Abstract
Sonic hedgehog (SHH) is essential for the development of the cochlear duct that harbors the organ of Corti. However, little is known about the molecular signaling pathway through which SHH promotes the development of the organ of Corti, especially cochlear sensory epithelial cells. In this study, we demonstrated that SHH contributes to the differentiation of cochlear neural progenitors (CNPs), which are derived from the postnatal day 1 organ of Corti in mice. Addition of SHH to CNPs increased the formation of epithelial cell islands, simultaneously activated the expression of Math1 that is a transcription factor for the initial differentiation of auditory hair cells. The increased expression of Math1 then regulated the promoter activity of Brn3.1, another transcription factor that controls the further differentiation and survival of auditory hair cells. Taken together, our data suggest that SHH plays an important role in the promotion of auditory hair cell differentiation via the Math1-Brn3.1 signaling pathway.
Keywords: SHH, cell differentiation, hair cell marker, cochlear neural progenitor, mouse
INTRODUCTION
Progenitors are en route to polarized or differentiated cells under guidance of tissue specific factors or cues. In the cochlea, progenitors become hair cells under the influence of certain factor(s) in the developing process or remain in an undifferentiated state awaiting to become hair cells. However, soluble factors or cytokines that control the hair cell differentiation in the organ of Corti remain to be elucidated.
Recently, progenitors from the postnatal day 1 mouse organ of Corti with the potential to become hair cell- and neuron-like cells have been isolated and maintained in cell culture (Lin et al. 2007). These progenitors are different from prior sensory epithelial cell lines (Ozeki et al. 2003) or primary hair cell cultures (Doetzlhofer et al. 2004; Malgrange et al. 2002) in several aspects. Firstly, they are renewable for a relatively long period of time in appropriate cell culture media. Secondly, these cells are capable of differentiating into distinct phenotypes: hair cell- and neuron-like cells under the influence of a cytokine cocktail (SERB), e.g., sonic hedgehog (SHH), epidermal growth factor (EGF), retinoic acid (RA), and brain-derived neurotrophic factor (BDNF). These cells were originally thought of as cochlear stem cells/progenitors (Lin et al. 2007; Lin et al. 2008) but were recently thought as CNPs because of their neural differentiation potential. Similar cochlear stem/progenitor cells with the potential to become hair cells and other phenotypes have been isolated from the rodent postnatal cochlear tissue (Lopez et al. 2004; Lou et al. 2007; Oshima et al. 2007; Yerukhimovich et al. 2007).
A major reason for degenerative hearing disorders is loss of auditory hair cells and spiral ganglion neurons. CNPs are intriguing because of their capability to differentiate into neuron- and hair cell-like phenotypes, which is of importance in cell replacement for degenerative hearing disorders. CNPs are able to differentiate into a hair cell-like phenotype in vitro at a percentage of 11~35% in the presence of SERB (Feng et al. 2009b). However, it is not clear which cytokine or factor in the SERB cocktail contributes to differentiation of CNPs.
SHH plays an important role in the inner ear development (Bok et al. 2005; Liu et al. 2002; Riccomagno et al. 2002); (Driver et al. 2008). SHH knockout mice developed no ventral vesicle derivatives of the otic vesicle including cochlear duct and cochleovestibular ganglia (Riccomagno et al. 2002; Wu et al. 1998), pointing to the specification of SHH on the mammalian inner ear. However, the biological function of SHH on CNPs is poorly understood.
In the developing cochlea, the action of SHH on the cochlear progenitors is observed under the context of multiple cellular populations compounding with complex developmental cues. It is, therefore, difficult to pinpoint the biological function of SHH directly on cochlear stem cells/progenitors. In our recent studies, we found that SHH has the least power for cellular proliferation in vitro among a cytokine cocktail: SERB, suggesting that SHH is not mitogenic to CNPs. Consistent with this, Driver et al demonstrated that SHH does not contribute to hair cell proliferation (Driver et al. 2008).
In this study, we hypothesized that SHH commits to the differentiation of CNPs by regulating the expression of Math1, a transcription factor controlling the initial differentiation of auditory hair cells and Math1, in turn, regulates the expression of Brn3.1 in CNPs. Brn3.1 is known as an important transcription factor that is responsible for the terminal differentiation and survival of hair cells (Clough et al. 2004; Xiang et al. 1997). To test this hypothesis, we studied the effects of SHH on differentiation of CNPs in vitro. The results indicate that SHH regulated the differentiation of mouse CNPs via regulation of Math1-Brn3.1 signaling pathway.
MATERIALS AND METHODS
Culture Media
Animal use in this study was approved by the Institutional Animal Care and Use Committee (IACUC) of University of Minnesota. CNPs from the postnatal day 1 C57BJ/6 mice were isolated as previously described (Lin et al. 2007) and maintained in Minimal Essential Medium (MEM, Sigma) supplemented with 20 mM HEPES, 2 mM L-glutamine, 10 mL/L non-essential animo acid, 0.4 μg/mL hydrocortisone, 5 μg/mLinsulin, 2.5 μg/mL transferrin, 10 μg/mL EGF, and 10% FBS (MEM media). MEM media were chosen because of their capability to maintain the growth of CNPs (Hasina et al. 2008). DMEM/F12 or 1% N2 (Gibco) was used as media for starvation of CNPs in this study and referred to as a positive control for cellular differentiation because of its effects on Math1 expression (Lin et al. 2007).
