Abstract
It is poorly understood if and how pain may modify the effect of opioids on neural systems that contribute to reward and addictive behavior. We hypothesized that the activation of ascending dopaminergic and serotonergic nuclei by morphine is modified by the presence of noxious stimulation. Immunohistochemical double-labeling technique with Fos was used to examine if an intraplantar formalin injection, an acute noxious input, changed the effect of morphine on dopaminergic neurons of the ventral tegmental area (VTA), and serotonergic neurons of the dorsal raphe nucleus (DR). Four groups of rats were analyzed: (1) CONTROL injected with normal saline subcutaneously, (2) rats treated with FORMALIN into the hind paw 30 minutes after normal saline injection, (3) rats injected with MORPHINE sulfate subcutaneously, and (4) rats treated with formalin into the hind paw 30 minutes after morphine injection (MORPHINE/FORMALIN). Following morphine injection, there was an increase in the number of dopaminergic neurons in the VTA with Fos immunolabeling. However, noxious stimulation did not detectably change morphine's effect on Fos expression in VTA dopamine neurons. In contrast, the number of serotonergic neurons containing Fos was increased in the morphine/formalin group compared to all other groups and this effect was topographically selective for the dorsal area of the DR at mid rostro-caudal levels. Therefore, morphine's activation of the VTA, which is associated with motivated behavior and reward seeking, appears similar in the context of pain. However, activation of the ascending serotonin system, which influences mood and has the capacity to modify reward pathways, appears different. In addition, these findings reveal interactions between nociceptive signaling and opioids that contrasts with the notion that opioids simply block access of nociceptive signaling to supraspinal structures.
Keywords: Dorsal raphe, Fos, Tryptophan hydroxylase, Formalin, Rat, Ventral tegmental area
1. Introduction
Opioid addiction is a problem with devastating individual and societal consequences. Moreover, the fear of promoting addiction can hamper adequate pain treatment. While a single acute exposure to opioids does not produce addiction, it is the required first step towards this state. It is commonly thought that individuals with acute pain are not as likely to become addicted to opioids then those free of pain (Porter and Jick, 1980; Ballantyne, 2007). This raises the following question: could the presence of pain modify the neurochemical consequences of an acute exposure to opioids within neural pathways influencing the affective, rewarding and motivational properties of opioids? If so, this could modify the subjective experience of opioids and the propensity of an individual to seek repeated exposures possibly leading to addiction.
Dopaminergic neurons of ventral tegmental area (VTA) and its major projection to the anterior part of the basal forebrain, the nucleus accumbens, comprise the mesolimbic dopamine system. This system is considered a key component to motivational and reward functions (see reviews (Wise, 2004; Van den Heuvel and Pasterkamp, 2008)) and is widely implicated in drug addiction (see reviews (Schmidt et al., 2005; Pierce and Kumaresan, 2006; Alcaro et al., 2007)). In addition, ascending serotonergic neurons in the dorsal raphe nucleus (DR) have also been associated with addiction-related behavior (see review (Rothman et al., 2008)). Serotonin influences mood and affective state, which may be dysregulated in individuals vulnerable to addiction. Serotonin contributes to behavioral sensitization, or the increased rewarding properties of drugs of abuse following stress or previous drug exposure. Furthermore, serotonin has the capacity to directly modify mesolimbic dopamine system function (Beart and McDonald, 1982; Minabe et al., 1996; Yan and Yan, 2001) and serotonin neurons project in part to both the VTA and it's forebrain targets such as the nucleus accumbens and extended amygdala (Imai et al., 1986a; Imai et al., 1986b; Van Bockstaele et al., 1993).
Noxious stimulation in the form of formalin injection has been reported to diminish reinforcing effects of morphine in rodents (Suzuki et al., 2001), as well as to increase Fos expression in dopaminergic neurons of VTA (Ma et al., 1993) and serotonergic neurons of DR (Lang and Li, 1998; Chen et al., 2003). Also, acute injection of morphine leads to increased Fos expression in VTA (Singh et al., 2004) as well as in ventrolateral periaqueductal gray (Loyd et al., 2007), a major pain modulatory area of the brainstem, where serotonergic neurons of DR are located. However, no studies have investigated how acute morphine activation of monoamine neurons located in regions that contribute to motivation, reward and addiction-related behavior may be modified by the presence of noxious input. The objective of the present study was to determine whether the presence of noxious stimulation produced by an intraplantar formalin injection modifies acute morphine's effect on dopaminergic neurons of VTA and serotonergic neurons of DR by using double-labeling immunohistochemistry for markers of these monoamines combined with Fos.
2. Methods
2.1. Animal Care and Use
Adult male Sprague-Dawley rats (250-300 g; derived from Sasco; Charles River Laboratories International, Inc. Wilmington, MA) were housed two to a cage on a 12-h light/dark cycle schedule while food and water were available at all times. This type of rat is selected because it represents typical experimental subject used in the literature. Rats were handled for at least 3 consecutive days before experimental procedures to minimize stress during experimental handling. A total of 24 unanesthetized animals in 4 experimental groups (n=6/group) were used in this study. They include: (1) control group that received normal saline subcutaneously in suprascapular location, (2) formalin group that was treated with formalin into the hind paw 30 minutes after subcutaneous normal saline injection, (3) morphine group that received only morphine injection subcutaneously, and (4) morphine/formalin group that was treated with formalin into the hind paw 30 minutes after subcutaneous morphine injection. Care and use of animals was approved by the Institutional Care and Use Committee at Children's Hospital Boston, and was consistent with the National Institutes of Health and Committee for Research and Ethical Issues of IASP (Zimmermann, 1983).
2.2. Drugs
Morphine sulfate (Bexter Healthcare Corp., Deerfield, IL) or equal volume of normal saline control was used subcutaneously in the suprascapular region. Morphine was given at 5 mg/kg dose. This dose was selected because it is antinociceptive and, if repeated, has the capacity to produce conditioned place preference suggesting it has rewarding properties (Abbott et al., 1995; Bardo et al., 1995; Cicero et al., 2000; Morgan et al., 2006). Thus, it represents a dose that could model an initial dose prescribed for pain, or an initial non-clinical exposure that if repeated could lead to addiction.
