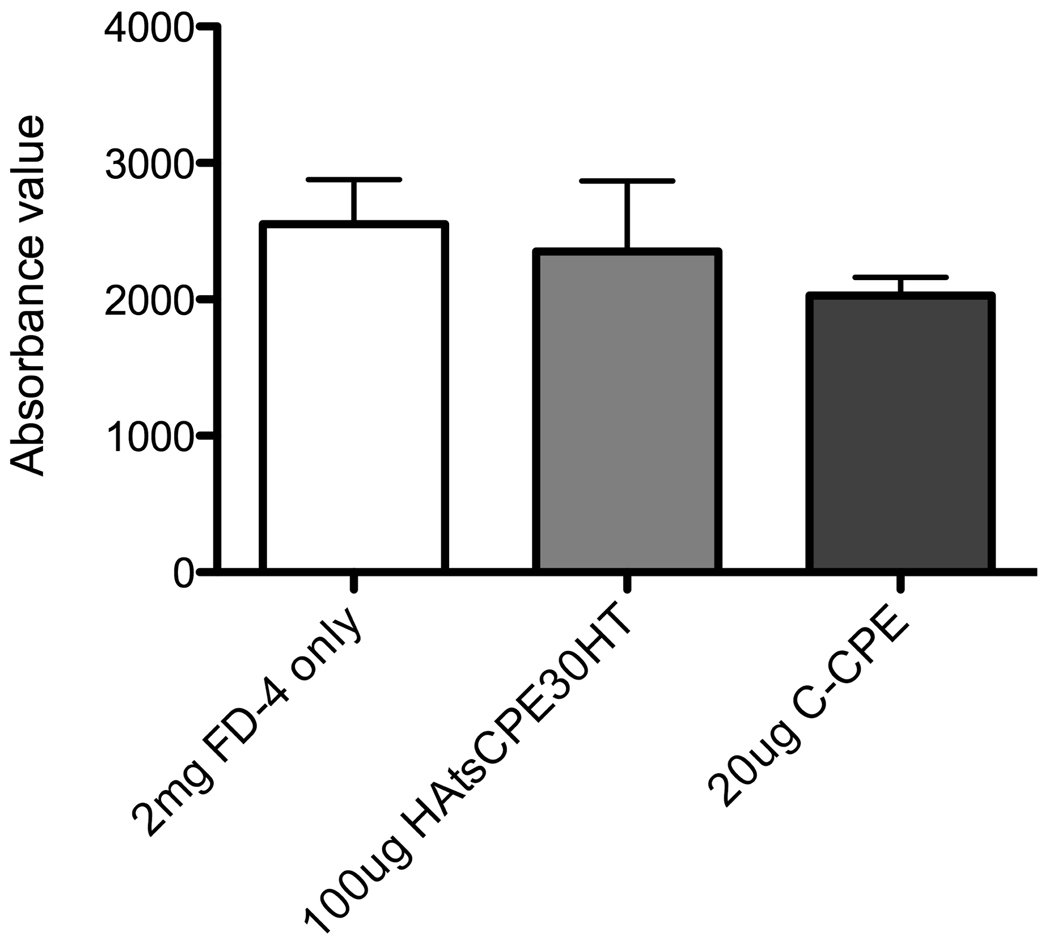
The toxicity of CPE peptides was evaluated in an in situ loop assay. Mice were fasted for two hours and 200 µl of 10 mg/ml fluorescent dextran (FD-4) was injected into the small intestine with 20 µg of C-CPE or 100 µg HA-CPE recombinant protein (HAtsCPE30HT). The control group was treated with FD-4 alone. Four hours post injection, serum was collected and level of FD-4 uptake was measured for each group. Effect of C-CPE and HA-CPE fusion protein on the intestinal barrier function is reported by plotting the absorbance reading for FD-4 for each condition in three different experiments.
2.3. In vivo and Ex vivo uptake of fluorescent beads
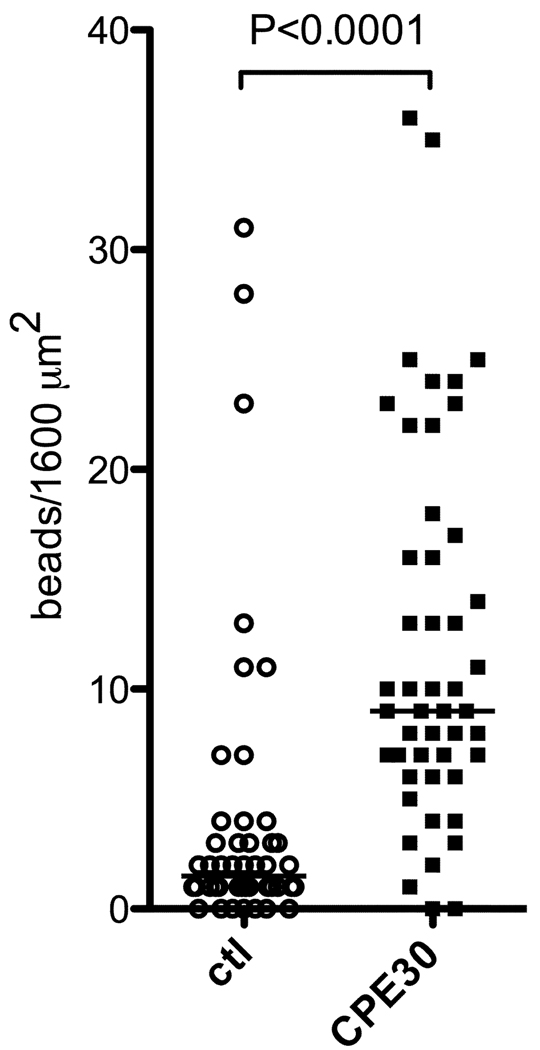
For fluorescent bead uptake studies, 0.2 µm 505/515 (yellow-green) Neutravidin FluoSpheres® were coated with biotinylated scramble sequence peptide or biotinylated CPE30 peptide. Under anesthesia, mice were given 6 × 109 beads in 40 µL of PBS intranasally (20 µL/nostril), and after 10 minutes; the NALT was dissected for microscopy. For very short time course studies (ex vivo), NALT was dissected first, and microparticle suspensions were administered to the NALT epithelial surface for one minute before fixation. The tissues were stained with mouse M cell marker, Ulex europaeus agglutinin 1 (UEA-1, green in Fig. 2A and 2B) and analyzed using a BD CARV II spinning disc confocal microscope. The images were deconvolved and the fluorescent bead (Red in Fig. 2A and 2B) uptake was measured from z-projection images of 1600 µm2 areas on NALT where UEA-1 positive cells were evident, using Volocity software. In Fig. 2C each symbol denotes the number of particles taken up in each 1600 µm2 area that was analyzed in two different NALT tissues dissected in two different experiments.