Full-length mouse Math1 cDNA was cloned into a protein-expressing vector (pEGFP, Clontech) using the similar method, as previously described (Ozeki et al. 2007). Briefly, the open reading frame of the Math1 gene was obtained by PCR using the following primer pair (sense 5'-ccagcacctcctctaacacg-3' and antisense 5'-acgatcaccacagaccaaaa-3'). The PCR product was inserted into a pGEM®-T-easy vector (Promega) and subcloned into the EcoRI site of the pEGFP (C2, Clontech) according to the manufacturer's instruction. The Math1 cDNA sequence in pEGFP (sense and antisense) was confirmed by sequencing and the sense cDNA for the Math1 was used in this study.
Construction of the Brn3.1 reporter was performed as follows according to standard cloning protocols. Briefly, the sequence for mouse Brn3.1 promoter from – 1244~ −1 (totaled 1,268 bp including both Kpn I endonuclease site at the 5'-end and Hind III endonuclease site at the 3'-end) was amplified from the mouse genomic DNA by PCR using the following complex primer pairs (containing Kpn I and Hind III endonuclease sites): 5'-atggccggtaccttgaaccgcattgg atcc-3/ 5'-ctcgccaagctttgtgtcccctatttccc-3'. The PCR-amplified cDNA fragments were sequenced, BLAST searched, digested with these two endonucleases, and then subcloned into pGL4 vectors (Promega) according to the manufacturer's instruction. Similarly, the Math1 reporter was made according to the above procedures. Briefly, the sequence for mouse Math1 (Atoh1) promoter from-1 to −1551 (totaled 1,576 bp including both end adaptors: Bgl II at the 5'- end and Hind III at the 3'- end). Specific complex primers used for the Math1 gene promoter are: 5'-tcagagcagatctgaagtctgacgataact-3'/ 5'-ctcgataagcttggttgctgaaggcgctgc-3'
Real-Time PCR (qPCR)
Cell cultures reaching 60% confluency were transfected with empty vector and Math1 cDNA constructs, respectively, by using Nucleofector II System (A-33 program, amaxa, Inc., Gaithersbury, MD). Cells were cultured in FGM for 2, 3, and 4 days and then harvested for qPCR experiments at the end of day 2, 3, and 4. qPCR was performed using RT2 SYBR Green qPCR Master Mix (PA-110, SuperArray) on Eppendorf Mastercycler® according to the manufacturer's instructions. qPCR data were analyzed by realplex (Eppendorf®) and plotted with Sigmaplot (SPSS Inc.) as previously described (Zheng et al. 2008).
Specific primers for the Math1 (5'- agatctacatcaacg ctctgtc-3'/5'-actggcctcatcagagtca ctg-3', 452 bp), Brn3.1 (5'- ctctggcggcggtggata-3'/5'-acggcat gcgggtgactc-3', 324 bp), myosin VIIa (5'-aagca cct gctcctgctcg tccacg-3'/5'-ctccctctacatcgctctgttcg-3', 628 bp), ; and glyceraldehydes 3-phosphate dedydrogenase (GAPDH, 5'-aacgggaagcccatcacc-3'/5'-cagccttggcagcaccag-3').
Immunohistochemistry
Clonal CNPs were cultured on 8-well chamber slides for 2 days, fixed with 100% methanol for 6 minutes at room temperature, pre-incubated with 3% normal donkey serum for 15 minutes, incubated with primary and secondary antibodies sequentially. Dual labeled immunohistochemistry was performed for cellular marker co-expression. Briefly, cell culture slides were incubated with a primary antibody, washed with 0.2% detergent (TWEEN) in PBS, incubated with a secondary antibody conjugated to fluorescein isothiocyanate (FITC, green) or tetramethylrhodamine isothiocyanate (TRITC, red), washed again, washed in 0.2% TWEEN in PBS for 5 minutes, and observed under a confocal or fluorescent microscope. Chamber slides incubated with non-specific IgG (Zymed) served as controls. Primary antibodies used in this study were Math1 (Abcam, 1:100) and myosin VIIa (Abcam, 1:100). Secondary antibodies used were FITC or TRITC-conjugated mouse anti-rabbit or goat anti-mouse IgG (Zymed). Counter-stain with 4',6-diamidino-2-phenylindole (DAPI) was performed on some slides.
Fluorescence-activated cell sorting (FACS)
Cells were preincubated with 0.3% saponin in PBS for 10 minutes, incubated with primary and secondary antibodies sequentially: Math1 antibody on ice for 20 minutes, washed with 0.3% saponin in PBS, incubated with FITC-conjugated secondary antibody on ice for 20 minutes, washed with 0.3% saponin in PBS, incubated with myosin VIIa antibody on ice for 20 minutes, washed with 0.3% saponin in PBS, followed by incubation with TRITC-conjugated secondary antibody, washed again, resuspended in PBS and analyzed on FACSCalibur using CellQuest Pro (BD Sciences) for identification of hair cell marker positive cells. Results are presented as a percentage of positive cells per 10,000 cells. Non-specific IgG was used as an antibody control.