2.3. Formalin Injection
Thirty minutes after either saline or morphine subcutaneous injection, at a time-point when morphine is known to have antinociceptive effects, half of each group of rats (12 total) received a subcutaneous injection of 50 μl of 2.5% formalin in the plantar surface of a single hindpaw (Dubuisson and Dennis, 1977; Abbott et al., 1995). Formalin was injected as quickly as possible using a 30-gauge needle. Formalin solution was prepared from commercially available stock formalin by further diluting it with isotonic saline to reach 2.5% concentration (Luccarini et al., 2006). Stock formalin solution is an aqueous solution of 37% formaldehyde (Sigma, St. Louis, MO). Although we did not quantitatively measure behavior following formalin injection alone, rats displayed spontaneous pain behavior, characterized by increased paw flinching, licking, and keeping the paw elevated as originally described by Dubuisson and Dennis (1977). Although post-formalin swelling was not measured, increase in edema and erythema of the formalin-injected paw has been noted. Furthermore, our qualitative behavioral observations agree with report by Dubuisson and Dennis (1977) who described that despite swelling and reddening of the formalin-injected paw, it was difficult to distinguish behavior between morphine/formalin treated rats from the ones in the control group. After formalin injection, animals were put back into the separate cages for two hours allowing Fos protein expression. Fos protein expression generally peaks at 1 hour and disappears by 3-4 hours (Herdegen and Leah, 1998) after either short or continuous stimulus. Therefore, time point selected for sampling perfusion was 2 hours after noxious stimulation and 2 hours and 30 minutes after systemic morphine administration. Experimental groups were matched and different groups were perfused on the same day. Tissue from animals in each group was processed in parallel.
2.4. Perfusion
Two hours after formalin injection and 2 hours and 30 minutes after saline or morphine subcutaneous injection, each animal was deeply anesthetized with sodium pentobarbital (100 mg/kg, intraperitoneally). Perfusion was done through ascending aorta with 50 ml of normal saline, followed by 250 ml of 4% paraformaldehyde in 0.1M phosphate buffer (PB, pH 7.4, room temperature). Brains were removed and stored in the same fixative solution overnight (4°C) before cryoprotection in 30% sucrose solution in 0.1M PB for at least 48 hours. Subsequently, brains were frozen and 40 μm coronal sections were cut on a freezing microtome. The free-floating sections were subsequently processed for immunolabeling.
2.5. Immunocytochemisty
We analyzed one third of collected coronal brain sections for Fos expression within serotonergic neurons using double-immunolabeling, another third for Fos expression within dopaminergic neurons, and the last third for triple-immunolabeling with markers of all three. Triple-immunolabeling was done to identify if any of the Fos labeling is present in known dopaminergic neurons that are located among serotonergic neurons of the DR (Trulson et al., 1985). Primary and secondary antisera were diluted in 0.1M PB with normal saline, 0.3% Triton X-100, 0.04% bovine serum albumin, and 0.1% sodium azide and incubated with the tissue for 2-3 days at 4 °C. Fos immunoreactivity was detected by incubating sections in rabbit anti-Fos antisera (Oncogene, Cambridge, MA) diluted 1:10,000. Dopaminergic neurons were labeled using an antiserum raised in mouse against tyrosine hydroxylase, the synthesizing enzyme for catecholamines (Chemicon International, Temecula, CA) diluted 1: 2000. Similarly, serotonin neurons were detected using an antiserum raised against tryptophan hydroxylase (TPOH), the synthesizing enzyme for serotonin, raised in sheep (Chemicon International, Temecula, CA) 1:1000. Although this antiserum has some cross-reactivity to tyrosine hydroxylase, which is present in some dopaminergic neurons in the area of the DR, it was selected for its very high sensitivity for TPOH. Free-floating sections were rinsed with 0.1 M PB in saline prior further processing. Secondary antiserum for Fos was raised in donkey and conjugated to CY3 (red fluorophore; Jackson ImmunoReasearch, West Grove, PA). Secondary antisera raised in donkey (anti-mouse or anti-sheep) were conjugated to Alexa-488 (green fluorophore; Invitrogen, Carlsbad, CA). All secondary antisera were diluted to 1:200. For triple-labeling experiments, secondary anti-TPOH antibody was detected with a donkey anti-sheep antisera conjugated for Alexa-647 (Invitrogen, Carlsbad, CA). Sections were rinsed in 0.1 M PB in saline solution prior to mounting on slides from a 0.05 M PB solution. After drying, mounted sections were coverslipped with 90% Glycerol solution.
2.6. Mapping and Cell Counts
All counting of neurons were done using a fluorescent microscope (Olympus IX81; Olympus America Inc. Melville, NY, USA) equipped with a camera and digital microscopy software (Slidebook v4.2, Olympus). Only dually immunolabeled neurons for both tyrosine hydroxylase or TPOH and Fos were counted in 8 different anatomical areas of the midbrain. The area defined by each subregion was based on the extent of the cellular groups comprising the region and specific landmarks (Fig.1). The rostrocaudal extent of the VTA area of quantification corresponded to plates 41- 46 of the Paxinos' atlas (Paxinos and Watson, 1997) while the DR area corresponded to plates 49-55. Furthermore, three rostrocaudal divisions of the DR were made: rostral DR, mid DR, and caudal DR, each of which was divided into dorsal and ventral subregions. The ventrolateral area of the DR comprised the last subregion and was sampled in sections of mid DR. For each rat, an average of 5-8 sections representing each of these areas were photographed. Fos immunolabeling was counted only in the vicinity of the monoamine neurons and dendrites comprising the specific nucleus. For each area, a minimum of 6 rats contributed to the mean number of double-immunolabeled profiles in each group. Double-labeled neurons were manually enumerated from the photographs by visualizing the individual and merged images of each fluorophore. Tyrosine hydroxylase- or TPOH-labeled neurons were considered Fos-positive if the nucleus was entirely filled with labeling for Fos, and the surrounding cell body and proximal dendrites filled with labeling for tyrosine hydroxylase or TPOH, as visualized by two different fluorophores. To ensure that immunolabeling was effectively detecting Fos, other brain regions besides those subject to analysis were examined for detectable Fos expression. An observer blind to the treatment group counted dual-immunolabeling of individual neurons.
Figure 1. Anatomical regions of analysis.