Fig. 2. NALT uptake of CPE30-coated fluorescent styrene beads.
(A) Confocal images of NALT showing epithelial UEA-1 positive M cells (green) and uptake of fluorescent styrene microparticles coated with control peptide (a scrambled peptide based on CPE) or CPE30 peptide. CPE30-coated particles (red, arrows) are more effectively taken into NALT than control particles.
(B) Confocal images of NALT taken after very brief exposure (1 minute) to CPE30-coated particles, showing that many beads (red, arrows) are visible within the UEA-1 positive M cells (green).
(C) Histological analysis of particle uptake into NALT. Each symbol denotes the number of particles taken up in each 1600 µm2 area that was analyzed in two different NALT tissues dissected in two different experiments. Data shows significantly higher numbers of beads taken up when beads are coated with CPE30 peptide (P<0.0001; one tailed Mann-Whitney test).
2.4. Nanoparticle preparation
PLGA nanoparticles containing targeting (HA-HT-CPE) and non-targeting (HA-HT) peptides were prepared from 85:15 PLGA using solvent evaporation/double emulsion (also known as water-in oil-in water, w/o/w) method. First, 4% PLGA polymer solution was prepared by dissolving 0.18 g of PLGA in 4.5 ml of methylene chloride. Then 0.5 ml of protein solution and 0.25 ml of 2% PVA solution in 10 mM HEPES buffer adjusted to pH 7.5 were added to 4.25 ml of the polymer solution and emulsified by probe sonication for 20 seconds (Branson® Sonifier 450, Duty cycle 20%, output control 3) [19, 25]and Dr. David Edwards, personal communication]. For labeled nanoparticles, 25 µl of 40 mg/ml Rhodamine 6 G (R6G) solutions was also added [22]. The resulting emulsion (w/o) was divided into two tubes, added 12.5 ml of 2% PVA solution to each tube and emulsified for 30 seconds to obtain the final w/o/w emulsion. The final w/o/w was then combined in a 50 ml beaker and stirred for 20 hours with a magnetic stirrer at 400 rpm at 4°C to allow solvent evaporation. The nanoparticles were collected by centrifugation at 3800 rpm for 30 minutes, resuspended in double distilled water; the washing step was repeated three times. The nanoparticles were freeze-dried and the final product was stored at 4°C until used.
2.5. Nanoparticle characterization
2.5.1. Scanning Electron Microscopy
The morphology of the protein-loaded nanoparticles was visualized by Scanning Electron Microscopy (SEM). The nanoparticles were placed on a double-sided adhesive tape attached to an aluminum stub and sputter coated with gold/palladium beam for 2 minutes. The coated sampled were imaged with Philips XL30-FEG SEM at 10kV.
2.5.2. Particle size measurements
The particle size of the nanoparticles was measured with ImageJ® software using the obtained SEM images. The diameter of approximately 150 nanoparticles per preparation was measured for three different preparations of nanoparticles, and the size distribution was plotted using Prism software. The particle size was also measured by dynamic light scattering using Zetasizer Nano ZS90 (Malvern Instruments, UK). Samples of PLGA nanoparticle dispersion in PBS (1 mg/ml concentration) was placed in a cuvette for size measurements. Each sample was measured for three times for triplicate preparations of nanoparticles and is reported as mean ± standard deviation.
2.5.3. Determination of protein loading
Total protein loading was estimated using BCA assay. Approximately 5 – 8 mg of freeze-dried nanoparticles were measured and added to 2 ml of 5% SDS in 0.1 M NaOH solution and incubated with shaking for 24 hours at room temperature until a clear solution was obtained. The protein content was measured in triplicates for each sample using BCA protein assay. The protein loading (%, w/w) was expressed as the amount of protein relative to the weight of the nanoparticles assayed [26]. In a separate experiment, blank PLGA nanoparticles were prepared and protein loading for these “non-protein loaded particles” was measured using BCA assay. The protein loading calculations showed negligible interference in BCA assay by blank particles.
2.5.4. Stability and integrity of protein loaded nanoparticles
The structural integrity of proteins incorporated in nanoparticles were first analyzed by SDS-PAGE and compared to non-encapsulated proteins. The nanoparticles were dissolved in 5% SDS in 0.1 M NaOH for one hour at room temperature and run on a gradient gel and tested for protein degradation by Coomassie stain. SDS-PAGE analysis; however, does not allow determining the presence of protein aggregates due to anionic detergent SDS, which dissociates protein aggregates. Therefore, we performed native PAGE analysis to confirm the absence of protein aggregates in nanoparticles. Nanoparticles prepared six months prior and stored at 4°C were used in this study. To extract detectable levels of targeting protein, HA-HT-CPE loaded nanoparticles were shaken at 150 rpm at 4°C with 0.2 M NaOH for four days. NaOH is known to catalyze the hydrolysis of PLGA co-polymer. The nanoparticle solution was centrifuged and the supernatant was run on a native gel under non-denaturing conditions. After migration, gels were stained with Commassie stain to reveal protein. In addition, same gel was transferred onto nitrocellulose membrane and blotted with antibody against HT to evaluate the structural integrity of recombinant protein containing HA and CPE.
2.5.5. In vitro release of protein
The release rate of HA-HT and HA-HT-CPE from nanoparticles were measured in phosphate buffered saline (PBS) at 37°C. Approximately 15 mg of protein loaded nanoparticles from three different experiments were measured and dispersed in 0.3 ml of PBS containing 0.02% sodium azide as a bacteriostatic agent. The samples were shaken at 200 rpm inside a 37°C incubator. At 4 hours, 24 hours and then at predetermined time intervals, the tubes were taken out of the shaker, centrifuged at 6000 rpm for 5 minutes. The supernatant was removed completely and the protein content of the supernatant was measured in triplicate using the BCA assay. Fresh PBS was added to the nanoparticles after each measurement. The release profile was calculated in terms of cumulative release (% w/w) with incubation time.
2.6. In vitro uptake studies and confocal microscopy
In vitro uptake studies of R6G-labeled protein-loaded nanoparticles were performed in Green Fluorescence Protein (GFP) tagged Claudin 4 transfected Chinese Hamster Ovarian (CHO) cells [10]. Cells were maintained in F-12 Kaighn’s medium supplemented with 10% Fetal Bovine Serum and 0.8 mg/ml geneticin. For the confocal studies, cells were plated on cover slides placed in 6-well plates and were grown at 37°C in 5% CO2 incubator for 48 hours. The cells were washed with PBS and the medium was replaced by 1 ml of nanoparticle solution in culture medium pre-warmed to 37°C (10 µg of protein/well). The cells were incubated at 37°C in 5% CO2 incubator for one or two hours. Upon incubation, cells were washed three times with PBS to remove unbound nanoparticles. Cells were then fixed with 4% paraformaldehyde in PBS for 20 minutes at room temperature and washed with PBS + 0.1% Tween20 for 3 – 5 minutes, two times. The cover slides were mounted on glass slides with Prolong Gold antifade reagent with DAPI and incubated for 24 hours at room temperature. Cells were imaged using a BD CARV II spinning disc confocal microscope, using IPLab software. Histological analysis of particle uptake was performed by counting the number of particles taken up per cell in randomly selected fields of the slides for three different experiments. Each data point on Fig. 5B denotes the number of particles taken up by each analyzed cell, approximately 130 cells per group. One tailed Mann-Whitney test was used for statistical analysis.