For apoptosis analysis by Annexin-V/7-AAD, 300 μL of 1.33x Annexin buffer was added to cells, followed by addition of 2 μL Annexin-V-APC with a final concentration of 0.5 ng/μL (Cat# 550474, BD Biosciences) to cells for 10 minutes at room temperature and finally by addition of 7-AAD to a final concentration of 50 μg/μL (Cat# 51-68981E, BD Biosciences) for additional 5 minutes prior to analysis (Annexin-V staining for early apoptotic cells and 7-AAD staining for late apoptotic/necrotic cells).
Luciferase assays
Cells cultured at a 6- or 12-well plate and reached at 40% confluence were transfected with the Math1 reporter at 1.4 μg/mL and co-transfected with β-galactodase reporter, respectively, at 0.7 μg/mL for 16 hours in the transfection medium and incubated in the growth media for 24 hours (recovery), and challenged with SHH at 5–50ng/mL, or incubated with MEM media (which promotes cellular proliferation and serves as a negative control for cellular differentiation), and DMEM/F12 (which contains no serum and growth factor and thus serves as a positive control for differentiation) for 6 and 24 hours. Cells were then harvested for luciferase assays. To study the effects of various inhibitors (PD98059, SB203580, LY294002, and cyclopamine) on SHH-induced Math1 promoter activity, cells were transfected with Math1 reporter for 16 hours, recovered in MEM media for 24 hours, incubated with individual inhibitors for 1 hour before addition of SHH, and then incubated with SHH plus individual inhibitors for 23 hours, and harvested for luciferase assays. To study the relationship between Math1 and Brn3.1, Math1 cDNA was transfected into CNPs and the promoter activity of Brn3.1 was evaluated by luciferase assays. Briefly, cells cultured in a 12-well plate with 60% confluence were transfected with the Math1 cDNA at 1.4 μg/mL and co-transfected with Brn3.1 reporter at 1.4 μg/mL and β-galactocidase at 0.7 μg/mL for 16 hours in the transfection media and recovered in culture media for 24 hours. Cells were harvested for luciferase assays, as previously described (Tsuchiya et al. 2005). Luciferase activity was measured using dual-light luciferase assay kit (Tropix) on a microplate luminometer (Model TR717, Tropix). β-galactocidase reporter activity was used as an internal control. The activity of luciferase reporter over β-galactocidase is presented as a relative luciferase activity (RLA).
Proliferation assays
For BrdU incorporation, cells were cultured on chamber slides beginning at 3×104 cells/per well in growth media, incubated with SHH at 5 nM, PBS control, and positive control (MEM media) for 24 hours, and chased with BrdU at 5 μM concentration for 1 hour prior to the end points of experiment, fixed with 100% methanol, and stained sequentially with anti-BrdU antibody (sheep IgG, cat# ab1893), and then counterstained with DNA dye (4'-6-diamidino-2-phenylindole, DAPI). Percentage of BrdU+ cells against total cell numbers (DAPI stained nuclei) were calculated from 5 high power fields (HPF, 4 corners plus 1 center of each slide) and representative BrdU incorporation data was obtained.
For Trypan blue exclusion, cells were cultured in growth media starting at 5×105 cells per T-25 flask, incubated with SHH at 5 nM, PBS control, and MEM media for 14 days, and harvested for cell counts after staining with Trypan blue dye as previously described (Ozeki et al. 2007).
For cell cycle progression, cells in flasks were incubated with SHH at 5 nM, PBS control, and MEM media for 14 days and harvested for cellular DNA quantitation by flow cytometry. For cell cycle analysis, approximately 3~5×105 cells were suspended in 100 μL of 40 μg/mL DNase-free RNase A with 100 μL of 200 μg/mL propidium iodide (fluorescent DNA dye) added, incubated for 30 minutes at room temperature, and analyzed on FACSCalibur with CellQuest Pro (BD Sciences). Singlets with DNA amount at 2x were defined as Go/G1 phase cells, >2x and <4x as S-phase cells, 4x as G2/M phase cells, and <2x as subG1 cells (e.g., apoptotic cells). Approximately 20,000 cells were measured per specimen and 6 separate specimens were run per treatment. Data was analyzed with Flowjo (version 7.0, Tree Star Inc) and is presented as mean ± SD with n=6.
Statistics
The differences between control and experiment in cell counts, immunohistochemistry, and cell marker positive counts were calculated using unpaired student t-tests when two groups of experiments were involved and/or analysis of variance (ANOVA) was used when multiple groups of experiments were involved. The confidence level at 95% was taken as significant.