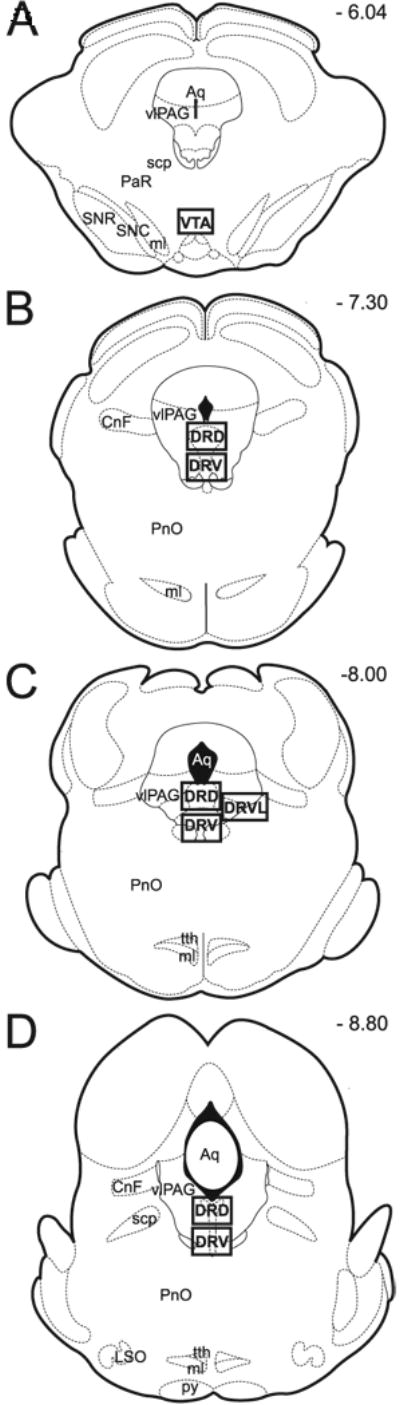
Schematic representation of rat brain representative transverse brainstem sections modified from Paxinos Atlas (Paxinos and Watson, 1997). Panels A-D correspond to Paxinos Atlas plates 44, 49, 52, and 55, respectively. Anatomical areas of analysis are marked by square throughout the midbrain sections. Anatomical regions of interest include: tyrosine hydroxylase-immunoreactive dopaminergic neurons of the ventral tegmental area (VTA) (Panel A) and tryptophan hydroxylase-immunoreactive serotonergic neurons of the dorsal raphe nucleus (DR; Panel B - D). Please note that DR is divided into ROSTRAL (Panel B), MID (Panel C) and CAUDAL (Panel D) subgroups. Each of these subgroups is further divided into dorsal (DRD) and ventral part (DRV), and in the case of mid portion of the DR, also into the ventrolateral part (DRVL) that is located in the area of the ventrolateral periaqueductal gray (vlPAG) (Panel C). Numbers in the right upper corner represent distance from Bregma (in mm). Abbreviations: Aq, aqueduct (Sylvius); CnF, cuneiform nucleus; DRD, dorsal raphe nucleus, dorsal part; DRV, dorsal raphe nucleus, ventral part; DRVL, dorsal raphe nucleus, ventrolateral part; LSO, lateral superior olive; ml, medial lemniscus; PaR, pararubral nucleus; PnO, pontine reticular nucleus, oral part; py, pyramidal tract; scp, superior cerebellar peduncle; SNC, substantia nigra, compact part; SNR, substantia nigra, reticular part; tth, trigeminothalamic tract; vlPAG, ventrolateral periaqueductal gray; VTA, ventral tegmental area.
2.7. Statistical Analysis
The mean density (# of double-labeled profiles/section/brain +/- SD) was determined for each experimental group and for each anatomical region (See Fig. 1). Estimated density of double-labeled neurons (Fig. 2) was compared among four different treatment groups (control, formalin, morphine, and morphine/formalin group). Data (density of double-labeled profiles) were tested for normality in each brain region using Kolmogorov-Smirnov Test and were found to follow a normal distribution closely. Significant F-tests were followed by post-hoc Tukey comparisons for pairwise tests. Therefore, density was compared between treatment groups using one-way analysis of variance (ANOVA) with Tukey adjustment to account for multiple group comparisons. This test was selected to protect against false positive results (type I error). Two-tailed p value less than 0.05 with Tukey correction was considered statistically significant. Statistical analysis was performed using the SPSS software package, v.16.0 (SPSS Inc., Chicago, IL).
Figure 2. Estimated density of double-labeled monoamine neurons with Fos.
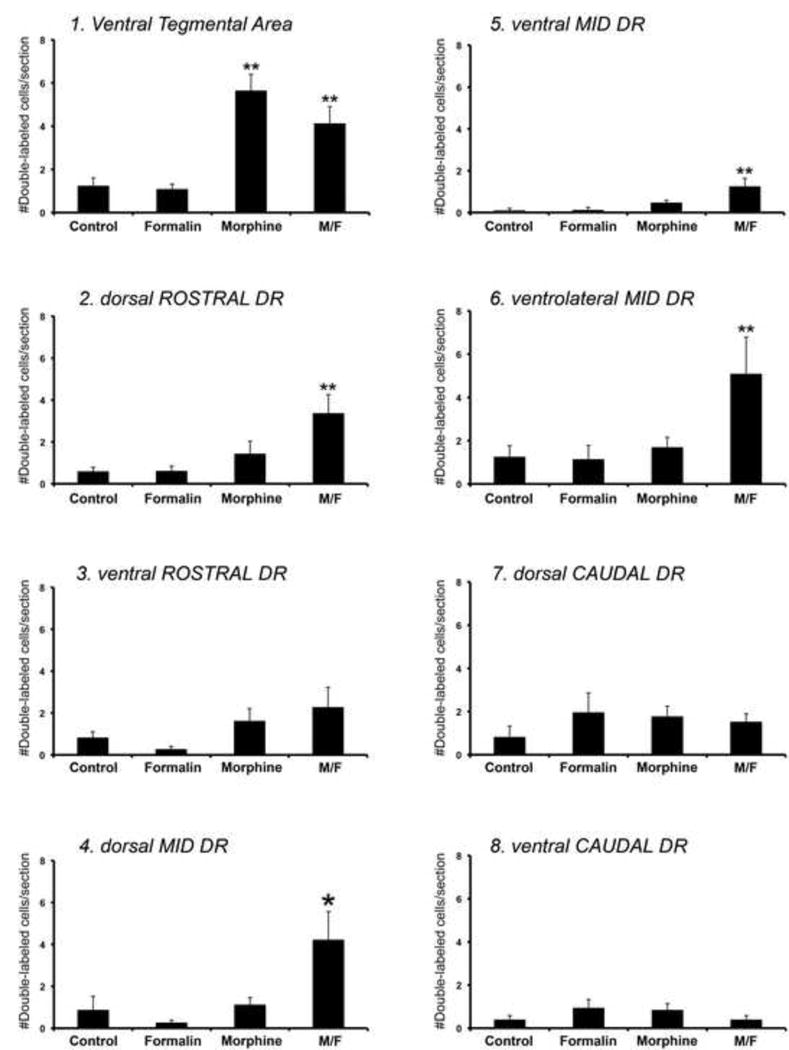
Each graph represents biased estimates of density (# double-labeled cells/section/brain) ± SD of double-labeled immunoreactive monoamine neurons with Fos in different brain regions that include dopaminergic neurons of the ventral tegmental area (Panel 1), and serotonergic neurons of the dorsal raphe nucleus (DR; Panels 2-8). The latter is divided into three subgroups: rostral (Panels 2 and 3), mid (Panels 4-6), and caudal (Panels 7 and 8), which are then further divided into dorsal and ventral parts, and in the case of mid DR also into the ventrolateral part (Panel 6). Graphs are numbered and correspond to anatomical regions distributed from the most rostral to the most caudal midbrain regions (see Fig. 1 for schematic illustrations of matching anatomical regions of interest). Analysis included four different experimental groups: (1) control, (2) formalin, (3) morphine, and (4) morphine/formalin group (M/F). Statistically significant difference in estimated density among 4 different experimental groups is found in following anatomical regions of interest: VTA (F(3,20)=14.625; p<0.001; Panel 1), dorsal part of rostral DR (F(3,20)=5.568; p=0.006; Panel 2), dorsal part of the mid DR (F(3,20)=5.411; p=0.007; Panel 4), ventral part of the mid DR (F(3,20)=6.261; p=0.004; Panel 5), and ventrolateral part of the mid DR (F(3,20)=3.776; p=0.027; Panel 6). Two-tailed p value less than 0.05 with Tukey correction was considered statistically significant. Double asterisks (**) denote statistically significant difference from control and formalin groups only, whereas single asterisk (*) denotes statistically significant