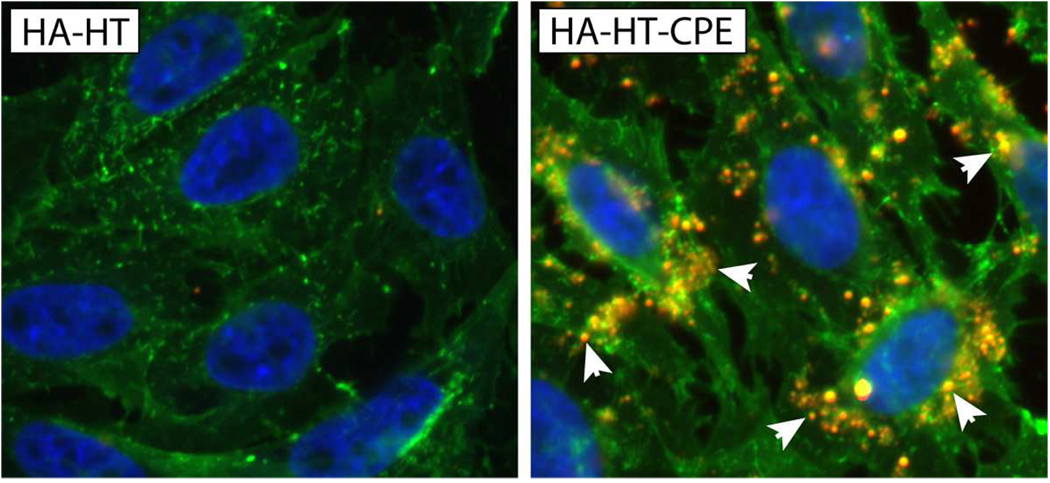
Fig. 5. PLGA nanoparticle uptake by GFP-Claudin 4 transfected CHO cells.
(A) In vitro uptake of HA-HT/R6G and HA-HT-CPE/R6G PLGA nanoparticles; R6G labeled nanoparticles were given to GFP-Claudin 4 transfectants (green) for one hour, and cells were processed for confocal microscopy. HA-HT-CPE/R6G particles were found to be readily bound and ingested, with particles shown to colocalize internally with GFP-Claudin 4 (yellow spots, arrows), while HA-HT nanoparticles are not taken up. Cell nuclei, blue.
(B) Histological analysis of particle uptake. Histological analysis of particle uptake was performed by counting the number of particles taken up per cell in randomly selected fields of the slides for three different experiments. Each data point denotes the number of particles taken up by each analyzed cell, approximately 130 cells per group. Highly significant uptake of HA-HT-CPE/R6G particles was evident (P < 0.0001, one tailed Mann-Whitney test).
2.7. In vivo uptake studies
For in vivo uptake studies, R6G-labeled, HA-HT or HA-HT-CPE loaded nanoparticles were used. Under anesthesia, mice were given 20 µg in 40 µl PBS intranasally (20 µl/nostril), and 100 µg in 200 µl by gavage for oral uptake. The NALT was dissected after one minute of nanoparticle incubation while Peyer’s Patches were dissected after four hours of incubation. Dissected NALT or Peyer’s Patch tissue were fixed in 4% paraformaldehyde/30% sucrose/PBS, which were then stained with UEA-1 (Vector). Casein/PBS (Fisher) with a final concentration of 0.1% Tween was used for blocking and for dilutions of antibody. DAPI was used as a nuclear stain. The slides were post fixed for 10 minutes with 4% paraformaldehyde, and mounted using ProLong Gold (Molecular Probes). Tissues were mounted in wells built from reinforcement rings on microscope slides for confocal microscopy studies. Cells were analyzed using a BD CARV II spinning disc confocal microscope, using IPLab software. Image deconvolution and analysis was performed using Volocity software. Nanoparticle uptake was measured from z-projection images from UEA-1-stained NALT taking 7850 µm2 areas where UEA-1 positive cells were evident. Nanoparticle counts were performed using Volocity software, and number of particles taken up in each 7850 µm2 area was plotted for two independent experiments. Each symbol denotes the number of particles taken up in each 7850 µm2 area that was analyzed in two different NALT and Peyer’s Patch tissues dissected in two different experiments. One tailed Mann-Whitney test was used for statistical analysis. All animals were purchased from Jackson Labs, housed at the UC Riverside vivarium under Specific pathogen-free (SPF) conditions, and were handled under an approved protocol in accordance with institutional IACUC and NIH guidelines.
3. Results
3.1. HA-HT-CPE30 protein shows specific binding to a Claudin 4 receptor protein
Ling et al. [10] demonstrated the ability of CPE30 to maintain its ability to bind to Claudin 4 receptor in a fusion protein with influenza HA. In this study, we modified the structure of the recombinant protein to include the CPE30 peptide at the C terminus to increase the effect of targeting (Fig. 1A). As shown in the schematic of the gene constructs, the control protein HA-HT contains the external domain of influenza HA and a HT for purification purposes. The targeted protein HA-HT-CPE contains influenza HA, a ts, HT, and C-terminal CPE30 peptide. The influenza HA protein normally forms trimers, and this can be reinforced in the recombinant protein by the inclusion of the trimerization peptide from Fibritin-C. Thus, the fusion protein with the CPE30 targeting peptide will actually be a trivalent particle; thus, it is possible that avidity effects of the trimer may enhance the binding and uptake by M cells in vivo to a greater degree than any single polypeptide. The ability of the targeted protein to bind to Claudin 4 receptor was analyzed by Biacore binding studies and was compared to the positive control C-CPE (a 135 amino acid C-terminal fragment of the CPE). As shown in the sensorgram in Fig. 1B, the targeted protein, HA-HT-CPE showed specific binding to Claudin 4 receptor compared to non-targeted HA-HT protein which behaved similar to the buffer control. The positive control C-CPE showed the highest binding as expected.
Fig. 1. HA-HT-CPE binding to Claudin 4 and in vivo toxicity of CPE.
(A) Schematic diagram of the Biacore sensorgrams showing the structure of the control HA-HT recombinant protein, including the first 528 aa of Influenza A/PR8/34 Hemagglutinin, and the His tag (HT) used for protein purification. The HA-HT-CPE contains an additional trimerization sequence (ts) from Fibritin-C, and the terminal 30 amino acids from CPE.