RESULTS
SHH promotes the differentiation of CNPs
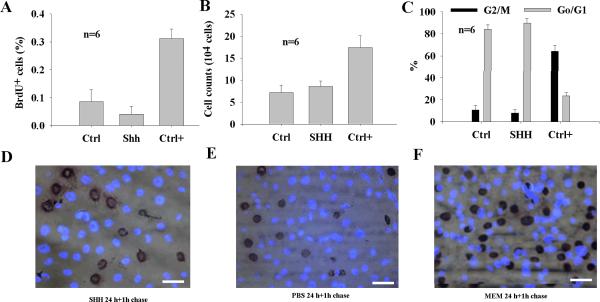
The action of SHH is cellular context-dependent. To study whether SHH is mitogenic to CNPs, BrdU incorporation was performed on cells after treatment with SHH at 5 nM for 24 hours with1-hour BrdU chase. It was found that SHH did not increase BrdU incorporation compared with PBS (Fig. 1A, representative BrdU incorporation data is shown in Fig. 1D–F). To study whether SHH has a mitogenic effect on CNPs on a long run, cell cycle progression and cell counts were performed with cell flow cytometry and Trypan blue exclusion. It was demonstrated that cells treated with SHH for 14 days were not significantly higher in numbers than those treated with no SHH for 14 days and cell cycle progression was not significantly different from those treated with PBS (Fig. 1B–C).
Fig. 1.
SHH has no significant influence on cellular proliferation of CNPs in vitro. At day 1 (24 hour), BrdU incorporation in CNPs was not increased in SHH-treated cell cultures compared with PBS- treated cell cultures (A). SHH had no significant influence on cell counts at day 14 cell cultures continuously with addition of SHH in a two-day interval compared with PBS (B). At day 14, cell cycle progression in SHH-treated CNPs showed 7.8±3.0% of cells in the G2/M+S phase and 89.7±3.8% in the Go/G1 phase (B) compared with MEM media (Ctrl+, 64.2±5.5% of cells in the G2/M+S phase and 23.6±3.0% in the Go/G1 phase) and PBS (Ctrl, 10.7±4.3% of cells in the G2/M+S phase and 84.0±4.2% in the Go/G1 phase). BrdU (1-hour chase experiment) verified that SHH did not increase BrdU+ cells compared with PBS- and MEM-treated CNPs (C) and representative BrdU+ cells after treatment with PBS, SHH, and MEM are shown in (D–F). bar=10 μM applying to D–F. Ctrl, control; Ctrl+, positive control.
SHH increases the expression of Math1 and formation of epithelial islands in CNP cultures
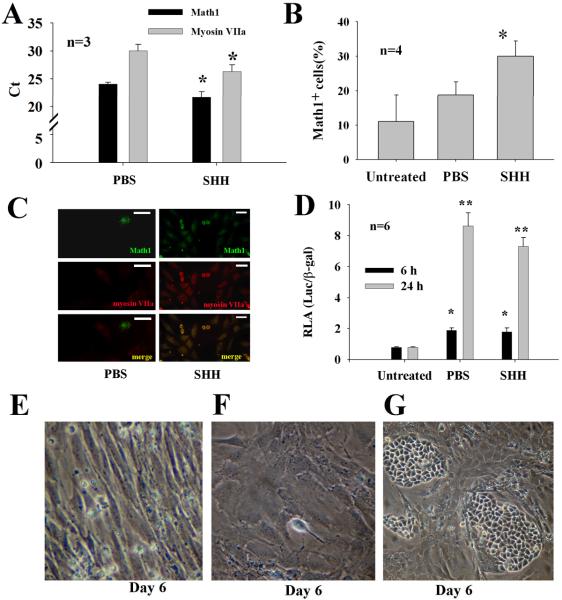
We questioned whether SHH is involved in cellular differentiation of CNPs. To this end, the effect of SHH on cellular differentiation of CNPs was performed. Briefly, cells were incubated with SHH at 5~50 nM for 6~48 hours and harvested for evaluation of Math1 expression by qPCR, immunohistochemistry, FACS, and luciferase assays. As expected, addition of SHH to CNPs led to a significant increase of the Math1 and myosin VIIa mRNA transcripts by qPCR (Fig. 2A), as well as Math1 positive cell population by FACS (Fig. 2B), and immunohistochemistry (Fig. 2C). In addition, the promoter activity of the Math1 gene significantly increased by luciferase assays by SHH (Fig. 2D). Interestingly, PBS-treated cells (starvation) also triggered the Math1 promoter activity but not so in the Math 1 protein production (Fig. 2B–D). During the development of the organ of Corti, there is a cellular band that contains hair cells. To study whether SHH plays a role in the formation of cellular band, we performed the morphological experiments. It was found that SHH promoted the formation of cobblestone-like epithelial islands in cell cultures (Fig. 2E–G).
Fig. 2.
SHH increases the differentiation of CNPs in vitro. Addition of SHH to CNPs significantly increased the expression of the Math1 mRNA transcripts by qPCR (A), the percentage of Math1+ cells by FACS (B), and the expression of Math1 and myosin VIIa in cells by immunohistochemistry (C), and the promoter activity of the Math1 gene by luciferase assays (D). Note that cell starvation (PBS, omitting EGF, FBS, and SHH) also significantly increases the promoter activity of Math1 compared with untreated cells. In comparison with PBS (E) and MEM-treated (F), SHH increased the formation of epithelial cell islands (G). Bar in C=10 μM; *p<0.05, **p<0.05.