difference in values from control, formalin, as well as morphine treatment groups. More specifically, in the VTA (Panel 1), pairwise comparisons with Tukey corrections of all groups showed statistically significant increase in density of double-labeled neurons in the morphine group compared to control (p<0.001) and formalin (p<0.001), and M/F group compared to control (p=0.011) and formalin (p=0.008). However, morphine and M/F groups were not statistically different between each other (p=0.287) in the VTA. Note that in the DR, morphine treatment did not cause any change in density of double-labeled serotonergic neurons with Fos. Furtheromore, in the DR, pairwise comparisons with Tukey corrections of experimental groups showed statistically significant increase in estimated density of double-labeled neurons in the morphine/formalin group compared to control (p=0.026), formalin (p=0.008), as well as morphine group (p=0.042) only in the dorsal area of the mid DR (Panel 4). In addition, statistically significant increase in density of double-labeled serotonergic neurons in morphine/formalin treatment group in comparison to control and formalin but not morphine treatment groups, were found in dorsal part of rostral DR (p=0.010 versus control; p=0.011 versus formalin; Panel 2), ventral part of the mid DR (p=0.006 versus control; p=0.007 versus formalin; Panel 5), and ventrolateral part of the mid DR (p=0.049 versus control; p=0.042 versus formalin; Panel 6). Finally, the estimated density of serotonergic neurons that were double-labeled with Fos showed no statistically significant difference among the four treatment groups in the following subgregions of the DR: ventral part of the rostral DR (F(3,20)=2.337; p=0.104; Panel 3), dorsal part of the caudal DR (F(3,20)=0.709; p=0.559; Panel 7), and ventral part of the caudal DR (F(3,20)=1.126; p=0.362; Panel 8).
3. Results
A total of 4 different treatment groups were used in this study: (1) control, (2) formalin, (3) morphine, and (4) morphine/formalin group. Analyzed anatomical areas included dopaminergic neurons of the VTA and serotonergic neurons of the DR distributed within seven different subregions of the midbrain (Fig. 1). The number of tyrosine-hydroxylase or TPOH immunoreactive neurons that were double-labeled for Fos was counted within each of these regions. Graphic representation and statistical analysis of estimated density (# double-labeled neurons/section/brain +/- SD) of double-labeled profiles in different anatomical regions per different treatment groups is illustrated in Fig. 2.
3.1. Fluorescent Microscopic Analysis of Fos Expression in the Ventral Tegmental Area
Anatomical location of the VTA in the ventral midbrain is schematically illustrated in Figure 1A. Estimated density in the VTA is graphically presented in Fig. 2 (Panel 1) and there is a statistical difference among four different treatment groups (F(3,20)=14.625; p<0.001). Pairwise comparisons with Tukey corrections of all groups showed statistically significant increase in density of double-labeled neurons in the morphine group compared to control (p<0.001) and formalin (p<0.001), and morphine/formalin group compared to control (p=0.011) and formalin (p=0.008). Therefore, animals treated with morphine in the absence and presence of noxious stimulation (in the form of plantar formalin injection) had statistically significant increase in density of double-labeled neurons (Fig. 2, Panel 1) when compared to controls or formalin treated animals. Although in animals treated with formalin following morphine administration (morphine/formalin group), density of double-labeled neurons was slightly lower than those treated with morphine alone, they were not statistically different from each other (p=0.287). More specifically, estimated density of double-labeled dopaminergic neurons with Fos (± SD) in VTA was 1.24 ± 0.88 for control, 1.09 ± 0.56 for formalin, 5.65 ± 1.84 for morphine, and 4.13 ± 1.91 for morphine/formalin treatment group. As illustrated in Fig. 3, double-labeled dopaminergic neurons of the VTA were distinguished by the presence of both tyrosine hydroxylase immunoreactivity of the neuronal cell body (green) and Fos labeling of the nucleus (red) in individual neurons.
Figure 3. Immunohistochemical labeling of dopaminergic neurons in the ventral tegmental area.