(B) Surface Plasmon Resonance sensorgram showing the binding of recombinant proteins (HA-HT, and HA-HT-CPE) to a GST-Claudin 4 fusion protein. It is compared to a positive control C-CPE (a subunit of the CPE), and buffer as a negative control. Note that HA-HT-CPE shows significant binding to Claudin 4, while HA-HT shows poor binding, similar to the buffer control.
(C) In vivo toxicity of CPE was evaluated in an in situ loop assay. The FD-4 uptake was measured in the presence of 20 µg of C-CPE or 100 µg of HA-CPE recombinant protein (HAtsCPE30HT). FD-4 alone was used as the control. The absorbance reading for FD-4 for each condition in triplicate experiments is plotted. Presence of C-CPE or HA-CPE recombinant protein did not affect the intestinal barrier function suggesting no toxicity of CPE peptides both in its native form and in recombinant protein.
The toxicity of different CPE peptides has been investigated in vitro [10]. Ling et al. [10] showed that CPE peptides do not affect epithelial barrier function when applied to the apical side, though reductions occurred when added to the basolateral side of M cell model. To investigate the toxicity of CPE peptides and the recombinant proteins containing CPE peptides in vivo, we measured the uptake of fluorescent dextran both in the absence and the presence of C-CPE and HA-CPE recombinant protein. As shown in the Fig. 1C, presence of C-CPE or HA-CPE recombinant protein (HAtsCPE30HT) did not affect the intestinal barrier function suggesting no toxicity of CPE peptides both in its native form and in recombinant protein.
3.2. Claudin 4-targeting peptide can mediate effective uptake of fluorescent beads by M cells
To enhance the avidity effect of trimerized HA proteins with CPE targeting even further, we sought to incorporate the recombinant protein into a particle displaying the targeting peptides across the surface. To establish proof of principle with the targeting peptide alone, Neutravidin-labeled FluoSpheres® beads were used. These polystyrene beads were coated with biotinylated scramble sequence peptide or biotinylated CPE30 peptide and intranasal uptake studies were performed in NALT. As shown in Fig. 2A, beads coated with CPE30 were taken up readily into the NALT after a very short time (10 minutes), while beads coated with control peptide were only very poorly taken up. Images taken from studies after very brief (<1 minute) ex vivo application of bead suspensions confirmed that beads were in fact taken up through M cells (Fig. 2B), as fluorescent beads (red) could be seen contained within UEA-1 positive M cells (green) on their way across the NALT epithelium. It is certainly also possible that some beads could cross without M cells, though their uptake is in any case still dependent on the Claudin 4 targeting peptide. Histological analyses of the bead uptake (Fig. 2C) showed a highly significant difference in the numbers of fluorescent beads taken up into NALT follicles; beads with CPE30 targeting peptide were taken up in greater numbers compared to beads coated with control peptide.
Having shown that the CPE30 targeting peptide can indeed mediate measurable uptake of nanoparticles by M cells, we next sought to develop nanoparticles that could incorporate the recombinant HA-HT-CPE30 fusion protein. It was critical that such particles would retain the proteins long enough for delivery to the target tissue, yet display enough of the protein on the surface to enable the CPE30 targeting peptide moiety to mediate uptake by M cells. PLGA polymer nanoparticles seemed to be an appropriate choice, since they could be produced with incorporated protein, yet they are biodegradable and can release the protein over time in vivo once delivered.
3.3. HA protein can be encapsulated into nanoscale PLGA particles
We developed a new method for PLGA nanoparticle production that provides a few important novel features that would increase mucosal targeting of vaccines. First, the particles are in a narrow size range less than 1 µm, which has been suggested by our own studies and the scientific literature [21, 22, 27] as being an optimal size range for mucosal M cell uptake. As shown in Fig. 3A, the SEM images revealed the uniform spherical form of both HA-HT and HA-HT-CPE loaded nanoparticles. These nanoparticles had a very narrow size distribution ranging from 0.1 to 0.7 µm as plotted in Fig. 3B. The particle size measured by Zetasizer Nano (ZS90) using dynamic light scattering method turned out to be slightly larger than values obtained by SEM images using ImageJ® software (Table 1). A number of studies also report higher particle diameter values reported by dynamic light scattering method due to the hydration of PLGA nanoparticles in solution [28, 29]. Our nanoparticle diameter remained within the desired size range for M cell uptake (less than 1 µm) in solution conditions used in uptake studies (1 × PBS), which is similar to physiological conditions.
Fig. 3. Influenza HA loaded PLGA nanoparticles.
(A) Scanning electron microscopy images of lyophilized PLGA nanoparticles produced with incorporated HA-HT or HA-HT-CPE protein, with or without Rhodamine 6G. Nanoparticles were prepared by double emulsion, solvent evaporation method as outlined in Section 2.4.
(B) Size distribution of lyophilized nanoparticles produced with HA-HT or HA-HT-CPE. Particle diameter was measured by ImageJ® using SEM images obtained for three different preparations of nanoparticles. The diameter of approximately 150 particles was measured for each preparation. The frequency distribution of particle size was plotted with Prism software. X-axis represents the particle diameter in µm while y-axis represents the percentage of particles at given diameter.
Table 1.
Properties of HA protein loaded nanoparticles
| Diameter of lyophilized particles (nm) |
Diameter of particles in solution (PBS) (nm) |
Protein loading (%, w/w) |
|
|---|---|---|---|
| HA-HT | 296 ± 10 | 455 ± 17 | 2.02 ± 0.39 |
| HA-HT-CPE | 280 ± 4 | 472 ± 25 | 2.16 ± 0.38 |
The Diameter of the lyophilized particles was measured by ImageJ® software using SEM images. The diameter of the particles in solution was measured by Zetasizer nano (ZS90) by dynamic light scattering method. The particle size expressed as mean ± standard deviation for three different preparations of nanoparticles as described in Section 2.5.3. The protein loading was measured as outlined in Section 2.5.3 using BCA protein assay. Control experiments revealed that there is negligible interference by blank PLGA particles on BCA assay. Percent protein (% w/w) loading for blank particles was approximately 0.031%.
Several parameters including the type of stabilizer, pH of the stabilizer solution, and protein concentration seemed to play a role in determining the morphology of PLGA nanoparticles. Thus each parameter was optimized to achieve uniform HA protein-loaded nanoparticles. In addition to the addition of stabilizer in the second emulsion, a low concentration PVA stabilizer (0.1%) solution was used in the first sonication to stabilize emulsion and to eliminate the diffusion of water-soluble substances into the second emulsion [25]. The pH of the stabilizer solution was adjusted to 7.5 to obtain uniform particles without debris. When the stabilizer solution pH was left at pH 4.5, significant debris was formed (data not shown). Both targeted and non-targeted nanoparticles exhibited a protein loading of approximately 2% (w/w) as listed in Table 1. PLGA nanoparticles in the same size range were reported to have lower loading efficiencies [30].