Math1 increases the expression of Brn3.1 in CNPs in vitro
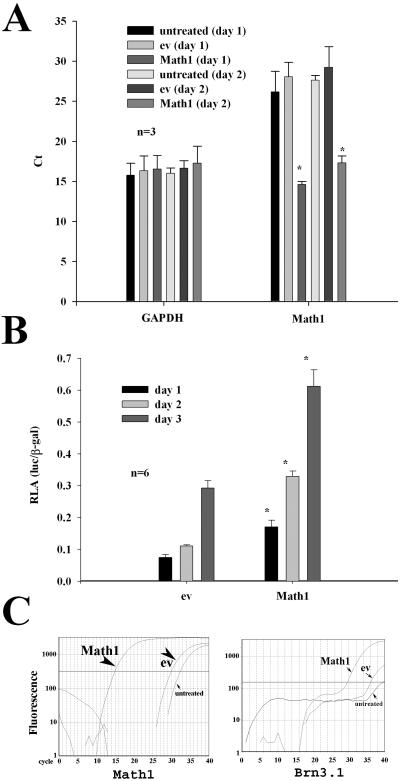
It is known from the mutant mouse model that the Brn3.1 gene is involved in the differentiation and survival of auditory hair cells. To study whether Math1 is involved in the regulation of Brn3.1, we constructed the reporter construct for the Brn3.1 gene and then performed luciferase assays. It was found that transfection of Math1 cDNA into CNPs increased the expression of the Math1 mRNA transcripts in CNPs as judged by qPCR, indicating that the transfection of Math1 in CNPs is successful in days 1 and 2 (Fig. 3A). Next, in these Math1-transfected cells, the promoter activity for the Brn3.1 gene was examined for evaluation of whether the transfection of Math1 leads to the upregulation of Brn3.1. It was found that Math1 significantly increased the promoter activity of the Brn3.1 gene in a time-dependent manner from day 1 to 3 (Fig. 3B). To verify the luciferase assay data, qPCR was performed for evaluation of the Brn3.1 mRNA transcripts. It was found that Math1 transfection in CNPs resulted in a significant increase of the Brn3.1 mRNA transcripts (lower cycles) compared with empty vector transfection (higher cycles), namely, a “theshold” cycle reduction from 36.0±2.1 to 30.9±1.6 cycles (p<0.03). Representative “threshold” cycle data is presented in (Fig. 3C).
Fig. 3.
Math1 activates the expression of Brn3.1 in CNPs. In comparison with ev transfection, Math1 cDNA transfection in CNPs successfully induced the expression of the Math1 mRNA transcripts at day 1 or 2 (A). Note that the higher the mRNA transcripts are, the lower the “threshold” cycles (Ct) are. Luciferase assays demonstrated that Math1 cDNA transfection in CNPs significantly increased the promoter activity of Brn3.1 in a time-dependent manner (B). The expression level of Brn3.1 mRNA transcripts was higher in those Math1-transfected CNPs than in ev-transfected CNPs for 2–4 days (C). Note that Math1-transfected CNPs have a remarkable increase of the mRNA transcripts for Math1 and a detectable increase of the mRNA for Brn3.1. Y axis, fluorescent intensity; X axis, cycle; solid line, the fluorescent level (approximately 200~500) being used as the “threshold” cycle of qPCR in this study (true threshold cycle at 100 or below).
Specific inhibitors for PI3K/Akt and Erk suppress SHH-induced expression of Math1 in CNPs
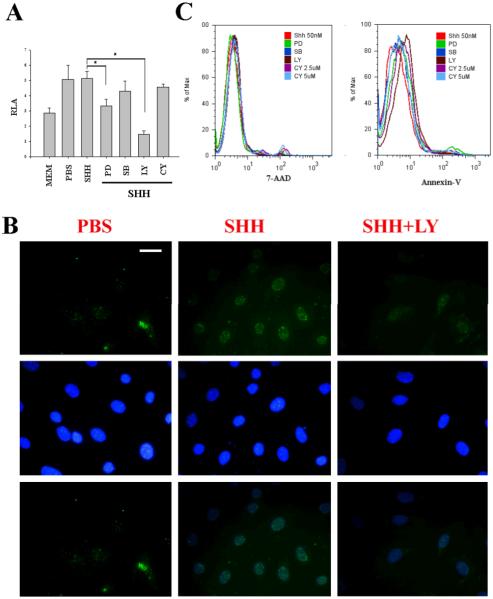
To study the signaling pathway through which SHH promotes the hair cell differentiation, we screened several chemical inhibitors: LY294002 for the PI3K/Akt signaling pathway, PD98059 for the Erk signaling pathway, SB230580 for the p38 pathway, and cyclopamine for the SHH receptor signaling pathway) on CNPs. It was found that LY294002 and PD98059 significantly inhibited the promoter activity of Math1 by SHH as judged by luciferase assays (Fig. 4A). To verify the increase of mRNA transcripts, CNPs were treated with SHH at 50 nM for 48 hours in the presence and absence of LY294002 at 20 μM and evaluated with the expression of Math1 protein by immunohistochemistry. It was found that the expression of Math1 protein was obviously inhibited in LY294002-treated cells compared with those not treated with LY294002 (Fig. 4B). Immunohistochemistry control (non-specific IgG control) showed negative stain (data not shown). It is known in our recent study that LY294002 is involved in cellular survival of auditory progenitor cells isolated from the P1 organ of Corti (Lin et al. 2008). We, therefore, asked whether this is due to cellular apoptosis in CNPs after administration of LY294002. The data indicated that reduced expression of Math1 was not due to cellular apoptosis because there was no significant increase in apoptotic cells by PD98059, SB230580, LY294002, and cyclopamine, as judged by Annexin-V and 7-AAD (Fig. 4C). The data, taken together, indicate that the PI3K/Akt and Erk signaling pathways are involved in the expression of Math1 in CNPs by SHH.