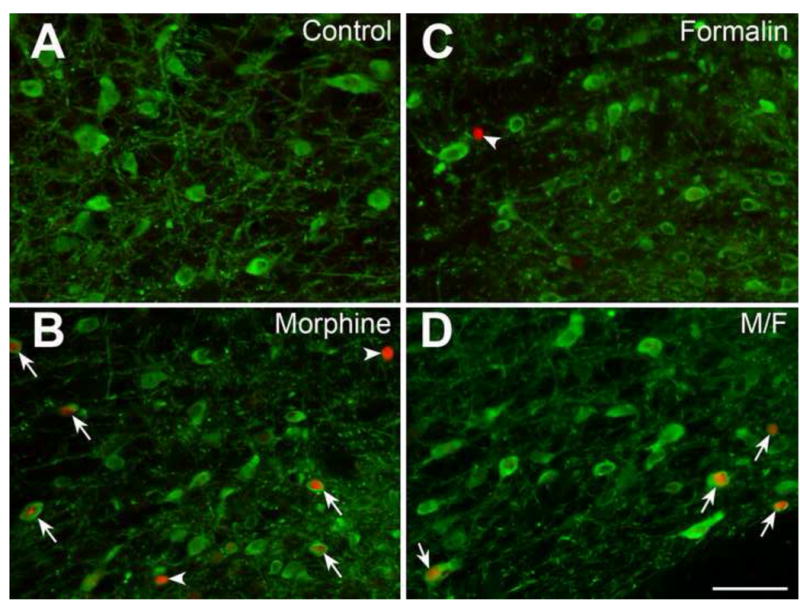
Fos immunolabeling (red) and tyrosine hydroxylase containing dopaminergic neurons (green) of the ventral tegmental area in four different experimental groups: (A) Control, (B) Formalin, (C) Morphine, and (D) Morphine/Formalin group (M/F). See Fig. 1A for schematic anatomical location of ventral tegmental area distribution. Arrowheads illustrate Fos immunolabeling only. Note the increased number of double-labeled neurons that are depicted both in morphine (C) and M/F group (D) as illustrated by arrows. No significant differences in number of double-labeled neurons were found between morphine (C) and M/F (D) treatment groups (see also Fig. 2, Panel 1 for statistical analysis). Scale bar = 100 μm throughout.
3.3. Fluorescent Microscopic Analysis of Fos Expression in the Dorsal Raphe Nucleus
Anatomical distribution and divisions of DR along the rostrocaudal axis of the midbrain is schematically represented in Fig. 1 (B-D). Analysis included total of 7 subregions of DR: rostral DR (dorsal and ventral part; Fig. 1B), mid DR (dorsal, ventral, and ventrolateral part; Fig. 1C), and caudal DR (dorsal and ventral part; Fig. 1D). Double-labeled serotonergic neurons of the DR were distinguished by the presence of TPOH immunoreactive cell bodies and dendrites and Fos immunoreactive nuclear labeling within individual neurons. Statistically significant difference in average density among four different treatment groups was topographically organized and appeared only within the following subregions: dorsal part of rostral DR (F(3,20)=5.568; p=0.006; Fig. 2, Panel 2), dorsal part of the mid DR (F(3,20)=5.411; p=0.007; Fig.2, Panel 4), ventral part of the mid DR (F(3,20)=6.261; p=0.004; Fig. 2, Panel 5), and ventrolateral part of the mid DR (F(3,20)=3.776; p=0.027; Fig. 2, Panel 6). In contrast, the estimated density of serotonergic neurons that were double-labeled with Fos showed no statistically significant difference among the four treatment groups in the following subgregions of the DR: ventral part of the rostral DR (F(3,20)=2.337; p=0.104; Fig. 2, Panel 3), dorsal part of the caudal DR (F(3,20)=0.709; p=0.559; Fig. 2, Panel 7), and ventral part of the caudal DR (F(3,20)=1.126; p=0.362; Fig. 2, Panel 8). Unlike the findings in dopaminergic neurons of the VTA, morphine administration alone did not lead to increase in number of double-labeled serotonergic neurons in any of the areas of the DR.
Furthermore, pairwise comparisons with Tukey corrections of all experimental groups showed statistically significant increase in estimated density of double-labeled neurons in the morphine/formalin group compared to control (p=0.026), formalin (p=0.008), as well as morphine group (p=0.042) only in the dorsal area of the mid DR (Fig.2, Panel 4). More specifically, estimated density of double-labeled serotonergic neurons with Fos (± SD) in dorsal area of the mid DR was 0.88 ± 1.58 for control, 0.27 ± 0.27 for formalin, 1.13 ± 0.82 for morphine, and 4.23 ± 3.27 for morphine/formalin treatment group. In addition, statistically significant increase in density of double-labeled serotonergic neurons in morphine/formalin treatment group in comparison to control and formalin but not morphine treatment groups, were found in dorsal part of rostral DR (p=0.010 versus control; p=0.011 versus formalin; Fig. 2, Panel 2), ventral part of the mid DR (p=0.006 versus control; p=0.007 versus formalin; Fig. 2, Panel 5), and ventrolateral part of the mid DR (p=0.049 versus control; p=0.042 versus formalin; Fig. 2, Panel 6). Although the estimated density of double-labeled neurons (± SD) in morphine/formalin group in the ventrolateral mid DR was the greatest (5.09 ± 4.14) and larger than that in the dorsal mid DR (4.23 ± 3.27), it was not statistically different from the value in morphine treatment group (1.7 ± 1.11), probably due to a large animal to animal variability in response within this area. Representative photomicrographs of labeling that included serotonergic neurons of the DR (green) and Fos labeling of nuclei (red) in four different treatment groups is illustrated in Fig. 4 for dorsal and ventrolateral areas of mid DR. Finally, for the purpose of identifying dopaminergic neurons that might be present in the rostral DR, triple labeling using Fos, tyrosine hydroxylase, and TPOH was used to determine the localization of those neurons and confirm that cells dually labeled for TPOH and Fos were not dopaminergic. Tyrosine-hydroxylase neurons were clearly detectable, and were found in the area of ventral periaqueductal gray corresponding to the dopaminergic neurons of the A10 dorsal-caudal cell group. Although those dopaminergic neurons contained light immunolabeling for TPOH, none were dually labeled with Fos immunoreactivity.
Figure 4. Immunohistochemical labeling of serotonergic neurons in the dorsal raphe nucleus.