3.4. Stability and long-term release of HA protein
Use of organic solvents such as methylene chloride, use of high-speed sonication and freeze-drying procedure during PLGA particle preparation can result in protein degradation. Thus to evaluate the stability of non-targeted and targeted HA protein encapsulated in nanoparticles SDS-PAGE and native-PAGE analyses were performed. The stability of the encapsulated protein was compared to the non-encapsulated protein. As shown in Fig. 4A, SDS-PAGE analysis reveals that the encapsulated proteins were stable inside the nanoparticles and did not degrade during the production process. Native-PAGE analysis can preserve either covalently or non-covalently bonded aggregates. To evaluate the presence or absence of protein aggregates during storage of HA-HT-CPE loaded nanoparticles, native-PAGE analysis was performed for particles prepared six months prior and stored at 4°C. Fig. 4B shows the Coomassie stain and the anti-HT stain for HA-HT-CPE extracted from nanoparticles and soluble (control) proteins used for nanoparticle preparation. Both Coomassie stain and the anti-HT blot revealed similar band patterns for both freshly purified soluble protein and protein extracted from the stored nanoparticles suggesting that no protein aggregation resulted from nanoparticle production nor prolonged storage. The band at approximately 480 kD (based on MW standards) corresponds to the HA-HT-CPE trimer and is visible in both soluble and extracted protein. (Note that the estimated molecular weights of large complexes in native-PAGE analysis are likely to be crude estimates as different large proteins will migrate at different rates depending on their folded conformation.) The only additional band present in protein extracted from nanoparticles compared to soluble protein is visible below 146 kD (~ 66 kD). This may be due to some protein hydrolysis at low pH resulting from catalyzed PLGA polymer hydrolysis [31, 32], since a 0.2 M NaOH solution was used to catalyze the hydrolysis of PLGA in order to extract a detectable amount of protein for native-PAGE analysis. Thus, this problem is unlikely to arise during in vivo release of protein. In addition, protein integrity was also evaluated after prolonged storage of lyophilized particles at room temperature. Proteins remained intact without evidence for degradation after two months storage at room temperature (data not shown).
Fig. 4. Stability of protein in PLGA nanoparticles and protein release.
(A) Coomassie gel showing protein before and after incorporation into PLGA nanoparticles. Minimal protein degradation was evident after particle production.
(B) Coomassie stain and anti-HT Western blot of HA-HT-CPE extracted from nanoparticles (NP) and soluble (control) proteins used for nanoparticle preparation. Both Coomassie stain and the anti-HT blot revealed similar band patterns for both soluble protein and protein extracted from nanoparticles suggesting no protein degradation resulted from nanoparticle production or extended storage. Band at 480 kD corresponds to the HA-HT-CPE and visible in both soluble and extracted protein.
(C) In vitro protein release profile from PLGA nanoparticles. The release rate of HA-HT and HA-CPE30 from nanoparticles was measured in PBS at 37°C using the BCA assay. The release profile was calculated in terms of cumulative release (% w/w) with incubation time. Each point presents the mean ± standard deviation for triplicate samples. Rapid protein release of up to 6% occurred within the first day, with gradual release over the next 4 – 6 weeks up to 12 – 14%.
We also analyzed the long-term release profile of the protein loaded in the nanoparticles and the characteristics of protein release are shown in Fig. 4C. The nanoparticles exhibited a 4 – 6% initial burst release of the protein within the first 24 hours. The burst release is likely due to the protein at the surface of the nanoparticles. Beyond the initial burst release, a steady and sustained release protein was observed for both control and targeted HA protein-loaded particles. Within the next 4 to 6 weeks, up to 12 – 14 % of total loaded protein was released from the particles.
3.5. Targeted nanoparticles were readily taken up by GFP-Claudin 4 CHO transfectants
To confirm the ability of our targeted nanoparticles to successfully target Claudin 4 receptor in M cells, we produced control and targeted HA protein-loaded nanoparticles that also incorporated a fluorescent dye, R6G. Incorporation of R6G did not affect the particle morphology or the protein loading characteristics of the particles (Fig. 3A). Particle uptake studies were first performed in vitro using CHO cells that were stably transfected with GFP-Claudin 4. GFP-Claudin 4-transfected CHO cells were incubated with R6G labeled nanoparticles for 1 hour at 37°C. Nanoparticles with HA-HT-CPE protein were readily taken up by GFP-Claudin 4 CHO transfectants, while HA-HT loaded particles were not taken up well at all (Fig. 5A), showing both the function of the targeting peptide and the accessibility of the functional targeting peptide in the nanoparticles. Most of the HA-HT-CPE nanoparticles that were taken up by CHO cells were localized to the peri-nuclear region of the cells and generally co-localized with GFP-Claudin 4 staining (yellow color particles; arrows). Some excess particles can also be seen stuck to the coverslips between cells (visible as orange particles). Quantitative analysis of particle uptake showed highly significant uptake of targeted particles (~ 30 particles per cell) compared to control particles (~ 1 – 2 particles per cell) as shown in Fig. 5B. Actual particle uptake may be underestimated here, since in some instances, a few particles were in a very close proximity or even appeared to be fused into larger vesicles upon internalization.
3.6. Increased uptake of targeted particles by NALT and Peyer’s Patch M cells
To test whether the HA-HT-CPE nanoparticles can be taken up by M cells in vivo, the fluorescent R6G-labeled HA-HT and HA-HT-CPE nanoparticles were given intranasally and orally to assess uptake into NALT and Peyer’s Patches. As in the previous experiment with fluorescent styrene beads, microscopy images in Fig. 6A show nanoparticles taken into follicles; in some cases they are still evident within M cells (green). Histological analysis showed significant enhancement of targeted particle uptake in both NALT and Peyer’s Patches (Fig. 6B). Interestingly, the enhanced uptake is more evident for Peyer’s Patch, where the slower transit time of the intestinal contents may allow for the effect of the targeting peptide on M cell uptake.
Fig. 6. In vivo uptake of PLGA nanoparticles.
(A) Confocal images showing HA-HT-CPE/R6G nanoparticles (red, arrows) taken into NALT and Peyer’s patch tissues after intranasal or oral administration. In some cases, particles can be seen within Peyer’s patch M cells; UEA-1 positive M cells are stained in green.
(B) Histological analysis of particle uptake. Each symbol denotes the number of particles taken up in each 7850 µm2 area that was analyzed in two NALT tissues dissected in two different experiments. Data shows increased uptake of HA-HT-CPE/R6G particles relative to HA-HT/R6G particles (P = 0.0326 for NALT, P = 0.0005 for Peyer’s patch, one tailed Mann-Whitney test).