Fig. 4.
LY294002 significantly inhibits the expression of Math1 by SHH in CNPs. Specific inhibitors for the PI3K/Akt signaling pathway (LY at 20 μM) and the Erk signaling pathway (PD) at 24-hour significantly reduced the promoter activity of Math1 induced by SHH (A). In contrast, CY and SB did not show any effect on the Math1 promoter activity. LY at 48-hour incubation canceled out the expression of Math1 in the nuclei by SHH (SHH+LY) in CNPs compared with control (B). Reduction of luciferase activity by LY and PD did not cause cellular apoptosis as judged by Annexin-V and 7-AAD (C). PD, PD98059; SB203580; LY, LY294002; CY, cyclopamine. Bar=10 μM applying to all panels in B.
DISCUSSION
It is well recognized that SHH signaling is indispensable in the developmental process of the cochlea (Bok et al. 2005; Driver et al. 2008; Riccomagno et al. 2002). In the early embryonic days, developmental signals for cellular proliferation are active, SHH acts as a mitogen under such a context. For example, SHH has been shown to promote the proliferation of mouse early embryonic day (E9.5~E10.5) cochlear progenitor hair cells under cultured conditions (Zhao et al. 2006) but not at the embryonic day 13 cochlear progenitor hair cells in explant cultures (Driver et al. 2008). In the current study, we demonstrated that SHH plays a role in the differentiation of auditory progenitors that have the potential to become sensorineural phenotypes (immature hair cells and bipolar neurons) under cultured conditions. CNPs may be useful in cell replacement therapy.
The reason why SHH is important for hair cell differentiation is that SHH is capable of regulating the expression of Math1. Math1 initiates the differentiation of auditory hair cells in mammalians (Bermingham et al. 1999). Recent studies indicate that transfection of Math1 into the cochlear epithelial cells promotes the differentiation of hair cells possibly derived from the supporting cells in guinea pigs following antibiotic deafening (Izumikawa et al. 2005; Kawamoto et al. 2003). Math1 alone is sufficient for hair cell differentiation (Chen et al. 2002). Recently, functional hair cells have been produced in the mouse cochlea by in uetero gene transfer of Math1 (Gubbels et al. 2008). It is noted in the current study that treatment of CNPs with SHH at 50 nM causes potential cell death, as judged by Annixin-V (Supplemental data 1A–B).
In this study, we demonstrated that transfection of Math1 into CNPs led to cellular apoptosis (Supplemental data 1C) in addition to hair cell marker expression (myosin VIIa) that adds an additional piece of evidence to the notion that SHH is instructive for hair cell differentiation, based upon the fact that cell differentiation is associated with cell apoptosis. What happened is that Math1 in turn increases the transcription of Brn3.1 which drives the further differentiation of auditory hair cells. The time sequence for Math1 and Brn3.1 expression in mice is at E13.5 and E14.5, respectively (Xiang et al. 1997; Zheng and Gao 2000). There is a one-day lag between Math1 and Brn3.1 after addition of SHH to cell cultures. Consistent with this temporal pattern, SHH-induced expression of Math1 occurs within 24 hours whereas SHH-induced expression of Brn3.1 occurs within 72 hours in the current study. It is well known that the expression of Brn3.1 is essential for the differentiation and survival of auditory hair cells (Vahava et al. 1998; Xiang et al. 1997; Xiang et al. 1998).
The action for survival is perhaps via BDNF because Brn3.1 regulates the promoter activity of BDNF (Clough et al. 2004) and we showed recently that BDNF is involved in the survival of cochlear stem cells/progenitors in vitro through NF-κB and Bcl-2 (Feng et al. 2009a). Accompanied with this cellular differentiation process is that many cells survive and some cells undergo apoptosis.
An interesting phenomenon is that only a small portion of CNPs responded to SHH with an increase of both Math1 at the beginning and Brn3.1 subsequently when the entire culture is treated with SHH. That is the reason why qPCR, when examined on the entire cell culture, showed a limited increase of both Math1 and Brn3.1 mRNA transcripts. Luciferase assay data verifies that an mRNA transcript increase in both Math1 and Brn3.1 is based upon the promoter activity of the both genes, which explains why the mRNA transcripts increased. FACS also demonstrated that approximately 10-15% of CNPs turned into Math1 positive cells from 24 to 48 hours after treatment with SHH. After that, Math1 restores to a relatively low level that is comparable to that in control cells. Math1 is usually observed in the post-mitotic cells or prior to the terminal differentiation. Our data demonstrate that the upregulation of Math1 is transient and usually has a spike around 24 hours after SHH addition and then restores to a baseline level which may not be detectable by immunohistochemistry. The level of Math1 is an important determinant for hair cell differentiation. CNPs themselves express the both Math1 and Brn3.1 at the low level without growth factor priming that does not appear sufficient for driving cellular differentiation.