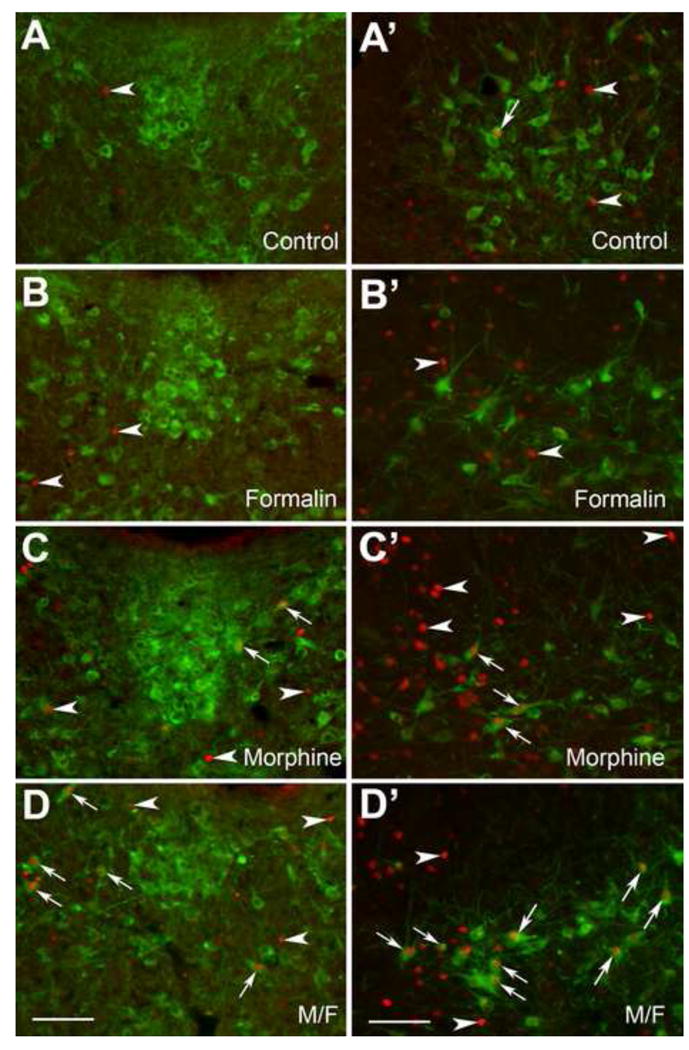
Panels A-D represent area of the mid dorsal raphe nucleus, whereas Panels A′-D′ illustrate ventrolateral part of the mid nucleus raphe magnus located in the ventrolateral periaqueductal gray. See Fig. 1C for schematic anatomical location of dorsal and ventrolateral part of the mid dorsal raphe nucleus. Fos immunolabeling (red) and tryptophan hydroxylase (TPOH) containing serotonergic neurons (green) of the dorsal raphe nucleus are shown in four different experimental groups: (A, A′) Control, (B, B′) Formalin, (C, C′) Morphine, and (D, D′) Morphine/Formalin group (M/F). Note individual nuclear Fos immunolabeling in each of the treatment groups as indicated by arrowheads. In control rats (A′), occasional TPOH-immunoreactive serotonergic neuron was double-labeled with Fos (arrow). In morphine injected rats (C, C′), some TPOH-immunoreactive serotonergic neurons were double-labeled with Fos (arrows). Note the increased number of double-labeled neurons that are depicted in M/F group (D, D′) as illustrated by arrows (see also Fig. 2, Panel 4 and 6 for statistical analysis). Scale bar = 100 μm throughout.
4. Discussion
The present study demonstrates that the effect of acute morphine on the DR differs when there is concurrent noxious stimulation. This effect is topographically organized and selectively impacts serotonergic neurons from the dorsal areas of the mid DR. However, increased Fos expression in dopaminergic neurons of the VTA by systemic morphine was not different in the presence or absence of acute noxious stimulation. Our results suggest that morphine administration may have similar effect on ascending dopaminergic systems of the VTA regardless of pain state, thus retaining the potential for activating motivation and reward circuits. In contrast, morphine effects on ascending serotonergic neurons are distinct in the context of pain, suggesting the potential for differential regulation of affective state. These findings suggest that morphine does not simply block access of nociceptive signals to supraspinal brain areas, but rather there are interactions between opioid and nociceptive stimulation within areas coordinating affective state and motivated behavior.
4.1. Methodological Considerations
Fos expression is thought to correlate with biochemical activity of cells (Hunt et al., 1987; Morgan et al., 1987) and has been used as a marker of neuronal activity (Sagar et al., 1988; Sharp et al., 1993). However, Fos expression is vulnerable to both false-negative and false positive error. For example, some neurons when activated may activate other immediate-early genes but not the gene for Fos protein, c-fos. Likewise, the amount of activation that is sufficient to initiate expression of c-fos may be different for different types of neurons. Both of these factors together might lead to under-detection of activation (Dragunow and Faull, 1989). However, the major advantage of Fos immunolabeling is that it is robustly expressed and can be easily detected and quantified. More specifically, Fos protein expression has been used to understand the effects of sensory (Hunt et al., 1987) and noxious stimulation (Hunt et al., 1987; Leah et al., 1989; Bullitt, 1990), as well as the effects of analgesics and drugs of abuse including morphine (Chang et al., 1988; Liu et al., 1994).
4.2. Dopaminergic Neurons of the VTA
The results of the present study did not show any increase in Fos expression in dopaminergic neurons of the VTA following noxious stimulation alone in the form of intraplantar formalin injection. This is in agreement with report that noxious stimulation selectively depresses dopaminergic neurons (Ungless et al., 2004). However, our findings are in contrast to the study by Ma et al. (1993), which reported significant increase in number of Fos immunoreactive dopaminergic neurons of the VTA following noxious stimulation with plantar injection of 5% formalin. Since we used a lower concentration of formalin (2.5%), it is plausible that in our study stimulation is subthreshold to produce Fos expression in dopaminergic neurons of the VTA.
Acute administration of morphine produces Fos expression in the VTA (Singh et al., 2004) and nucleus accumbens (Liu et al., 1994; Garcia et al., 1995), brain areas that have been associated with drug reinforcement and addiction processes. Our study is consistent with previously published study by Singh et al. (2004), and extended these results by providing evidence that increased number of double-labeled dopaminergic neurons with Fos following morphine treatment appears unaffected by noxious stimulation. This suggests that regardless of pain state, morphine retains the capacity to activate dopaminergic pathways that are implicated in motivation, reward and addiction-related behavior.