4. Discussion
The notion of targeted delivery of proteins and particles through M cells into mucosal lymphoid follicles is not new; several studies have used this principle for delivery of antigens to M cells using a variety of targeting strategies. For example, in the mouse, a fucose moiety is displayed on the apical surface of M cells, so groups have used the fucose-binding lectin UEA-1 or an antibody specific to fucose for targeted delivery [23, 24, 33, 34]. Similarly, groups have used the reovirus sigma protein, which also binds a target on mouse M cells [35–37]. However, these approaches are mainly limited to use in the mouse, as the targets are not present in human M cells. So while the focus of this study was not on targets expressed in human M cells, our previous studies focused on Claudin 4 as a target receptor because of its expression in both human and mouse M cells [4, 10]. Thus, the results of the present study are likely to have application to human clinical situations.
Our studies are more specifically focused on establishing proof of principle for the use of Claudin 4 binding peptides to mediate targeted delivery of nanoparticles to M cells and mucosal lymphoid tissues. Moreover, the production of recombinant fusion proteins with the targeting peptides and their incorporation into PLGA nanoparticles is accomplished reasonably simply, once the effects of different proteins on particle production are taken into account. In our nanoparticles, since the recombinant fusion proteins already have the targeting peptide, delivery of these proteins is still possible even if the protein is released from the nanoparticles. A few other studies also report incorporation of M cell targeting moieties in antigen loaded PLGA micro/nanoparticle preparations [23, 24]. However, it is most encouraging that the proteins remaining on the surface of the nanoparticles is sufficient to mediate delivery of the intact particles, even though they only comprise two percent of the mass of the particles. It is reported that PLGA particles stabilized with PVA stabilizer results in higher surface loading of protein compared to particles stabilized with other stabilizers [26].
Protein distribution, release of protein from PLGA delivery vehicles has been extensively studied [12, 38–41] and the adjuvant effect of PLGA encapsulated vaccines has also been investigated [42, 43]. Sustained and complete delivery of protein encapsulated in PLGA micro and nanoparticles in its native form still remains one of the most challenging tasks for development of PLGA based drug/vaccine delivery system [44]. While our nanoparticle formulation resulted in sustained delivery of targeted and non-targeted influenza HA antigens in vitro, complete release of protein was not achieved. The incomplete release of protein can be due to a several reasons, including, the slow hydrolysis rate of PLGA 85:15 co-polymer which causes the protein to remain in the polymer without being released [45], or non-release of protein due to formation of water-insoluble aggregations of protein inside the nanoparticles [44]. In addition, physical instability of released protein due to aggregation or hydrolysis can result in non-detection of released protein, which can also account for the slow release profile. Jiang and Schwendeman [46] provided a systematic evaluation of different excipients such as trehalose, sorbitol and MgCO3 that can be used to enhance the protein stability and achieve better release profile for PLGA encapsulated proteins. Similarly, Gupta et al., [23] reported the use of trehalose and Mg(OH)2 to stabilize and attain better release profiles. While a very slow rate of protein release is undesirable, a very fast rate of release is also undesirable. A long-term sustained release of antigen is beneficial in terms of vaccine delivery in eliciting a long-term immune response.
The actual uptake of particles in the polystyrene bead versus PLGA nanoparticle experiments suggests that the targeting peptide coated styrene beads may have been taken up in greater numbers than the PLGA nanoparticles. This might be due to a number of factors, such as the density and orientation of the targeting peptide on the surface of the particle, overall surface charge (i.e., zeta potential) of the particles [21], effects of the pH of the luminal fluid, and the presence of mucus. For example, it is notable that in our experience, the Neutravidin-coated styrene beads were not taken up well at all by mucosal M cells without targeting peptide (e.g., Fig. 2); while uncoated latex beads are readily taken up by M cells even without the use of targeting peptides (not shown). There might also be an effect of the transit time of contents in the nasal passages (probably seconds to minutes) versus the intestine (probably minutes to hours). Accordingly, preliminary observations suggest that NALT M cells take up particles at a much higher rate than Peyer’s patch M cells (not shown). Thus, the slower transit time in the intestine may help explain why the enhancement of delivery by the targeting peptide was more significant in the Peyer’s patch. Interestingly, the PLGA nanoparticles and their incorporated proteins survived both the stomach acid and upper small intestine digestive enzymes with the targeting function largely intact; it is possible that the nanoparticle polymer matrix was helpful in protecting the proteins from degradation for at least a few hours [47, 48]. Fievez et al. [49] investigated the use of nanoparticles with non-peptidic ligands for oral M cell targeted delivery. Further study will be necessary to establish particle features that may further enhance targeted uptake.
5. Conclusions
The use of PLGA-encapsulated proteins for vaccine or drug delivery has been investigated for the past 20 years; however, successful mucosal-targeted delivery has not yet been introduced to the clinic. While it is important to consider the physiochemical properties that affect the preparation of successful PLGA-based delivery systems, it is also equally important to pay attention to the targeting and uptake properties of mucosal M cells. With the use of M cell-targeting peptides incorporated into recombinant fusion proteins, we have produced PLGA nanoparticles that can be specifically targeted to M cells in vivo and this protocol can be adapted to incorporate nearly any new protein.