One thing we would like to discuss is the effect of DMEM (basal media) on the differentiation of CNPs. We have shown in our previous study that cell starvation is effective for induction of cellular differentiation as judged by hair cell marker expression, especially for Math1 and myosin VIIa(Lin et al. 2007). Later on, we found that starvation alone is limited for cellular differentiation unless cells are primed with growth factors or cytokines before starvation. In the current study, we observed that in our control groups (when cells treated with PBS, withdrawal of all growth factors and cytokines in culture media, that is equivalent to cell starvation) can trigger the promoter activity of both Math1 and Brn3.1. One may argue that cell starvation is as good as SHH in terms of Math1 and Brn3.1 promoter activity and why SHH is needed for CNP differentiation. Based upon our observations, cell starvation can trigger the promoter activity of Math1 in CNPs, but that action alone is not sufficient for the differentiation of auditory hair cells. The generation of mature hair cell is not only involved in the upregulation of both Math1 and Brn3.1 but also related to synergistic actions of multiple cell markers such as ion channels and other important proteins which are yet-to-be defined but they are essential for the function of mature hair cells. The effect of SHH on these markers remain to be elucidated in the future studies.
CNPs, capable of both neuron- and hair cell-like differentiation, are intriguing in terms of cell replacement in hearing degenerative disorders. At a minimum, these CNPs are inducible for the expression of hair cell markers (Math1 and myosin VIIa) under the influence of SHH in vitro. Whether these CNPs are able to replace lost hair cells and neurons in animal models remains to be elucidated.
In summary, SHH is essential for the specification of auditory hair cell progenitors by upregulation of Math1 at the first 24 hours that follows by an induction of Brn3.1 in cultured CNPs. This signaling pathway commits CNPs to a hair cell lineage and eventually drives CNPs to become nascent auditory hair cells which are immature but have the potential to become hair cells and replace lost hair cells in degenerating hearing disorders.
Supplementary Material
ACKNOWLEDGEMENTS
This study is in part supported by the National Institutes of Health (grant # R01DC008165, R03CA107989), the Lions 5M International Hearing Foundation, and the Fujian International Collaboration project of Science and Technical Reserch # 2008I0017. We would like to thank Ellalane Bearth for her editorial assistance in the preparation of this manuscript.
REFERENCES
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284(5421):1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bok J, Bronner-Fraser M, Wu DK. Role of the hindbrain in dorsoventral but not anteroposterior axial specification of the inner ear. Development. 2005;132(9):2115–2124. doi: 10.1242/dev.01796. [DOI] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Clough RL, Sud R, Davis-Silberman N, Hertzano R, Avraham KB, Holley M, Dawson SJ. Brn-3c (POU4F3) regulates BDNF and NT-3 promoter activity. Biochem Biophys Res Commun. 2004;324(1):372–381. doi: 10.1016/j.bbrc.2004.09.074. [DOI] [PubMed] [Google Scholar]
- Doetzlhofer A, White PM, Johnson JE, Segil N, Groves AK. In vitro growth and differentiation of mammalian sensory hair cell progenitors: a requirement for EGF and periotic mesenchyme. Dev Biol. 2004;272(2):432–447. doi: 10.1016/j.ydbio.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Driver EC, Pryor SP, Hill P, Turner J, Ruther U, Biesecker LG, Griffith AJ, Kelley MW. Hedgehog signaling regulates sensory cell formation and auditory function in mice and humans. J Neurosci. 2008;28(29):7350–7358. doi: 10.1523/JNEUROSCI.0312-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Fukudome S, Wang D, Schlentz E, Lin J. BDNF increases the survival of rat sensory epithelial cells via the PI3K/AKT and NF-kB/BCL-2 signaling pathways submitted. 2009a [Google Scholar]
- Feng L, Hamajima Y, Komori M, Anderson J, Wang D, Burns TC, Verfaillie CM, Low WC, Lin J. Differentiation of cochlear neural progenitors with SV40 in vitro. Mol Cell Pharm. 2009b;1:11–22. doi: 10.1152/ajpcell.00324.2008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gubbels SP, Woessner DW, Mitchell JC, Ricci AJ, Brigande JV. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature. 2008;455(7212):537–541. doi: 10.1038/nature07265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasina R, Whipple ME, Martin LE, Kuo WP, Ohno-Machado L, Lingen MW. Angiogenic heterogeneity in head and neck squamous cell carcinoma: biological and therapeutic implications. Lab Invest. 2008;88(4):342–353. doi: 10.1038/labinvest.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11(3):271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23(11):4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Feng L, Fukudome S, Hamajima Y, Huang T, Levine S. Cochlear stem cells/progenitors and degenerative hearing disorders. Curr Med Chem. 2007;14(27):2937–2943. doi: 10.2174/092986707782360051. [DOI] [PubMed] [Google Scholar]