4.3. Serotonergic neurons of the Dorsal Raphe Nucleus
The results of the present study did not show any increase in Fos expression in serotonergic neurons in any part of the DR following noxious stimulation in the form of intraplantar 2.5% formalin injection alone or morphine injection alone. Previous studies reported increased Fos immunoreactivity in the region of periaqueductal gray where serotonergic neurons of DR are distributed following noxious stimulation alone (Bullitt, 1990; Keay et al., 1994), as well as following systemic administration of morphine alone (Loyd et al., 2007). This suggests that neither stimulus alone may be robust enough to activate Fos expression in serotonergic neurons of the DR. Supporting this contention, Chen et al. (2003) reported a high proportion of double-labeled serotonergic neurons with Fos in DR after somatic noxious stimulation with 10% formalin solution but not 1 or 5% solutions.
Dorsal and Ventrolateral Region of the Mid Dorsal Raphe Nucleus
In the present study we demonstrated that increased number of serotonergic neurons with Fos in the DR is topographically organized following morphine administration in the presence of the noxious stimulus (morphine/formalin group). More specifically, it was only the area of the dorsal region of the mid DR that had statistically significant increase in number of double-labeled neurons following noxious stimulation in the presence of morphine in comparison to other groups. Although the greatest number of double-labeled serotonergic neurons was found in the ventrolateral region of mid DR in the morphine/formalin treatment group, there was no statistically significant difference from morphine alone treated group. Greater animal-to-animal variability in response to noxious stimulus in the presence of morphine within ventrolateral part of mid DR could explain the lack of statistically significant difference.
4.4. Functional significance
Drugs of abuse have been hypothesized to produce their rewarding effects by neuropharmacological action on a common brain reward circuit called the extended amygdala that involves the mesolimbic dopamine system (Spanagel and Weiss, 1999), and several other subregions of the basal forebrain (shell of the nucleus accumbens, the bed nucleus of the stria terminalis, and the central nucleus of the amygdala) (Leshner and Koob, 1999; Weiss and Koob, 2001). Serotonergic neurons of DR are implicated as a modulator of the mesolimbic dopamine system (Jacobs et al., 1978; Guan and McBride, 1989; Hagan et al., 1990; Benloucif and Galloway, 1991; Yoshimoto and McBride, 1992). Furthermore, anatomical studies have identified that serotonergic neurons within dorsal area of the mid DR (also described as dorsomedial subdivision of DR, around 8 mm from bregma) project to the central nucleus of the amygdala (Commons et al., 2003; Petrov et al., 1994), a forebrain structure involved in the processing and memorizing of emotional responses. Surrounding these neurons are cells that project to the basolateral amygdala (Abrams et al., 2005), an important area for emotionally-motivated learning (see review (McGaugh, 2004)). Thus, our results raise the possibility that the presence of the noxious stimulation can modify acute morphine's effect on serotonergic neurons originating from the dorsal region of the mid DR that are involved in modulation of affective state and emotional learning.
4.5. Conclusions
The results of the present studies suggest that morphine administration may have a similar effect on ascending dopaminergic system originating from the VTA regardless of the pain state. In contrast, our results suggest that serotonergic influence from the dorsal area of the mid DR to the forebrain areas involved in mood and motivated behavior is likely distinct in the context of pain. Therefore, while morphine may retain the potential for reward, this function may be differentially modified in the context of pain by serotonergic activation. While addiction and dependence are processes that require continued exposure to opioids, these observations raise the possibility that the neurochemical consequences of acute exposure may be different when an individual is in pain. This could modify the subjective experience produced by opioids as well as the propensity for repeated usage after pain has subsided.
Acknowledgments
This work was supported by the National Institute on Drug Abuse grant, DA-021801. Thoughtful comments on the manuscript by Dr. Charles Berde were greatly appreciated. Authors would also like to acknowledge Mr. David Zurakowski for the help with statistical analysis.
Abbreviations
- DR
dorsal raphe nucleus
- PB
phosphate buffer
- TPOH
tryptophan hydroxylase
- VTA
ventral tegmental area
Footnotes
Section Editor: Dr. Miles Herkenham
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott FV, Franklin KBJ, Westbrook RF. The formalin test: scoring properties of the first and second phases of the pain response in rats. Pain. 1995;60:91–102. doi: 10.1016/0304-3959(94)00095-V. [DOI] [PubMed] [Google Scholar]
- Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133(4):983–97. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Alcaro A, Huber R, Panksepp J. Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective. Brain Res Rev. 2007;56:283–321. doi: 10.1016/j.brainresrev.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne JC. Opioid analgesia: perspectives on right use and utility. Pain Physician. 2007;10:479–491. [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drungs: a meta-analysis. Neurosci Biobehavior Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Beart PM, McDonald D. 5-Hydroxytryptamine and 5-hydroxytryptaminergic-dopaminergic interactions in the ventral tegmental area of rat brain. J Pharm Pharmacol. 1982;34:591–593. doi: 10.1111/j.2042-7158.1982.tb04801.x. [DOI] [PubMed] [Google Scholar]
- Benloucif S, Galloway MP. Facilitation of dopamine release in vivo by serotonin agonists: studies with microdialysis. Eur J Pharmacol. 1991;200:1–8. doi: 10.1016/0014-2999(91)90658-d. [DOI] [PubMed] [Google Scholar]
- Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol. 1990;296:517–530. doi: 10.1002/cne.902960402. [DOI] [PubMed] [Google Scholar]
- Chang SL, Squinto SP, Harlan RE. Morphine activation of c-fos expression in rat brain. Biochem Biophys Res Commun. 1988;157:698–704. doi: 10.1016/s0006-291x(88)80306-1. [DOI] [PubMed] [Google Scholar]
- Chen T, Dong YX, Li YQ. Fos expression in serotonergic neurons in the rat brainstem following noxious stimuli: an immunohistochemical double-labelling study. J Anat. 2003;203:579–588. doi: 10.1046/j.1469-7580.2003.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero J, Ennis T, Ogden J, Meyer ER. Gender differences in the reinforcing properties of morphine. Pharm Bioch Behav. 2000;65:91–96. doi: 10.1016/s0091-3057(99)00174-4. [DOI] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28:206–215. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brainstem stimulation in rats and cats. Pain. 1977;4:161–164. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Garcia MM, Brown HE, Harlan RE. Alterations in immediate-early gene proteins in the rat forebrain induced by acute morphine injection. Brain Res. 1995;692:23–40. doi: 10.1016/0006-8993(95)00625-z. [DOI] [PubMed] [Google Scholar]