Acknowledgements
The authors thank Dr. Victor Rodgers, UC Riverside, and Dr. David Edwards, Harvard University for technical advice, and Mandy Wong and Nancy Appleby for technical assistance. This work was funded by a Grant from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jepson MA, Clark MA, Foster N, Mason CM, Bennett MK, Simmons NL, Hirst BH. Targeting to intestinal M cells. J Anat. 1996;189(Pt 3):507–516. [PMC free article] [PubMed] [Google Scholar]
- 2.Sansonetti PJ, Phalipon A. M cells as ports of entry for enteroinvasive pathogens: mechanisms of interaction, consequences for the disease process. Semin Immunol. 1999;11(3):193–203. doi: 10.1006/smim.1999.0175. [DOI] [PubMed] [Google Scholar]
- 3.Ermak TH, Dougherty EP, Bhagat HR, Kabok Z, Pappo J. Uptake and transport of copolymer biodegradable microspheres by rabbit Peyer's patch M cells. Cell Tissue Res. 1995;279(2):433–436. doi: 10.1007/BF00318501. [DOI] [PubMed] [Google Scholar]
- 4.Kuo WL, Lee LY, Wu CM, Wang CC, Yu JS, Liang Y, Lo CH, Huang KH, Hwang TL. Differential expression of claudin-4 between intestinal and diffuse-type gastric cancer. Oncol Rep. 2006;16(4):729–734. [PubMed] [Google Scholar]
- 5.Lo D, Tynan W, Dickerson J, Scharf M, Cooper J, Byrne D, Brayden D, Higgins L, Evans C, O'Mahony DJ. Cell culture modeling of specialized tissue: identification of genes expressed specifically by follicle-associated epithelium of Peyer's patch by expression profiling of Caco-2/Raji co-cultures. Int Immunol. 2004;16(1):91–99. doi: 10.1093/intimm/dxh011. [DOI] [PubMed] [Google Scholar]
- 6.Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120(2):411–422. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- 7.Katahira J, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Molecular cloning and functional characterization of the receptor for Clostridium perfringens enterotoxin. J Cell Biol. 1997;136(6):1239–1247. doi: 10.1083/jcb.136.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katahira J, Sugiyama H, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J Biol Chem. 1997;272(42):26652–26658. doi: 10.1074/jbc.272.42.26652. [DOI] [PubMed] [Google Scholar]
- 9.Sonoda N, Furuse M, Sasaki H, Yonemura S, Katahira J, Horiguchi Y, Tsukita S. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: Evidence for direct involvement of claudins in tight junction barrier. J Cell Biol. 1999;147(1):195–204. doi: 10.1083/jcb.147.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ling J, Liao H, Clark R, Wong MS, Lo DD. Structural constraints for the binding of short peptides to claudin-4 revealed by surface plasmon resonance. J Biol Chem. 2008;283(45):30585–30595. doi: 10.1074/jbc.M803548200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsung MJ, Burgess DJ. Preparation and characterization of gelatin surface modified PLGA microspheres. AAPS PharmSci. 2001;3(2):E11. doi: 10.1208/ps030211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chong CS, Cao M, Wong WW, Fischer KP, Addison WR, Kwon GS, Tyrrell DL, Samuel J. Enhancement of T helper type 1 immune responses against hepatitis B virus core antigen by PLGA nanoparticle vaccine delivery. J Control Release. 2005;102(1):85–99. doi: 10.1016/j.jconrel.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Brandhonneur N, Chevanne F, Vie V, Frisch B, Primault R, Le Potier MF, Le Corre P. Specific and non-specific phagocytosis of ligand-grafted PLGA microspheres by macrophages. Eur J Pharm Sci. 2009;36(4–5):474–485. doi: 10.1016/j.ejps.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Brandhonneur N, Loizel C, Chevanne F, Wakeley P, Jestin A, Le Potier MF, Le Corre P. Mucosal or systemic administration of rE2 glycoprotein antigen loaded PLGA microspheres. Int J Pharm. 2009;373(1–2):16–23. doi: 10.1016/j.ijpharm.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Zhang JX, Chen D, Wang SJ, Zhu KJ. Optimizing double emulsion process to decrease the burst release of protein from biodegradable polymer microspheres. J Microencapsul. 2005;22(4):413–422. doi: 10.1080/02652040500098994. [DOI] [PubMed] [Google Scholar]
- 16.Eldridge JH, Staas JK, Meulbroek JA, Tice TR, Gilley RM. Biodegradable and biocompatible poly(DL-lactide-co-glycolide) microspheres as an adjuvant for staphylococcal enterotoxin B toxoid which enhances the level of toxin-neutralizing antibodies. Infect Immun. 1991;59(9):2978–2986. doi: 10.1128/iai.59.9.2978-2986.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang W, Gupta RK, Deshpande MC, Schwendeman SP. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv Drug Deliv Rev. 2005;57(3):391–410. doi: 10.1016/j.addr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Rafati H, Lavelle EC, Coombes AG, Stolnik S, Holland J, Davis SS. The immune response to a model antigen associated with PLG microparticles prepared using different surfactants. Vaccine. 1997;15(17–18):1888–1897. doi: 10.1016/s0264-410x(97)00134-5. [DOI] [PubMed] [Google Scholar]
- 19.Rosas JE, Hernandez RM, Gascon AR, Igartua M, Guzman F, Patarroyo ME, Pedraz JL. Biodegradable PLGA microspheres as a delivery system for malaria synthetic peptide SPf66. Vaccine. 2001;19(31):4445–4451. doi: 10.1016/s0264-410x(01)00192-x. [DOI] [PubMed] [Google Scholar]
- 20.Pappo J, Ermak TH. Uptake and translocation of fluorescent latex particles by rabbit Peyer's patch follicle epithelium: a quantitative model for M cell uptake. Clin Exp Immunol. 1989;76(1):144–148. [PMC free article] [PubMed] [Google Scholar]
- 21.Gaumet M, Gurny R, Delie F. Localization and quantification of biodegradable particles in an intestinal cell model: the influence of particle size. Eur J Pharm Sci. 2009;36(4–5):465–473. doi: 10.1016/j.ejps.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Shakweh M, Besnard M, Nicolas V, Fattal E. Poly (lactide-co-glycolide) particles of different physicochemical properties and their uptake by peyer's patches in mice. Eur J Pharm Biopharm. 2005;61(1–2):1–13. doi: 10.1016/j.ejpb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Gupta PN, Khatri K, Goyal AK, Mishra N, Vyas SP. M-cell targeted biodegradable PLGA nanoparticles for oral immunization against hepatitis B. J Drug Target. 2007;15(10):701–713. doi: 10.1080/10611860701637982. [DOI] [PubMed] [Google Scholar]
- 24.Manocha M, Pal PC, Chitralekha KT, Thomas BE, Tripathi V, Gupta SD, Paranjape R, Kulkarni S, Rao DN. Enhanced mucosal and systemic immune response with intranasal immunization of mice with HIV peptides entrapped in PLG microparticles in combination with Ulex Europaeus-I lectin as M cell target. Vaccine. 2005;23(48–49):5599–5617. doi: 10.1016/j.vaccine.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Sharma KK, Boeglin M, Ogier J, Mainard D, Voegel JC, Mely Y, Benkirane-Jessel N. Transfection ability and intracellular DNA pathway of nanostructured gene-delivery systems. Nano Lett. 2008;8(8):2432–2436. doi: 10.1021/nl801379y. [DOI] [PubMed] [Google Scholar]