- Lin J, Low WC, Verfaillie CM. Cochlear stem cells/progenitors. In: Low WCV CM, editor. Stem Cells and Regerative Medicine. World Scientific; Singapore: 2008. pp. 237–354. [Google Scholar]
- Liu W, Li G, Chien JS, Raft S, Zhang H, Chiang C, Frenz DA. Sonic hedgehog regulates otic capsule chondrogenesis and inner ear development in the mouse embryo. Dev Biol. 2002;248(2):240–250. doi: 10.1006/dbio.2002.0733. [DOI] [PubMed] [Google Scholar]
- Lopez IA, Zhao PM, Yamaguchi M, de Vellis J, Espinosa-Jeffrey A. Stem/progenitor cells in the postnatal inner ear of the GFP-nestin transgenic mouse. Int J Dev Neurosci. 2004;22(4):205–213. doi: 10.1016/j.ijdevneu.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Lou X, Zhang Y, Yuan C. Multipotent stem cells from the young rat inner ear. Neurosci Lett. 2007;416(1):28–33. doi: 10.1016/j.neulet.2006.12.061. [DOI] [PubMed] [Google Scholar]
- Malgrange B, Belachew S, Thiry M, Nguyen L, Rogister B, Alvarez ML, Rigo JM, Van De Water TR, Moonen G, Lefebvre PP. Proliferative generation of mammalian auditory hair cells in culture. Mech Dev. 2002;112:79–88. doi: 10.1016/s0925-4773(01)00642-6. [DOI] [PubMed] [Google Scholar]
- Oshima K, Grimm CM, Corrales CE, Senn P, Martinez Monedero R, Geleoc GS, Edge A, Holt JR, Heller S. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 2007;8(1):18–31. doi: 10.1007/s10162-006-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozeki M, Duan L, Obritch W, Lin J. Establishment and characterization of progenitor hair cell lines in rats. Hear Res. 2003;179:43–52. doi: 10.1016/s0378-5955(03)00077-7. [DOI] [PubMed] [Google Scholar]
- Ozeki M, Hamajima Y, Feng L, Ondrey FG, Zheng M, Schlentz EP, Lin J. Id1 induces the proliferation of cochlear sensorineural epithelial cells via the NF-kB/cyclin D1 pathway in vitro. J Neurosci Res. 2007;85:515–524. doi: 10.1002/jnr.21133. [DOI] [PubMed] [Google Scholar]
- Riccomagno MM, Martinu L, Mulheisen M, Wu DK, Epstein DJ. Specification of the mammalian cochlea is dependent on Sonic hedgehog. Genes Dev. 2002;16(18):2365–2378. doi: 10.1101/gad.1013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K, Kim Y, Ondrey FG, Lin J. Characterization of a temperature-sensitive mouse middle ear epithelial cell line. Acta Otolaryngol. 2005;125:823–829. doi: 10.1080/00016480510031533. [DOI] [PubMed] [Google Scholar]
- Vahava O, Morell R, Lynch ED, Weiss S, Kagan ME, Ahituv N, Morrow JE, Lee MK, Skvorak AB, Morton CC, Blumenfeld A, Frydman M, Friedman TB, King MC, Avraham KB. Mutation in transcription factor POU4F3 associated with inherited progressive hearing loss in humans. Science. 1998;279:1950–1954. doi: 10.1126/science.279.5358.1950. [DOI] [PubMed] [Google Scholar]
- Wu DK, Nunes FD, Choo D. Axial specification for sensory organs versus non-sensory structures of the chicken inner ear. Development. 1998;125:11–20. doi: 10.1242/dev.125.1.11. [DOI] [PubMed] [Google Scholar]
- Xiang M, Gan L, Li D, Chen ZY, Zhou L, O'Malley BWJ, Klein W, Nathans J. Essential role of POU-domain factor Brn-3c in auditory and vestibular hair cell development. Proc Natl Acad Sci U S A. 1997;94:9445–9450. doi: 10.1073/pnas.94.17.9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M, Gao WQ, Hasson T, Shin JJ. Requirement for Brn-3c in maturation and survival, but not in fate determination of inner ear hair cells. Development. 1998;125(20):3935–3946. doi: 10.1242/dev.125.20.3935. [DOI] [PubMed] [Google Scholar]
- Yerukhimovich MV, Bai L, Chen DH, Miller RH, Alagramam KN. Identification and characterization of mouse cochlear stem cells. Dev Neurosci. 2007;29(3):251–260. doi: 10.1159/000096415. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wang Y, Wang Z, Liu H, Shen Y, Li W, Heller S, Li H. Sonic hedgehog promotes mouse inner ear progenitor cell proliferation and hair cell generation in vitro. Neuroreport. 2006;17(2):121–124. doi: 10.1097/01.wnr.0000198439.44636.49. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Rayner M, Feng L, Hu X, Zheng X, Bearth E, Lin L. EGF mediates survival of rat cochlear sensory cells via an NF-kB dependent mechanism in vitro. Open Neurosci J. 2008;2:17–22. doi: 10.2174/1874082000802010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.