- Guan XM, McBride WJ. Serotonin microinfusion into the ventral tegmental area increases accumbens dopamine release. Brain Res Bull. 1989;23:541–547. doi: 10.1016/0361-9230(89)90198-6. [DOI] [PubMed] [Google Scholar]
- Hagan RM, Jones BJ, Jordan CC, Tyers MB. Effect of 5-HT3 receptor antagonists on responses to selective activation of mesolimbic dopaminergic pathways in the rat. Br J Pharmacol. 1990;99:227–232. doi: 10.1111/j.1476-5381.1990.tb14685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Imai H, Park MR, Steindler DA, Kitai ST. The morphology and divergent axonal organization of midbrain raphe projection neurons in the rat. Brain Dev. 1986a;8:343–354. doi: 10.1016/s0387-7604(86)80054-7. [DOI] [PubMed] [Google Scholar]
- Imai H, Steindler DA, Kitai ST. The organization of divergent axonal projections from the midbrain raphe nuclei in the rat. J Comp Neurol. 1986b;243:363–380. doi: 10.1002/cne.902430307. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Foote SL, Bloom FE. Differential projections of neurons within the dorsal raphe nucleus of the rat: a horseradish peroxidase (HRP) study. Brain Res. 1978;147:149–153. doi: 10.1016/0006-8993(78)90779-5. [DOI] [PubMed] [Google Scholar]
- Keay KA, CI C, Owler B, Depaulis A, Bandler R. Convergence of deep somatic and vesceral nociceptive information onto a discrete ventrolateral midbrain periaqueductal gray region. Neuroscience. 1994;61:727–732. doi: 10.1016/0306-4522(94)90395-6. [DOI] [PubMed] [Google Scholar]
- Lang B, Li YQ. Serotoninergic neurons in the brainstem expressing FOS protein after orofacial noxious stimulation: an immunocytochemical double-labeling study. J Hirnforsch. 1998;39:263–268. [PubMed] [Google Scholar]
- Leah JD, Herdegen T, Zimmermann M. Physiological and pharmacological induction of c-fos protein immunoreactivity in superficial dorsal horn neurons. In: Cervero F, editor. Processing of Sensory Information in the Superficial Dorsal Horn of the Spinal Cord. Vol. 176. New York: Plenum Press; 1989. pp. 307–310. [Google Scholar]
- Leshner AI, Koob GF. Drugs of abuse and the brain. Proc Assoc Am Physicians. 1999;111:99–108. doi: 10.1046/j.1525-1381.1999.09218.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Nickolenko J, Sharp FR. Morphine induces c-fos and junB in striatum and nucleus accumbens via D1 and N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 1994;91:8537–8541. doi: 10.1073/pnas.91.18.8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, Morgan MM, Murphy AZ. Morphine preferentially activates the periaqueductal gray-rostral ventromedial medullary pathway in the male rat: a potential mechanism for sex differences in antinociception. Neuroscience. 2007;147:456–468. doi: 10.1016/j.neuroscience.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luccarini P, Childeric A, Gaydier AM, Voisin D, Dallel R. The orofacial formalin test in the mouse: a behvioral model for studying physiology and modulation of trigeminal nociception. J Pain. 2006;7:908–914. doi: 10.1016/j.jpain.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Ma QP, Zhou Y, Han JS. Noxious stimulation accelerated the expression of c-fos protooncogene in cholecystokininergic and dopaminergic neurons in the ventral tegmental area. Peptides. 1993;14:561–566. doi: 10.1016/0196-9781(93)90145-7. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Minabe Y, Emori K, Ashby CR., Jr The depletion of brain serotonin levels by para-chlorophenylalanine administration significantly alters the activity of midbrain dopamine cells in rats: an extracellular single cell recording study. Synapse. 1996;22:46–53. doi: 10.1002/(SICI)1098-2396(199601)22:1<46::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping pattern of c-fos expression in the central nervous system after seizure. Science. 1987;237:192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Fossum EN, Stalding BM, King MM. Morphine antinociceptive potency on chemical, mechanical, and thermal nociceptive tests in the rat. J Pain. 2006;7:358–366. doi: 10.1016/j.jpain.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates Orlando. FL: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Petrov T, Krukoff TL, Jhamandas JH. Chemically defined collateral projections from the pons to the central nucleus of the amygdala and hypothalamic paraventricular nucleus in the rat. Cell Tissue Res. 1994;277:289–295. doi: 10.1007/BF00327776. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Porter J, Jick H. Addiction rare in patients treated with narcotics. N Engl J Med. 1980;302:123. doi: 10.1056/nejm198001103020221. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Dual dopamine/serotonin releasers: potential treatment agents for stimulant addiction. Exp Clin Psychopharmacol. 2008;16:458–474. doi: 10.1037/a0014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Sagar SM, Swanson RA. Metabolic mapping with cellular resolution: c-fos vs. 2-deoxyglucose. Crit Rev Neurobiol. 1993;7:205–228. [PubMed] [Google Scholar]
- Singh ME, Verty AN, Price I, McGregor IS, Mallet PE. Modulation of morphine-induced Fos-immunoreactivity by the cannabinoid receptor antagonist SR 141716. Neuropharmacology. 2004;47:1157–1169. doi: 10.1016/j.neuropharm.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kishimoto Y, Ozaki S, Narita M. Mechanism of opioid dependence and interaction between opioid receptors. Eur J Pain. 2001;5 A:63–65. doi: 10.1053/eujp.2001.0282. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Cannon MS, Raese JD. Identification of dopamine-containing cell bodies in the dorsal and median raphe nuclei of the rat brain using tyrosine hydroxylase immunochemistry. Brain Res Bull. 1985;15:229–234. doi: 10.1016/0361-9230(85)90142-x. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Biswas A, Pickel VM. Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Res. 1993;624:188–198. doi: 10.1016/0006-8993(93)90077-z. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel DM, Pasterkamp RJ. Getting connected in the dopamine system. Prog Neurobiol. 2008;85:75–93. doi: 10.1016/j.pneurobio.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Weiss F, Koob GF. Drug addiction: functional neurotoxicity of the brain reward systems. Neurotox Res. 2001;3:145–156. doi: 10.1007/BF03033235. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Yan QS, Yan SE. Activation of 5-HT(1B/1D) receptors in the mesolimbic dopamine system increases dopamine release from the nucleus accumbens: a microdialysis study. Eur J Pharmacol. 2001;418:55–64. doi: 10.1016/s0014-2999(01)00913-x. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ. Regulation of nucleus accumbens dopamine release by the dorsal raphe nucleus in the rat. Neurochem Res. 1992;17:401–407. doi: 10.1007/BF00969884. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