- 26.Coombes AG, Yeh MK, Lavelle EC, Davis SS. The control of protein release from poly(DL-lactide co-glycolide) microparticles by variation of the external aqueous phase surfactant in the water-in oil-in water method. J Control Release. 1998;52(3):311–320. doi: 10.1016/s0168-3659(98)00006-6. [DOI] [PubMed] [Google Scholar]
- 27.Fujimura Y, Akisada T, Harada T, Haruma K. Uptake of microparticles into the epithelium of human nasopharyngeal lymphoid tissue. Med Mol Morphol. 2006;39(4):181–186. doi: 10.1007/s00795-006-0335-6. [DOI] [PubMed] [Google Scholar]
- 28.Woo BH, Jiang G, Jo YW, DeLuca PP. Preparation and characterization of a composite PLGA and poly(acryloyl hydroxyethyl starch) microsphere system for protein delivery. Pharm Res. 2001;18(11):1600–1606. doi: 10.1023/a:1013090700443. [DOI] [PubMed] [Google Scholar]
- 29.Xie S, Wang S, Zhao B, Han C, Wang M, Zhou W. Effect of PLGA as a polymeric emulsifier on preparation of hydrophilic protein-loaded solid lipid nanoparticles. Colloids Surf B Biointerfaces. 2008;67(2):199–204. doi: 10.1016/j.colsurfb.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Betancourt T, Shah K, Brannon-Peppas L. Rhodamine-loaded poly(lactic-co-glycolic acid) nanoparticles for investigation of in vitro interactions with breast cancer cells. J Mater Sci Mater Med. 2009;20(1):387–395. doi: 10.1007/s10856-008-3594-z. [DOI] [PubMed] [Google Scholar]
- 31.Crotts G, Park TG. Protein delivery from poly(lactic-co-glycolic acid) biodegradable microspheres: release kinetics and stability issues. J Microencapsul. 1998;15(6):699–713. doi: 10.3109/02652049809008253. [DOI] [PubMed] [Google Scholar]
- 32.Igartua M, Hernandez RM, Esquisabel A, Gascon AR, Calvo MB, Pedraz JL. Enhanced immune response after subcutaneous and oral immunization with biodegradable PLGA microspheres. J Control Release. 1998;56(1–3):63–73. doi: 10.1016/s0168-3659(98)00077-7. [DOI] [PubMed] [Google Scholar]
- 33.Nochi T, Yuki Y, Matsumura A, Mejima M, Terahara K, Kim DY, Fukuyama S, Iwatsuki-Horimoto K, Kawaoka Y, Kohda T, Kozaki S, Igarashi O, Kiyono H. A novel M cell-specific carbohydrate-targeted mucosal vaccine effectively induces antigen-specific immune responses. J Exp Med. 2007;204(12):2789–2796. doi: 10.1084/jem.20070607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foster N, Clark MA, Jepson MA, Hirst BH. Ulex europaeus 1 lectin targets microspheres to mouse Peyer's patch M-cells in vivo. Vaccine. 1998;16(5):536–541. doi: 10.1016/s0264-410x(97)00222-3. [DOI] [PubMed] [Google Scholar]
- 35.Rubas W, Banerjea AC, Gallati H, Speiser PP, Joklik WK. Incorporation of the reovirus M cell attachment protein into small unilamellar vesicles: incorporation efficiency and binding capability to L929 cells in vitro. J Microencapsul. 1990;7(3):385–395. doi: 10.3109/02652049009021848. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki H, Sekine S, Kataoka K, Pascual DW, Maddaloni M, Kobayashi R, Fujihashi K, Kozono H, McGhee JR. Ovalbumin-protein sigma 1 M-cell targeting facilitates oral tolerance with reduction of antigen-specific CD4+ T cells. Gastroenterology. 2008;135(3):917–925. doi: 10.1053/j.gastro.2008.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Y, Wang X, Csencsits KL, Haddad A, Walters N, Pascual DW. M cell-targeted DNA vaccination. Proc Natl Acad Sci U S A. 2001;98(16):9318–9323. doi: 10.1073/pnas.161204098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al Haushey L, Bolzinger MA, Bordes C, Gauvrit JY, Briancon S. Improvement of a bovine serum albumin microencapsulation process by screening design. Int J Pharm. 2007;344(1–2):16–25. doi: 10.1016/j.ijpharm.2007.05.067. [DOI] [PubMed] [Google Scholar]
- 39.Mao S, Shi Y, Li L, Xu J, Schaper A, Kissel T. Effects of process and formulation parameters on characteristics and internal morphology of poly(d,l-lactide-co-glycolide) microspheres formed by the solvent evaporation method. Eur J Pharm Biopharm. 2008;68(2):214–223. doi: 10.1016/j.ejpb.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Sundaram S, Roy SK, Ambati BK, Kompella UB. Surface-functionalized nanoparticles for targeted gene delivery across nasal respiratory epithelium. FASEB J. 2009 doi: 10.1096/fj.09-129825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao A, Rodgers VG. Using TEM to couple transient protein distribution and release for PLGA microparticles for potential use as vaccine delivery vehicles. J Control Release. 2006;113(1):15–22. doi: 10.1016/j.jconrel.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 42.San Roman B, Irache JM, Gomez S, Tsapis N, Gamazo C, Espuelas MS. Co-encapsulation of an antigen and CpG oligonucleotides into PLGA microparticles by TROMS technology. Eur J Pharm Biopharm. 2008;70(1):98–108. doi: 10.1016/j.ejpb.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 43.Slutter B, Plapied L, Fievez V, Sande MA, des Rieux A, Schneider YJ, Van Riet E, Jiskoot W, Preat V. Mechanistic study of the adjuvant effect of biodegradable nanoparticles in mucosal vaccination. J Control Release. 2009;138(2):113–121. doi: 10.1016/j.jconrel.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 44.Giteau A, Venier-Julienne MC, Aubert-Pouessel A, Benoit JP. How to achieve sustained and complete protein release from PLGA-based microparticles? Int J Pharm. 2008;350(1–2):14–26. doi: 10.1016/j.ijpharm.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Corrigan OI, Li X. Quantifying drug release from PLGA nanoparticulates. Eur J Pharm Sci. 2009;37(3–4):477–485. doi: 10.1016/j.ejps.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Jiang W, Schwendeman SP. Stabilization of tetanus toxoid encapsulated in PLGA microspheres. Mol Pharm. 2008;5(5):808–817. doi: 10.1021/mp800027f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blanco D, Alonso MJ. Protein encapsulation and release from poly(lactide-co-glycolide) microspheres: effect of the protein and polymer properties and of the co-encapsulation of surfactants. Eur J Pharm Biopharm. 1998;45(3):285–294. doi: 10.1016/s0939-6411(98)00011-3. [DOI] [PubMed] [Google Scholar]
- 48.Shi L, Caulfield MJ, Chern RT, Wilson RA, Sanyal G, Volkin DB. Pharmaceutical and immunological evaluation of a single-shot hepatitis B vaccine formulated with PLGA microspheres. J Pharm Sci. 2002;91(4):1019–1035. doi: 10.1002/jps.10042. [DOI] [PubMed] [Google Scholar]
- 49.Fievez V, Plapied L, des Rieux A, Pourcelle V, Freichels H, Wascotte V, Vanderhaeghen ML, Jerome C, Vanderplasschen A, Marchand-Brynaert J, Schneider YJ, Preat V. Targeting nanoparticles to M cells with non-peptidic ligands for oral vaccination. Eur J Pharm Biopharm. 2009;73(1):16–24. doi: 10.1016/j.ejpb.2009.04.009. [DOI] [PubMed] [Google Scholar]